Chemical Composition and Biological Activities of Oregano Essential Oil and Its Fractions Obtained by Vacuum Distillation
Abstract
:1. Introduction
2. Results
2.1. Obtention of the Oregano Essential Oil and Fractions
2.2. Physicochemical Characteristics
2.3. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
2.4. Antioxidant Activity
2.5. Antimicrobial Activity
2.6. Analysis of Main Components
3. Discussion
4. Materials and Methods
4.1. Plant Material and Reagents
4.2. Acquisition of the Oregano Essential Oil and Fractions
4.3. Physicochemical Characteristics of the Oils
4.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
4.5. Antioxidant Activity
4.6. Antimicrobial Activity
4.6.1. Microbial Strains and Growth Conditions
4.6.2. Preliminary Antimicrobial Activity
4.6.3. Assessment of Minimum Inhibitory Concentration
4.7. Principal Component analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gooch, J.W. Essential oils. In Encyclopedic Dictionary of Plymers; Springer: New York, NY, USA, 2011; p. 274. [Google Scholar]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Nazzaro, F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven Lamiaceae essential oils. Molecules 2009, 14, 4213–4230. [Google Scholar] [CrossRef]
- Białon, M.; Krzysko-Łupicka, T.; Pik, A.; Wieczorek, P.P. Chemical composition of herbal macerates and corresponding commercial essential oils. Molecules 2017, 22, 1887. [Google Scholar]
- De Falco, E.; Mancini, E.; Roscigno, G.; Mignola, E.; Taglialatela-Scafati, O.; Senatore, F. chemical composition and biological activity of essential oils of Origanum vulgare L. subsp. vulgare L. under different growth conditions. Molecules 2013, 18, 14948–14960. [Google Scholar] [CrossRef]
- Olmedo, R.; Nepote, V.; Grosso, N. Antioxidant activity of fractions from oregano essential oils obtained by molecular distillation. Food Chem. 2014, 156, 212–219. [Google Scholar] [CrossRef]
- García-Pérez, J.S.; Cuéllar-Bermúdez, S.P.; de la Cruz-Quiroz, R.; Arévalo-Gallegos, A.; Esquivel-Hernandez, D.A.; Rodríguez-Rodríguez, J.; García-García, R.; Iqbal, H.M.N.; Parra-Saldivar, R. Supercritical CO2 based tailor made valorization of Origanum vulgare L. extracts: A green approach to extract high-value compounds with applied perspectives. J. Environ. Manag. 2019, 232, 796–802. [Google Scholar]
- Borgarello, A.V.; Mezza, G.N.; Pramparo, M.C.; Gayol, M.F. Thymol enrichment from oregano essential oil by molecular distillation. Sep. Purif. Technol. 2015, 153, 60–66. [Google Scholar] [CrossRef]
- Oniga, I.; Puscas, C.; Silaghi-Dumitrescu, R.; Olah, N.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Zhang, X.L.; Guo, Y.S.; Wang, C.H.; Li, G.Q.; Xu, J.J.; Chung, H.Y.; Ye, W.C.; Li, Y.L.; Wang, G.C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef]
- Dutra, T.V.; Castro, J.C.; Menezes, J.; Ramos, R.; Nunes do Parado, I.; Machinski, M.; Graton, J.M.; de Abreu Filho, B.A. Bioactivity of oregano (Origanum vulgare) essential oil against Alicyclobacillus spp. Ind. Crop. Prod. 2019, 129, 345–349. [Google Scholar] [CrossRef]
- Chuang, L.; Tsai, T.; Lien, T.; Huang, W.; Liu, J.; Chang, H.; Chang, M.; Tsai, P.-J. Ethanolic extract of origanum vulgare suppresses propionibacterium acnes -induced inflammatory responses in human monocyte and mouse ear edema models. Molecules 2018, 23, 1987. [Google Scholar] [CrossRef]
- Cheng, C.; Zou, Y.; Peng, J. Oregano essential oil attenuates RAW264.7 cells from lipopolysaccharide-induced inflammatory response through regulating NADPH Oxidase activation-driven oxidative stress. Molecules 2018, 23, 1857. [Google Scholar] [CrossRef]
- Gonceariuc, M.; Balmus, Z.; Benea, A.; Barsan, V.; Sandu, T. Biochemical diversity of the Origanum vulgare ssp. vulgare L. and Origanum vulgare ssp. hirtum (Link) letswaart genotypes from Moldova. J. ASM Life Sci. 2015, 2, 92–100. [Google Scholar]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; De Feo, V.; Camele, I. Cytotoxic activity of Origanum vulgare L. on hepatocellular carcinoma cell line HepG2 and evaluation of its biological activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef]
- Gîrd, C.E.; Dutu, L.E.; Costea, T.; Nencu, I.; Popescu, M.L.; Tudorel, O.O. Preliminary research concerning the obtaining of herbal extracts with potential neuroprotective activity note I. Obtaining and characterization of a selective Origanum vulgare L. dry extract. Farmacia (Bucharest Rom.) 2016, 64, 680–687. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Su, L.; Yin, J.; Charles, D.; Zhou, K.; Moore, J.; Yu, L. Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem. 2007, 990–997. [Google Scholar] [CrossRef]
- Torres-Alvarez, C.; Núñez-González, A.; Rodríguez, J.; Castillo, S.; Leos-Rivas, C.; Báez-González, J.G. Chemical composition, antimicrobial, and antioxidant activities of orange essential oil and its concentrated oils. CyTA J. Food 2017, 15, 129–135. [Google Scholar] [CrossRef]
- Baydar, H.; Sağdiç, O.; Özkan, G.; Karadoğan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Regalado-González, C.; Vázquez-Landaverde, P.; Guerrero-Legarreta, I.; García-Almendárez, B.E. Microencapsulation, chemical characterization, and antimicrobial activity of Mexican (Lippia graveolens H. B. K.) and European (Origanum vulgare L.) oregano essential oils. Sci. World J. 2014, 2014, 641814. [Google Scholar] [CrossRef]
- Vardar-Ünlü, G.; Candan, F.; Sökmen, A.; Daferera, D.; Polissiou, M.; Sökmen, M.; Dönmez, E.; Tepe, B. Antimicrobial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey. Var. pectinatus (Lamiaceae). J. Agric. Food Chem. 2003, 51, 63–67. [Google Scholar]
- Milos, M.; Makota, D. Investigation of antioxidant synergisms and antagonisms among thymol, carvacrol, thymoquinone and p-cymene in a model system using Briggs-Rauscher oscillating reaction. Food Chem. 2012, 131, 296–299. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Milos, M.; Mastelic, J.; Jerkovic, I. Chemical composition and antioxidant effect of glycosidically bound volatile compounds from oregano (Origanum vulgare L.ssp. hirtum). Food Chem. 2000, 71, 79–83. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Du, E.; Gan, L.; Li, Z.; Wang, W.; Liu, D.; Guo, Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotech. 2015, 6, 58. [Google Scholar] [CrossRef]
- García-García, R.; López-Malo, A.; Palou, E. Bactericidal action of binary and ternary mixtures of carvacrol, thymol, and eugenol against Listeria innocua. J. Food Sci. 2011, 76, M95–M100. [Google Scholar] [CrossRef]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the antimicrobial activity and cytotoxicity of different components of natural origin present in essential oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef]
- Lima, I.O.; Pereira, F.D.O.; Oliveira, W.A.D.; Lima, E.D.O.; Menezes, E.A.; Cunha, F.A.; Diniz, M.D.F.F.M. Antifungal activity and mode of action of carvacrol against Candida albicans strains. J. Essent. Oil Res. 2013, 25, 138–142. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012, 3, 1–24. [Google Scholar] [CrossRef]
- Bassolé, I.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebié, R.; Dicko, M. Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules 2010, 15, 7825–7839. [Google Scholar] [CrossRef]
- Rahman, A.; Shanta, Z.S.; Rashid, M.A.; Parvin, T.; Afrin, S.; Khatun, M.K.; Sattar, M.A. In vitro antibacterial properties of essential oil and organic extracts of Premna integrifolia Linn. Arab J. Chem. 2016, 9, S475–S479. [Google Scholar] [CrossRef]
- Pichette, A.; Larouche, P.L.; Lebrun, M.; Legault, J. Composition and antibacterial activity of Abies balsamea essential oil. Phytother. Res. 2016, 20, 371–373. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Q.; Liu, Y.; Zhou, X.; Wang, X. Isolation and biological activities of decanal, linalool, valencene, and octanal from sweet orange oil. J. Food Sci. 2012, 77, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Frassinetti, S.; Caltavuturo, L.; Cini, M.; DellaCroce, C.M.; Maserti, B.E. Antibacterial and antioxidant activity of essential oils from Citrus spp. J. Essent. Oil Res. 2011, 23, 27–31. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
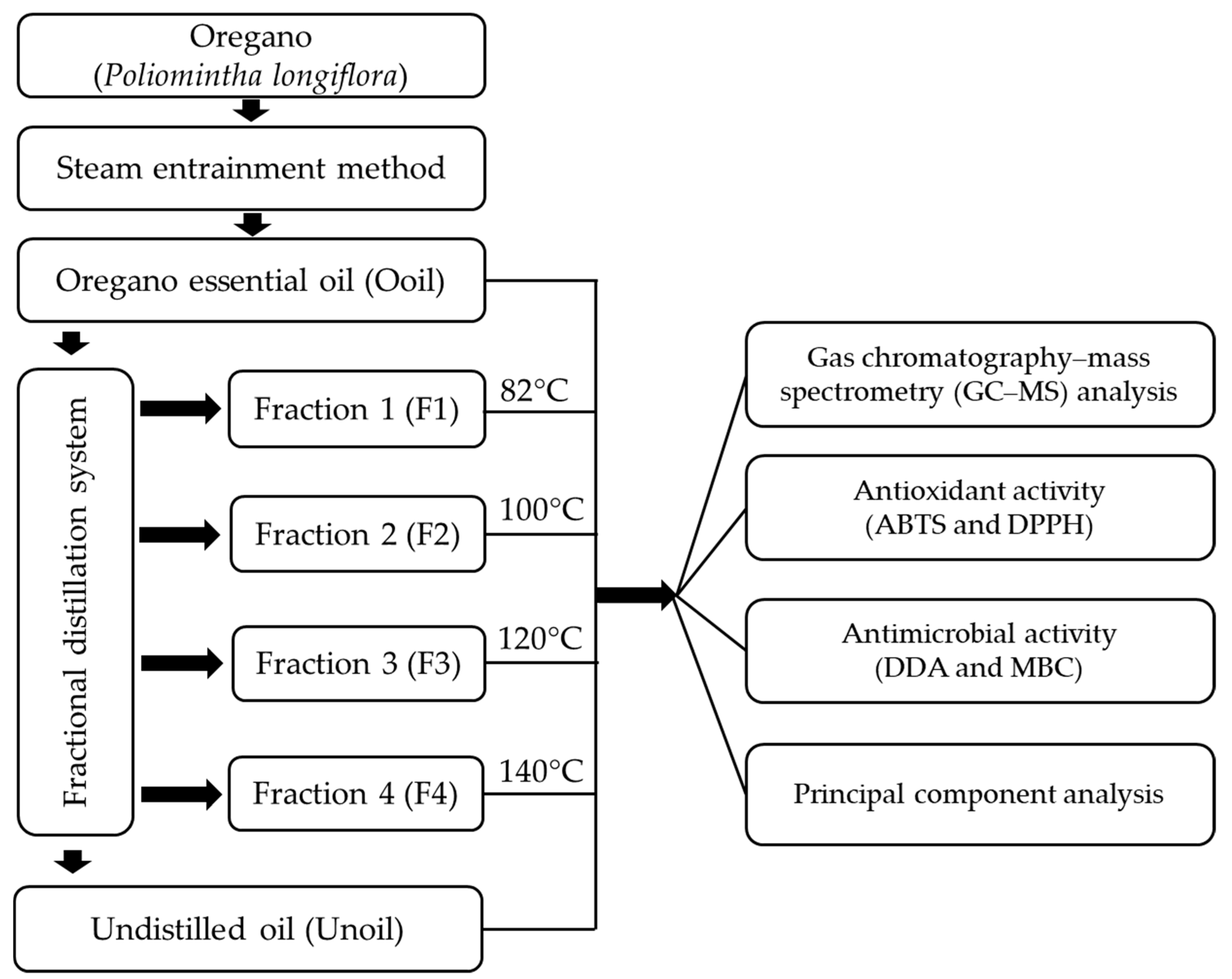
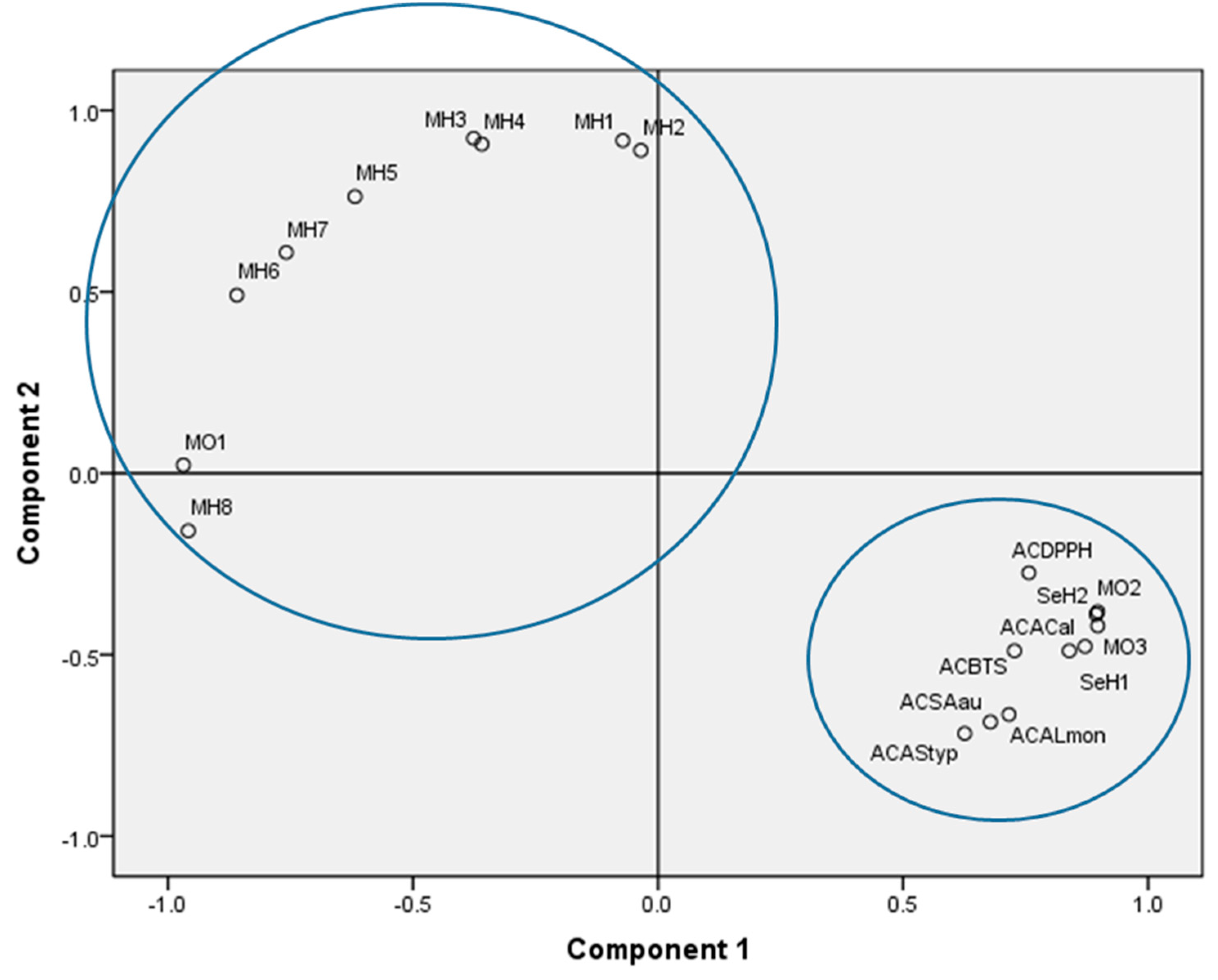
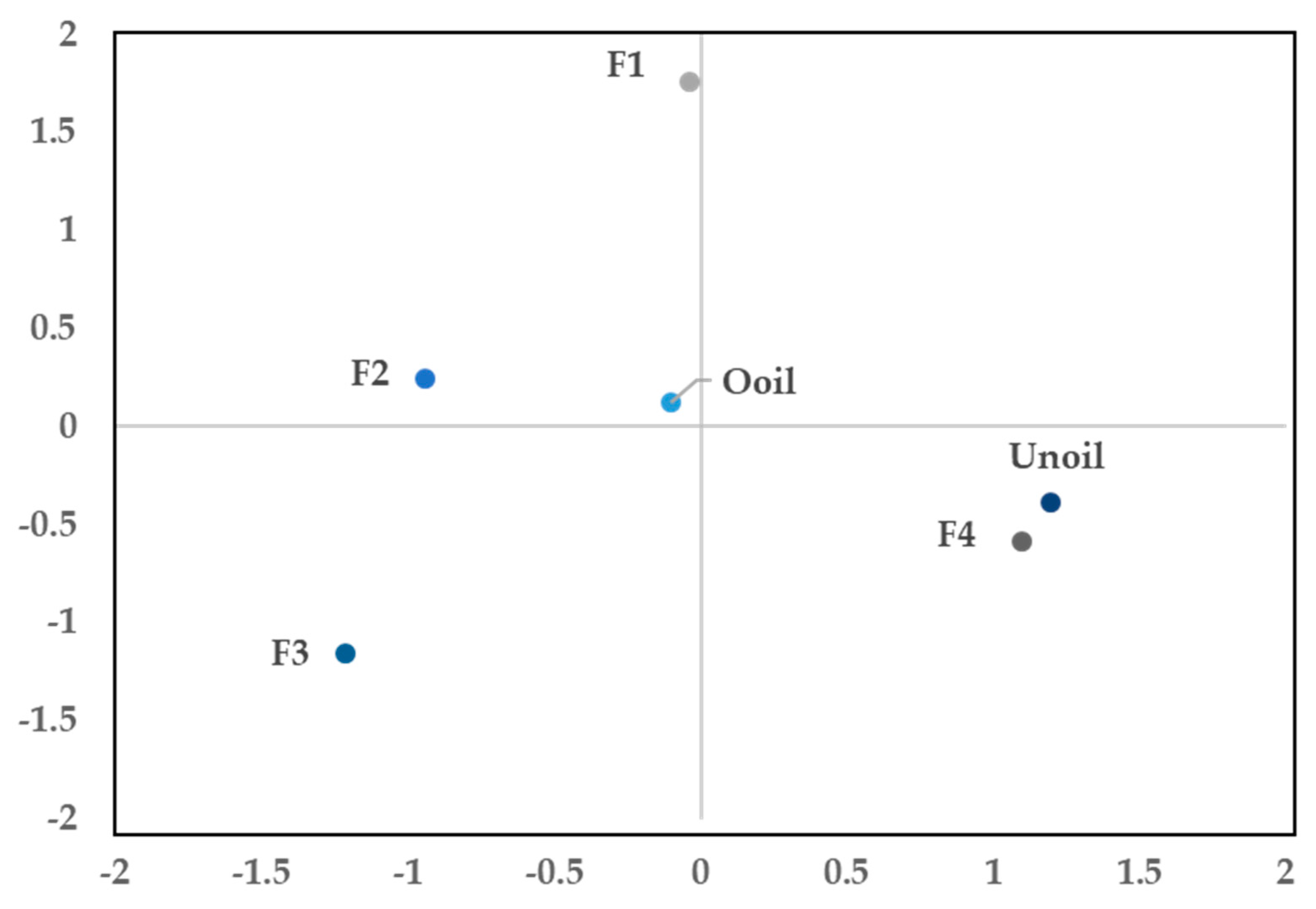
| Oil | Code | Color | Odor | Specific Gravity (20 °C) g/mL | Refractive Index (20 °C) | Brix |
|---|---|---|---|---|---|---|
| Fraction 1 | F1 | Colorless | Soft | 0.842 ± 0.0 | 1.47 ± 0.0 | 75.72 ± 0.0 |
| Fraction 2 | F2 | Colorless | Soft | 0.845 ± 0.0 | 1.48 ± 0.0 | 77.20 ± 0.0 |
| Fraction 3 | F3 | Colorless | Strong | 0.859 ± 0.0 | 1.48 ± 0.0 | 77.77 ± 0.0 |
| Fraction 4 | F4 | Colorless | Strong | 0.884 ± 0.0 | 1.51 ± 0.0 | 88.95 ± 0.0 |
| Undistilled oil | Unoil | Brown | Strong | 0.927 ± 0.0 | 1.51 ± 0.0 | 89.16 ± 0.0 |
| Oregano Oil | Ooil | Light Yellow | Strong | 0.778 ± 0.0 | 1.48 ± 0.0 | 76.06 ± 0.0 |
| Compound | Boiling Point °C | Code | % de Relative Area 1 | |||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | Unoil | Ooil | |||
| α-thujene | 150–152 | MH1 | 5.03 | 0.389 | ND | ND | ND | 1.74 |
| α-pinene | 156 | MH2 | 3.01 | ND | ND | ND | ND | 1.07 |
| β-myrcene | 166–168 | MH3 | 11.62 | 6.93 | 1.08 | ND | ND | 5.50 |
| Phellandrene | 172 | MH4 | 1.32 | 1.00 | ND | ND | ND | 0.72 |
| α-terpinene | 174 | MH5 | 8.91 | 8.32 | 2.90 | ND | ND | 5.57 |
| o-cymene | 174 | MH6 | 47.96 | 53.97 | 38.14 | 1.31 | 0.973 | 39.13 |
| Limonene | 175 | MH7 | 2.29 | 2.71 | 1.25 | ND | ND | 1.58 |
| 1,8-cineole | 177 | MO1 | 1.51 | 1.77 | 2.74 | ND | ND | 1.53 |
| γ-terpinene | 181–183 | MH8 | 15.59 | 24.43 | 40.57 | 1.40 | 0.94 | 22.34 |
| Thymol | 232 | MO2 | ND | ND | ND | 5.08 | 3.77 | 1.71 |
| Carvacrol | 237–238 | MO3 | ND | ND | 4.58 | 60.03 | 64.31 | 12.60 |
| Trans-caryophyllene | 268 | SeH1 | ND | ND | 2.97 | 18.96 | 13.78 | 3.47 |
| α-humulene | 276 | SeH2 | ND | ND | 0.34 | 6.16 | 8.36 | 1.56 |
| Monoterpene hydrocarbons (MH) | 95.73 | 97.75 | 83.94 | 2.71 | 1.91 | 77.65 | ||
| Monoterpene oxygenated (MO) | 1.51 | 1.77 | 7.32 | 65.11 | 68.08 | 15.84 | ||
| Sesquiterpene hydrocarbons (SeH) | ND | ND | 3.31 | 25.12 | 22.14 | 5.03 | ||
| Total identified components | 97.24 | 99.52 | 94.57 | 92.94 | 92.13 | 98.52 | ||
| Oil | Method | |
|---|---|---|
| DPPH | ABTS | |
| F1 | 2.91 ± 0.58 d | 14.07 ± 1.14 e |
| F2 | 6.71 ± 1.10 d | 10.76 ± 2.91 e |
| F3 | 161.83 ± 4.76 d | 26,002.33 ± 1220.15 d |
| F4 | 6025.03 ± 230.78 b | 177,016.31 ± 7369.93 a |
| Unoil | 22,129.54 ± 615.53 a | 150,310.58 ± 3609.10 b |
| Ooil | 4177.52 ± 181.62 c | 61,500.67 ± 522.20 c |
| Oil | Method | |
|---|---|---|
| DPPH | ABTS | |
| F1 | 114,507 ± 15,060 b | 106,621 ± 1454 b |
| F2 | 103,563 ± 13,021 b | 160,796 ± 2297 c |
| F3 | 3968 ± 271 a | 162 ± 10 a |
| F4 | 563 ± 51 a | 17 ± 1 a |
| Unoil | 117 ± 17 a | 22 ± 2 a |
| Ooil | 886 ± 23 a | 85 ± 3 a |
| Oil Fraction | Diameter of Inhibition Zone (cm) | |||
|---|---|---|---|---|
| Staphylococcus aureus | Listeria monocytogenes | Salmonella typhi | Candida albicans | |
| F1 | WI | WI | WI | WI |
| F2 | WI | WI | WI | WI |
| F3 | 1.0 ± 0.05 a | 1.2 ± 0.15 a | 1.2 ± 0.1 a | 1.4 ± 0.16 b |
| F4 | 2.0 ± 0.2 c | 2.4 ± 0.26 b | 2.19 ± 0.2 c | 4.5 ± 0.05 a |
| Unoil | 1.6 ± 0.2 b | 2.3 ± 0.2 b | 1.6 ± 0.1 b | 4.4 ± 0.1 a |
| Ooil | 1.1 ± 0.06 a | 1.4 ± 0.05 a | 1.15 ± 0.1 a | 4.3 ± 0.2 a |
| Gentamicin [10] | 1.8 ± 0.00 bc | 2.2 ± 0.00 b | 2.8 ± 0.7 d | - |
| Amphotericin B [10] | - | - | 2.1 ± 0.00 c | |
| Oil Fraction | Minimum Inhibitory Concentration (μg/mL) | |||
|---|---|---|---|---|
| Staphylococcus aureus | Listeria monocytogenes | Salmonella typhi | Candida albicans | |
| F1 | ND | ND | ND | ND |
| F2 | ND | ND | ND | ND |
| F3 | 482 ± 20 c | 162 ± 16 c | 480 ± 11 c | 450 ± 24 b |
| F4 | 660 ± 54 b | 164 ± 19 c | 135 ± 13 b | 84 ± 10 a |
| Unoil | 276 ± 25 a | 6.4 ± 0.8 b | 115 ± 13 b | 78 ± 10 a |
| Ooil | 280 ± 23 a | 77 ± 0.7 a | 770 ± 27 a | 94 ± 5 a |
| Gentamicin [10] | 3.2 ± 0.5 d | 2.0 ± 0.3 b | >122 ± 10 b | |
| Amphotericin B [10] | 0.25 ± 0.05 c | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostro-Alanis, M.d.J.; Báez-González, J.; Torres-Alvarez, C.; Parra-Saldívar, R.; Rodriguez-Rodriguez, J.; Castillo, S. Chemical Composition and Biological Activities of Oregano Essential Oil and Its Fractions Obtained by Vacuum Distillation. Molecules 2019, 24, 1904. https://doi.org/10.3390/molecules24101904
Rostro-Alanis MdJ, Báez-González J, Torres-Alvarez C, Parra-Saldívar R, Rodriguez-Rodriguez J, Castillo S. Chemical Composition and Biological Activities of Oregano Essential Oil and Its Fractions Obtained by Vacuum Distillation. Molecules. 2019; 24(10):1904. https://doi.org/10.3390/molecules24101904
Chicago/Turabian StyleRostro-Alanis, Magdalena de J., Juan Báez-González, Cynthia Torres-Alvarez, Roberto Parra-Saldívar, José Rodriguez-Rodriguez, and Sandra Castillo. 2019. "Chemical Composition and Biological Activities of Oregano Essential Oil and Its Fractions Obtained by Vacuum Distillation" Molecules 24, no. 10: 1904. https://doi.org/10.3390/molecules24101904
APA StyleRostro-Alanis, M. d. J., Báez-González, J., Torres-Alvarez, C., Parra-Saldívar, R., Rodriguez-Rodriguez, J., & Castillo, S. (2019). Chemical Composition and Biological Activities of Oregano Essential Oil and Its Fractions Obtained by Vacuum Distillation. Molecules, 24(10), 1904. https://doi.org/10.3390/molecules24101904







