Synthesis and Evaluation of Antidepressant Activities of 5-Aryl-4,5-dihydrotetrazolo [1,5-a]thieno[2,3-e]pyridine Derivatives
Abstract
:1. Introduction
2. Results and Discussion
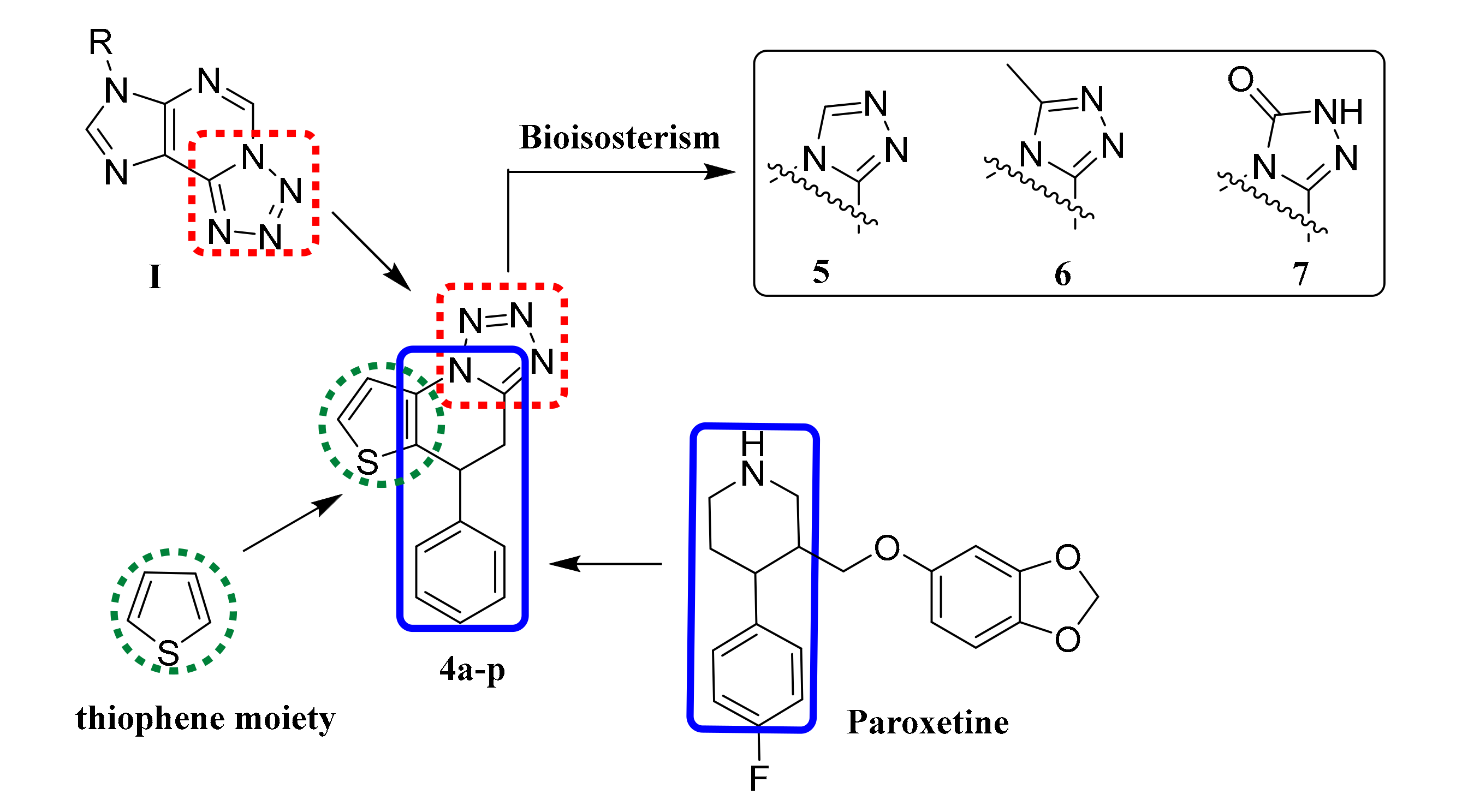
2.1. Chemistry
2.2. Pharmacology
2.2.1. Forced Swim Test (FST)
2.2.2. Tail Suspension Test (TST)
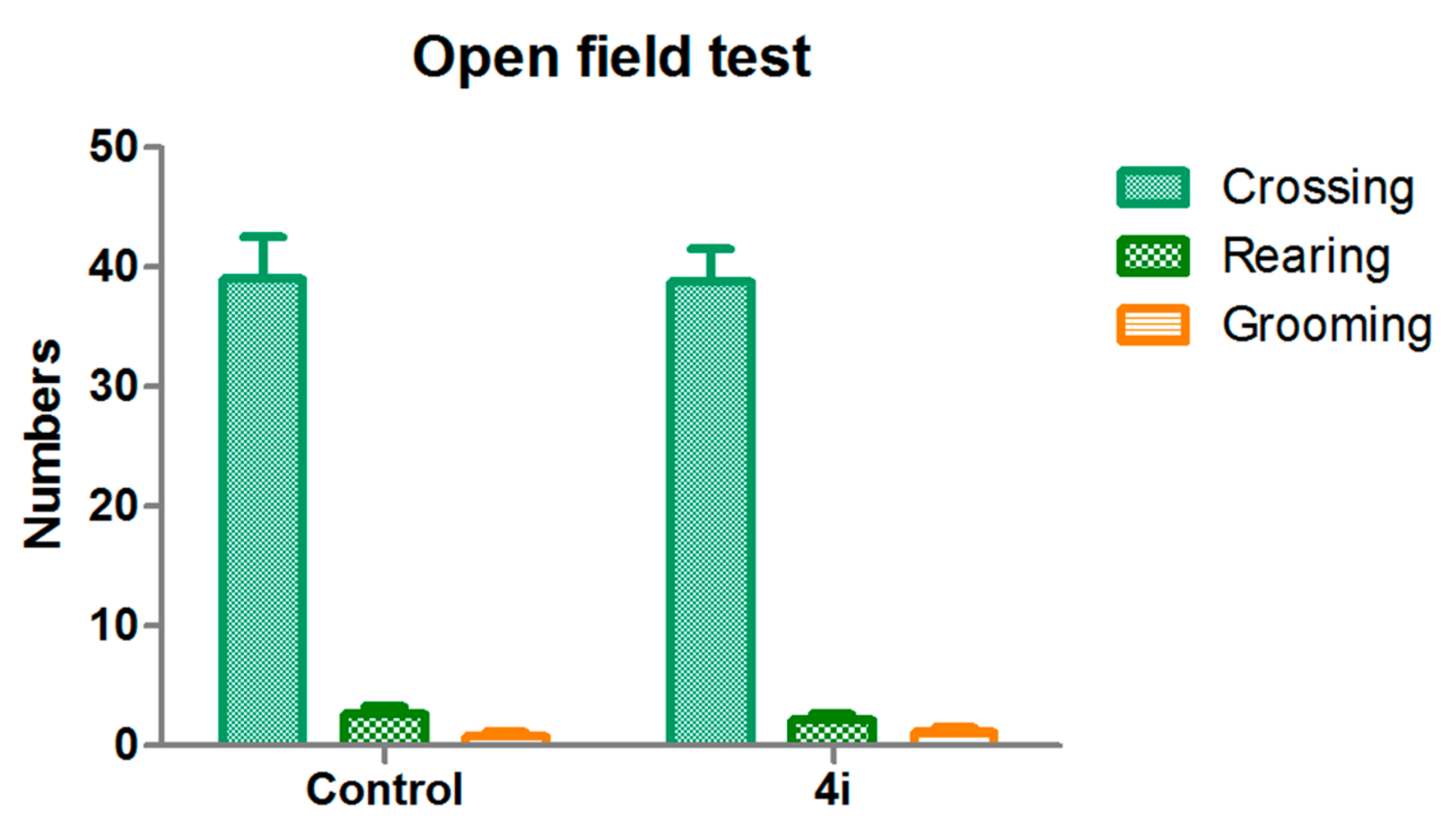
2.2.3. Open-Field Test
2.2.4. Determination of 5-HT Concentration
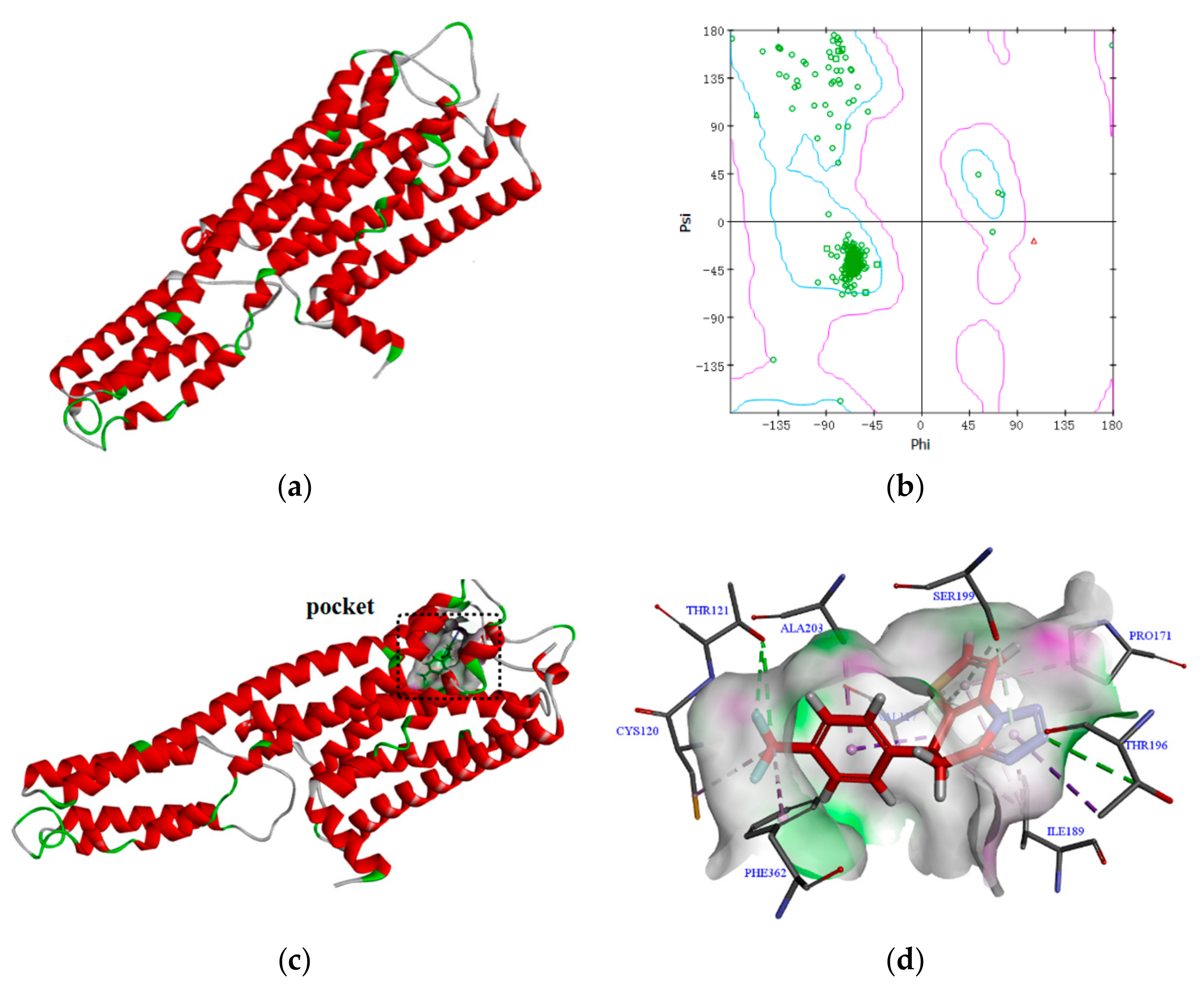
2.2.5. Docking Study
3. Experimental Section
3.1. Chemistry
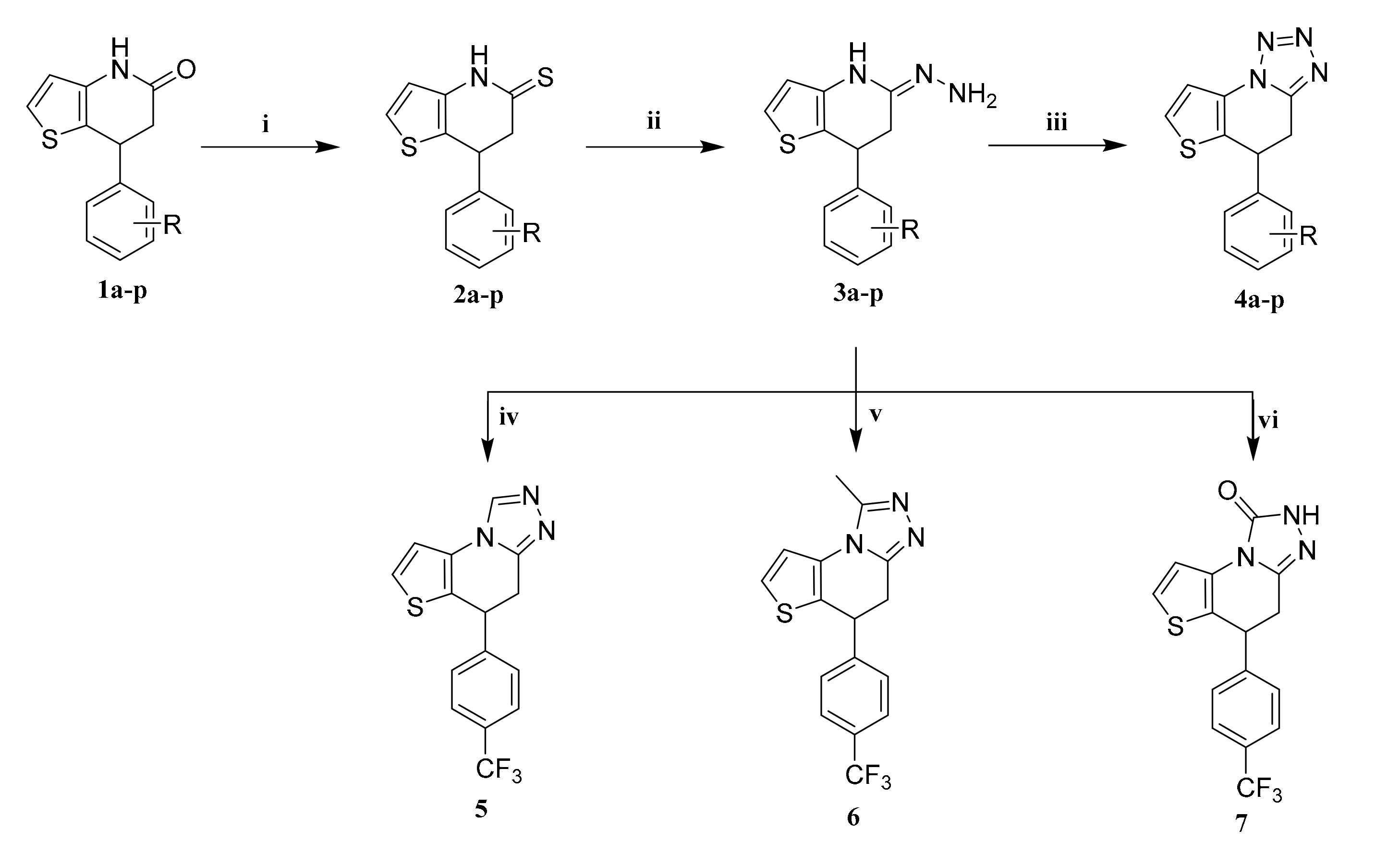
3.1.1. Synthesis of 7-substitutedphenyl-6,7-dihydrothieno[3,2-b]pyridine-5(4H)-thiones (2a–p)
3.1.2. Synthesis of 5-hydrazono-7-substitutedphenyl-4,5,6,7-tetrahydrothieno[3,2-b]pyridines (3a–p)
3.1.3. Synthesis of 5-aryl-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4a–p)
3.1.4. Synthesis of 5-[4-(trifluoromethyl)phenyl]-4,5-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyridine (5)
3.1.5. Synthesis of 1-methyl-5-[4-(trifluoromethyl)phenyl]-4,5-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyridine (6)
3.1.6. Synthesis of 5-[4-(trifluoromethyl)phenyl]-4,5-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyridin-1(2H)-one (7)
3.2. Pharmacology
3.2.1. Evaluation of Antidepressant Activity
3.2.2. Molecular Docking Studies
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Badr, S.M.I. Synthesis and anti-inflammatory activity of novel 2,5-disubstituted thiophene derivatives. Turk. J. Chem. 2010, 35, 131–143. [Google Scholar]
- Foroumadi, A.; Mansouri, S.; Kiani, Z.; Rahmani, A. Synthesis and in vitro antibacterial evaluation of N-[5-(5-nitro-2-thienyl)-1,3,4-thiadiazole-2-yl]piperazinyl quinolones. Eur. J. Med. Chem. 2003, 38, 851–854. [Google Scholar] [CrossRef]
- Amer, A.E.; Sherif, M.H.; Assy, M.G.; Al-Omar, M.A.; Ragab, I. Antiarrhythmic, serotonin antagonist and antianxiety activities of novel substituted thiophene derivatives synthesized from 2-amino-4,5,6,7-tetrahydro-N-phenylbenzo[b]thiophene-3-carboxamide. Eur. J. Med. Chem. 2010, 45, 5935–5942. [Google Scholar] [CrossRef]
- Fadda, A.A.; Abdel-Latif, E.; El-Mekawy, R.E. Synthesis of some new arylazothiophene and arylazopyrazole derivatives as antitumor agents. J. Pharm. Pharmacol. 2012, 3, 148–157. [Google Scholar] [CrossRef]
- Ali, K.A.; Abdalghfar, H.S.; Mahmoud, K.; Ragab, E.A. Synthesis and antitumor activity of new Polysubstituted Thiophenes and 1,3,4-thiadiazoles incorporating 2,6-pyridine moieties. J. Heterocycl. Chem. 2013, 50, 1157–1164. [Google Scholar] [CrossRef]
- Mathew, B.; Jerad, S.; Anbazhagan, S. Synthesis, in silico preclinical evaluation, antidepressant potential of 5-substituted phenyl-3-(thiophen-2-yl)-4,5-dihydro-1hpyrazole-1-carboxamides. Biomed. Aging Pathol. 2014, 4, 327–333. [Google Scholar] [CrossRef]
- Łukowska-Chojnacka, E.; Kowalkowska, A.; Gizińska, M.; Koronkiewicz, M.; Staniszewska, M. Synthesis of tetrazole derivatives bearing pyrrolidine scaffold and evaluation of their antifungal activity against Candida albicans. Eur. J. Med. Chem. 2019, 164, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Sribalan, R.; Banuppriya, G.; Kirubavathi, M.; Padmini, V. Synthesis, biological evaluation and in silico studies of tetrazole heterocycle hybrids. J. Mol. Struct. 2019, 1175, 577–586. [Google Scholar] [CrossRef]
- Aziz, H.; Saeed, A.; Jabeen, F.; Din, N.U.; Flörke, U. Synthesis, single crystal analysis, biological and docking evaluation of tetrazole derivatives. Heliyon 2018, 4, e00792. [Google Scholar] [CrossRef]
- Yan, Z.; Chong, S.; Lin, H.; Yang, Q.; Wang, X.; Zhang, W.; Zhang, X.; Zeng, Z.; Su, Y. Design, synthesis and biological evaluation of tetrazole-containing RXRα ligands as anticancer agents. Eur. J. Med. Chem. 2019, 164, 562–575. [Google Scholar] [CrossRef]
- Bommagani, S.; Penthala, N.R.; Balasubramaniam, M.; Kuravi, S.; Caldas-Lopes, E.; Guzman, M.L.; Balusu, R.; Crooks, P.A. A novel tetrazole analogue of resveratrol is a potent anticancer agent. Bioog. Med. Chem. Lett. 2019, 29, 172–178. [Google Scholar] [CrossRef]
- Deng, X.Q.; Wei, C.X.; Song, M.X.; Chai, K.Y.; Sun, Z.G.; Quan, Z.S. Synthesis and studies on anticonvulsant and antidepressant activities of 5-alkoxy-tetrazolo[1,5-a]quinolines. Bull. Korean. Chem. Soc. 2010, 31, 447–452. [Google Scholar] [CrossRef]
- Wang, S.B.; Piao, G.C.; Zhang, H.J.; Quan, Z.S. Synthesis of 5-Alkoxythieno[2,3-e][1,2,4]triazolo[4,3-c]pyrimidine Derivatives and Evaluation of Their Anticonvulsant Activities. Molecules 2015, 20, 6827–6843. [Google Scholar] [CrossRef]
- Sun, X.Y.; Wei, C.X.; Deng, X.Q.; Sun, Z.G.; Quan, Z.S. Synthesis and primary anticonvulsant activity evaluation of 6-alkyoxyl-tetrazolo[5,1-a]phthalazine derivatives. Arzneimittelforschung 2010, 60, 289–292. [Google Scholar] [CrossRef]
- Wang, S.B.; Deng, X.Q.; Liu, D.C.; Zhang, H.J.; Quan, Z.S. Synthesis and evaluation of anticonvulsant and antidepressant activities of 7-alkyl-7H-tetrazolo[1,5-g]purine derivatives. Med. Chem. Res. 2014, 23, 4619–4626. [Google Scholar] [CrossRef]
- Zhang, M.M.; Wang, X.S.; Li, Q.; Yao, C.S.; Tu, S.J. Three-Component One-Pot Synthesis of 1-Aryl-4-benzo[f]quinoline Derivatives in Aqueous Media. Chin. J. Org. Chem. 2008, 28, 881–884. [Google Scholar]
- Sun, X.Y.; Zhang, L.; Wei, C.X.; Piao, H.R.; Quan, Z.S. Design, synthesis of 8-alkoxy-5,6-dihydro-[1,2,4]triazino[4,3-a]quinolin-1-ones with anticonvulsant activity. Eur. J. Med. Chem. 2009, 44, 1265–1270. [Google Scholar] [CrossRef]
- Sun, X.Y.; Wei, C.X.; Chai, K.Y.; Piao, H.R.; Quan, Z.S. Synthesis and anti-inflammatory activity evaluation of novel 7-alkoxy-1-amino-4,5-dihydro[1,2,4]triazole[4,3-a]quinolines. Arch. Pharm. 2008, 341, 288–293. [Google Scholar] [CrossRef]
- Choi, B.D.; Lim, H.J.; Lee, S.Y.; Lee, M.H.; Kil, K.S.; Lim, D.S.; Jeong, S.J.; Jeong, M.J. Synthesis of Novel Benzazole Derivatives and Evaluation of Their Antidepressant-Like Activities with Possible Underlying Mechanisms. Molecules 2018, 23, 2881. [Google Scholar]
- Galdino, P.M.; Nascimento, M.V.; Sampaio, B.L.; Ferreira, R.N.; Paula, J.R.; Costa, E.A. Antidepressant-like effect of Lafoensia pacari A. St.-Hil. ethanolic extract and fractions in mice. J. Ethnopharmacol. 2009, 124, 581–585. [Google Scholar] [CrossRef]
- Sakakibara, H.; Ishida, K.; Grundmann, O.; Nakajima, J.; Seo, S.; Butterweck, V.; Minami, Y.; Saito, S.; Kawai, Y.; Nakaya, Y.; et al. Antidepressant effect of extracts from Ginkgo biloba leaves in behavioral models. Biol. Pharm. Bull. 2006, 29, 1767–1770. [Google Scholar] [CrossRef]
- Meyer, J.H.; McMain, S.; Kennedy, S.H.; Korman, L.; Brown, G.M.; DaSilva, J.N.; Wilson, A.A.; Blak, T.; Eynan-Harvey, R.; Goulding, V.S.; et al. Dysfunctional attitudes and 5-HT2A receptors during depression self-harm. Am. J. Psychiatry. 2003, 160, 90–99. [Google Scholar] [CrossRef]
- Ostrowska, K.; Grzeszczuk, D.; Głuch-Lutwin, M.; Gryboś, A.; Siwek, A.; Leśniak, A.; Sacharczuk, M.; Trzaskowski, B. 5-HT1A and 5-HT2A receptors affinity, docking studies and pharmacological evaluation of a series of 8-acetyl-7-hydroxy-4-methylcoumarin derivatives. Bioorg. Med. Chem. 2018, 26, 527–535. [Google Scholar] [CrossRef]
- Blier, P.; Ward, N.M. Is there a role for 5-HT1A agonists in the treatment of depression? Biol. Psychiatry. 2003, 53, 93–103. [Google Scholar] [CrossRef]
- Sherin, D.R.; Geethu, C.K.; Prabhakaran, J.; Mann, J.J.; Dileep, J.S.; Manojkumar, T.K. Molecular docking, dynamics simulations and 3D-QSAR modeling of arylpiperazine derivatives of 3,5-dioxo-(2H,4H)-1,2,4-triazine as 5-HT1AR agonists. Comput. Biol. Chem. 2019, 78, 108–115. [Google Scholar] [CrossRef]
- Porsolt, R.D. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Zomkowski, A.D.; Santos, A.R.; Rodrigues, A.L. Evidence for the involvement of the opioid system in the agmatine antidepressant-like effect in the forced swimming test. Neurosci. Lett. 2005, 381, 279–283. [Google Scholar] [CrossRef]
- Streu, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacol 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Sairanen, M.; Lucas, G.; Ernfors, P.; Castrén, M.; Castrén, E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronalturnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 2005, 25, 1089–1094. [Google Scholar] [CrossRef]
- Elliott, P.J.; Chan, J.; Parker, Y.M.; Nemeroff, C.B. Behavioral effects of neurotensin in the open field: Structure-activity studies. Brain. Res. 1986, 381, 259–265. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Compound | R | Antidepressant Activities a | |
|---|---|---|---|
| Duration of Immobility(s) | Change from Control | ||
| (mean ± S.E.M.) b | (%) | ||
| 4a | o-Cl | 87.21 ± 16.95 * | 24.25 |
| 4b | m-Cl | 99.02 ± 13.12 | 13.99 |
| 4c | p-Cl | 65.15 ± 12.73 ** | 43.41 |
| 4d | o-F | 85.22 ± 14.20 * | 25.98 |
| 4e | m-F | 79.72 ± 19.41 * | 30.76 |
| 4f | p-F | 53.61 ± 15.50 ** | 53.44 |
| 4g | o-CF3 | 68.43 ± 11.87 ** | 40.56 |
| 4h | m-CF3 | 73.63 ± 17.58 ** | 36.05 |
| 4i | p-CF3 | 51.43 ± 13.76 ** | 55.33 |
| 4j | o-OCH3 | 95.26 ± 18.83 | 17.26 |
| 4k | m-OCH3 | 86.54 ± 16.72 * | 24.83 |
| 4l | p-OCH3 | 66.32 ± 15.61 ** | 42.40 |
| 4m | p-Br | 83.53 ± 14.23 * | 27.45 |
| 4n | -H | 78.13 ± 20.91 * | 32.14 |
| 4o | 2-Cl-6-F | 98.28 ± 16.32 | 14.64 |
| 4p | 3,4,5-OCH3 | 102.41 ± 19.18 | 11.05 |
| 5 | p-CF3 | 89.38 ± 12.39 * | 22.37 |
| 6 | p-CF3 | 93.63 ± 16.81 | 18.70 |
| 7 | p-CF3 | 82.58 ± 15.15 * | 28.27 |
| Fluoxetine | - | 54.21 ± 11.97 ** | 52.91 |
| Control | - | 115.13 ± 18.67 | - |
| Compound | Dose (mg/kg) | Antidepressant Activities a | |
|---|---|---|---|
| Duration of Immobility(s) | Change from Control | ||
| (mean ± S.E.M.) b | (%) | ||
| 10 | 81.27 ± 16.13 * | 27.69 | |
| 4i | 20 | 67.41 ± 14.91 ** | 40.02 |
| 40 | 53.39 ± 12.85 ** | 52.53 | |
| 10 | 82.41 ± 15.72 ** | 27.56 | |
| Fluoxetine | 20 | 69.18 ± 14.56 ** | 38.44 |
| 40 | 55.02 ± 10.17 ** | 51.04 | |
| Control | - | 112.39 ± 12.14 | - |
| Compound | Dose (mg/kg) | Antidepressant Activities a | |
|---|---|---|---|
| Duration of Immobility (s) | Change from Control | ||
| (mean ± S.E.M.) b | (%) | ||
| 4i | 40 | 72.33 ± 14.61 ** | 43.73 |
| Fluoxetine | 40 | 77.18 ± 16.52 ** | 39.95 |
| Control | - | 128.54 ± 25.37 | - |
| Compound | Dose (mg/kg) | 5-HT (ng/mg) a |
|---|---|---|
| 4i | 40 | 1.276 ± 0.094 *,b |
| Fluoxetine | 40 | 1.328 ± 0.105 * |
| Control | - | 1.015 ± 0.098 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Liu, H.; Wang, X.; Lei, K.; Li, G.; Quan, Z. Synthesis and Evaluation of Antidepressant Activities of 5-Aryl-4,5-dihydrotetrazolo [1,5-a]thieno[2,3-e]pyridine Derivatives. Molecules 2019, 24, 1857. https://doi.org/10.3390/molecules24101857
Wang S, Liu H, Wang X, Lei K, Li G, Quan Z. Synthesis and Evaluation of Antidepressant Activities of 5-Aryl-4,5-dihydrotetrazolo [1,5-a]thieno[2,3-e]pyridine Derivatives. Molecules. 2019; 24(10):1857. https://doi.org/10.3390/molecules24101857
Chicago/Turabian StyleWang, Shiben, Hui Liu, Xuekun Wang, Kang Lei, Guangyong Li, and Zheshan Quan. 2019. "Synthesis and Evaluation of Antidepressant Activities of 5-Aryl-4,5-dihydrotetrazolo [1,5-a]thieno[2,3-e]pyridine Derivatives" Molecules 24, no. 10: 1857. https://doi.org/10.3390/molecules24101857
APA StyleWang, S., Liu, H., Wang, X., Lei, K., Li, G., & Quan, Z. (2019). Synthesis and Evaluation of Antidepressant Activities of 5-Aryl-4,5-dihydrotetrazolo [1,5-a]thieno[2,3-e]pyridine Derivatives. Molecules, 24(10), 1857. https://doi.org/10.3390/molecules24101857





