Sinomenine Protects Against Morphine Dependence through the NMDAR1/CAMKII/CREB Pathway: A Possible Role of Astrocyte-Derived Exosomes
Abstract
1. Introduction
2. Results
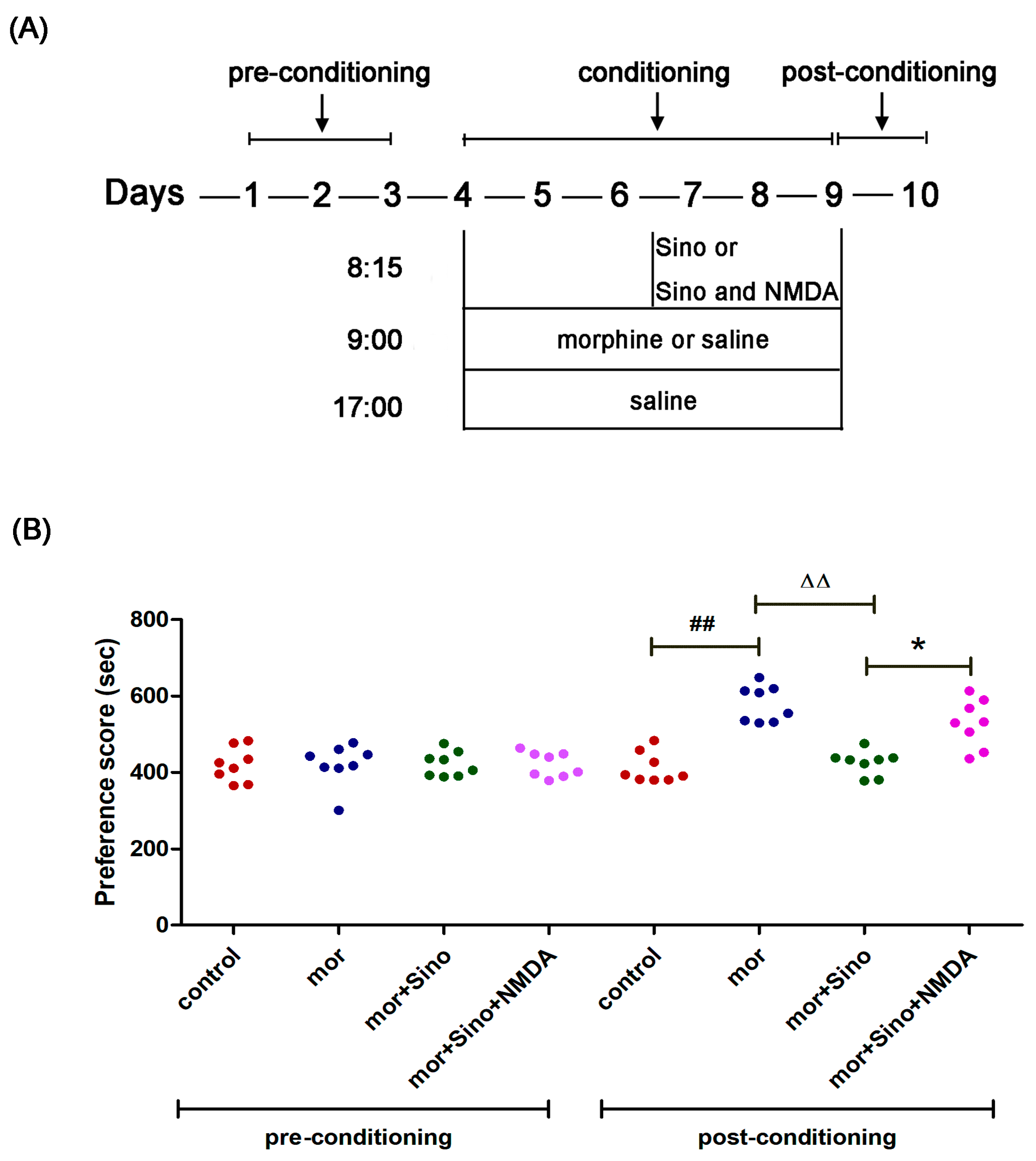
2.1. Sinomenine Inhibited Morphine-Induced Conditioned Place Preference in Mice
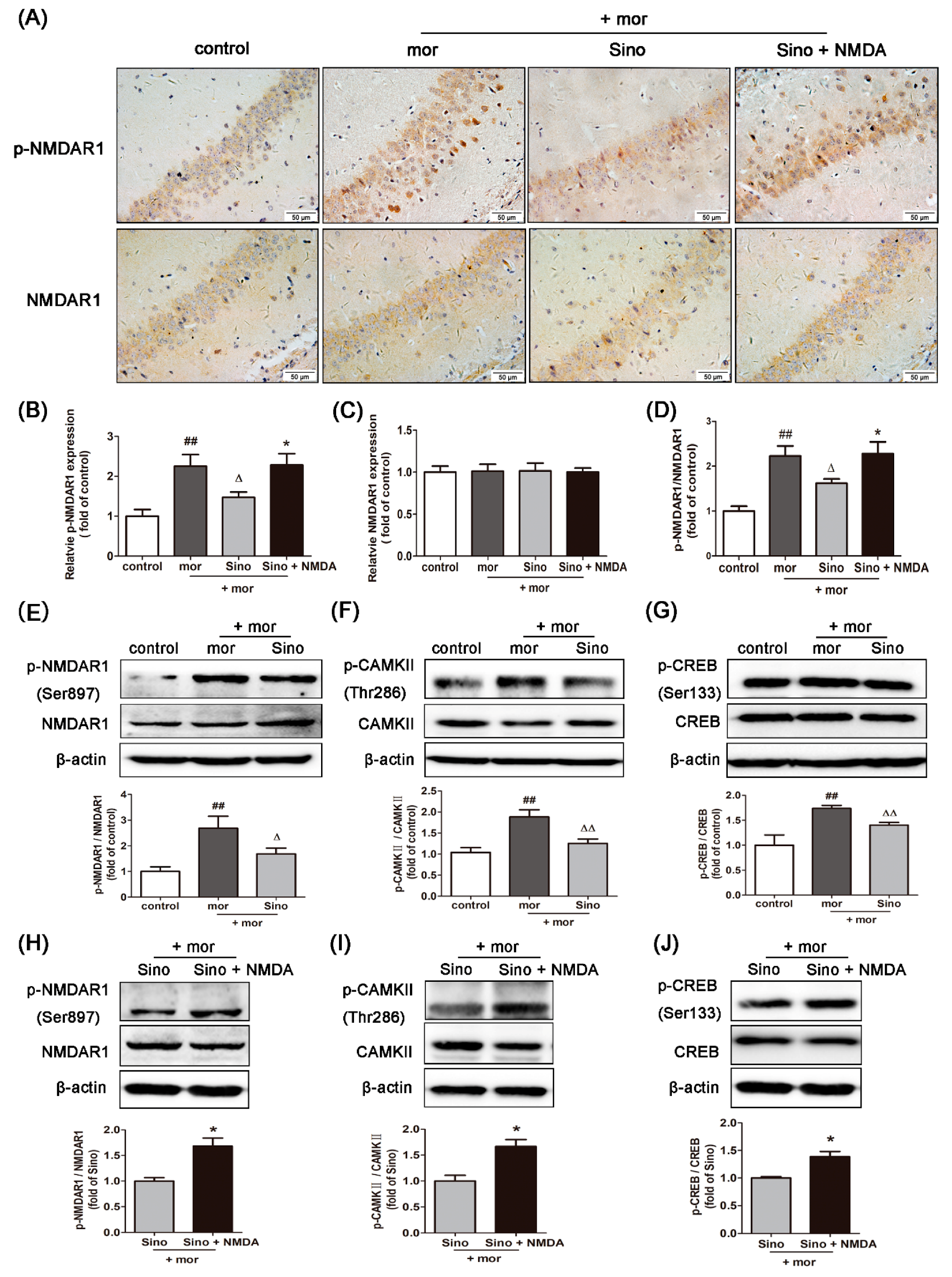
2.2. Sinomenine Inhibited the Expression of p-NMDAR1/NMDAR1, p-CAMKII/CAMKII, and p-CREB/CREB in the Hippocampus of Morphine-Dependent Mice
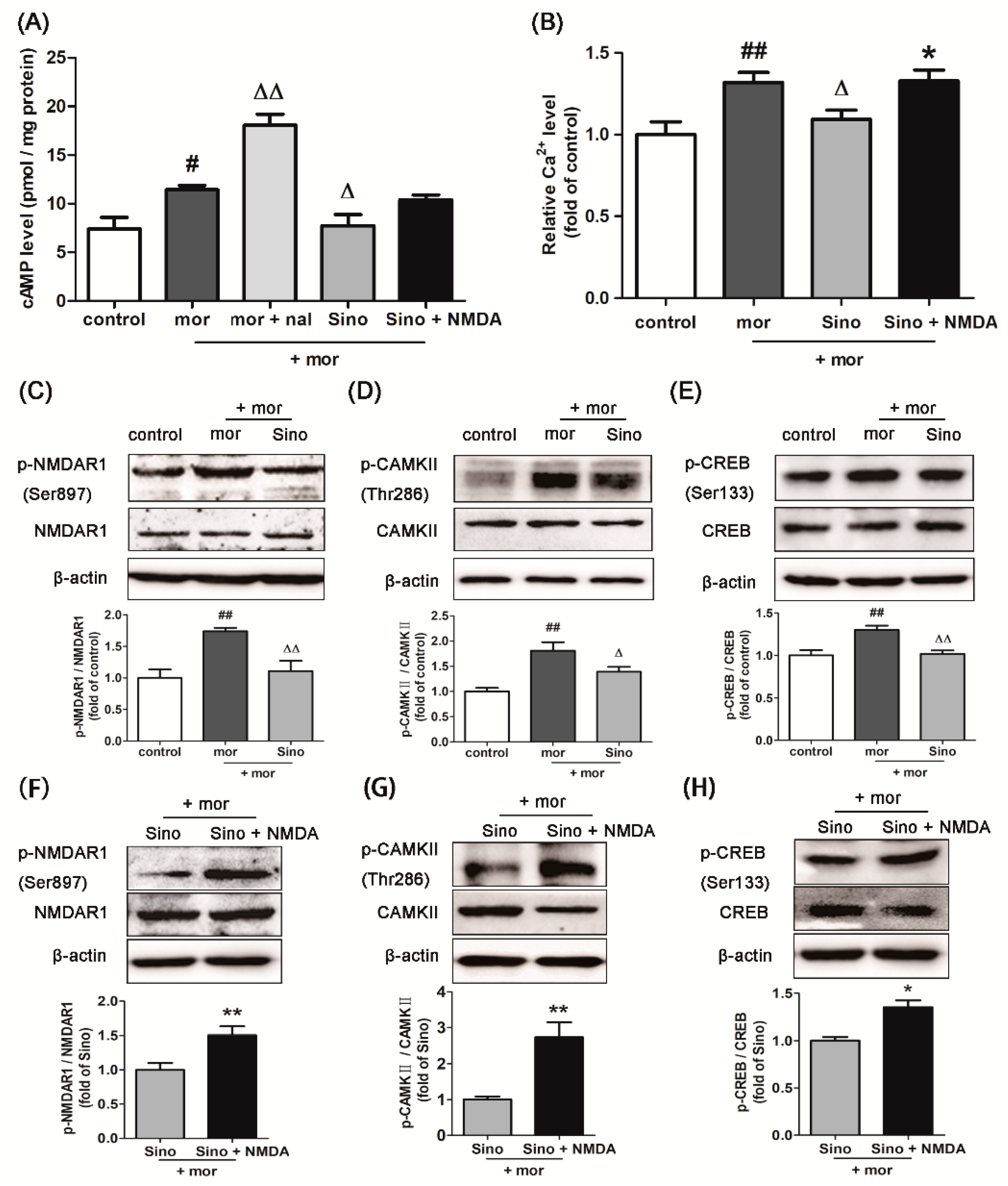
2.3. Sinomenine Decreased the Level of cAMP in Morphine-Treated SH-SY5Y Cells
2.4. Sinomenine Decreasedthe Level of Intracellular Ca2+ in Morphine-Treated SH-SY5Y Cells
2.5. Sinomenine Inhibitedthe Expressions of p-NMDAR1/NMDAR1, p-CAMKII/CAMKII, and p-CREB/CREB in Morphine-Treated SH-SY5Y Cells
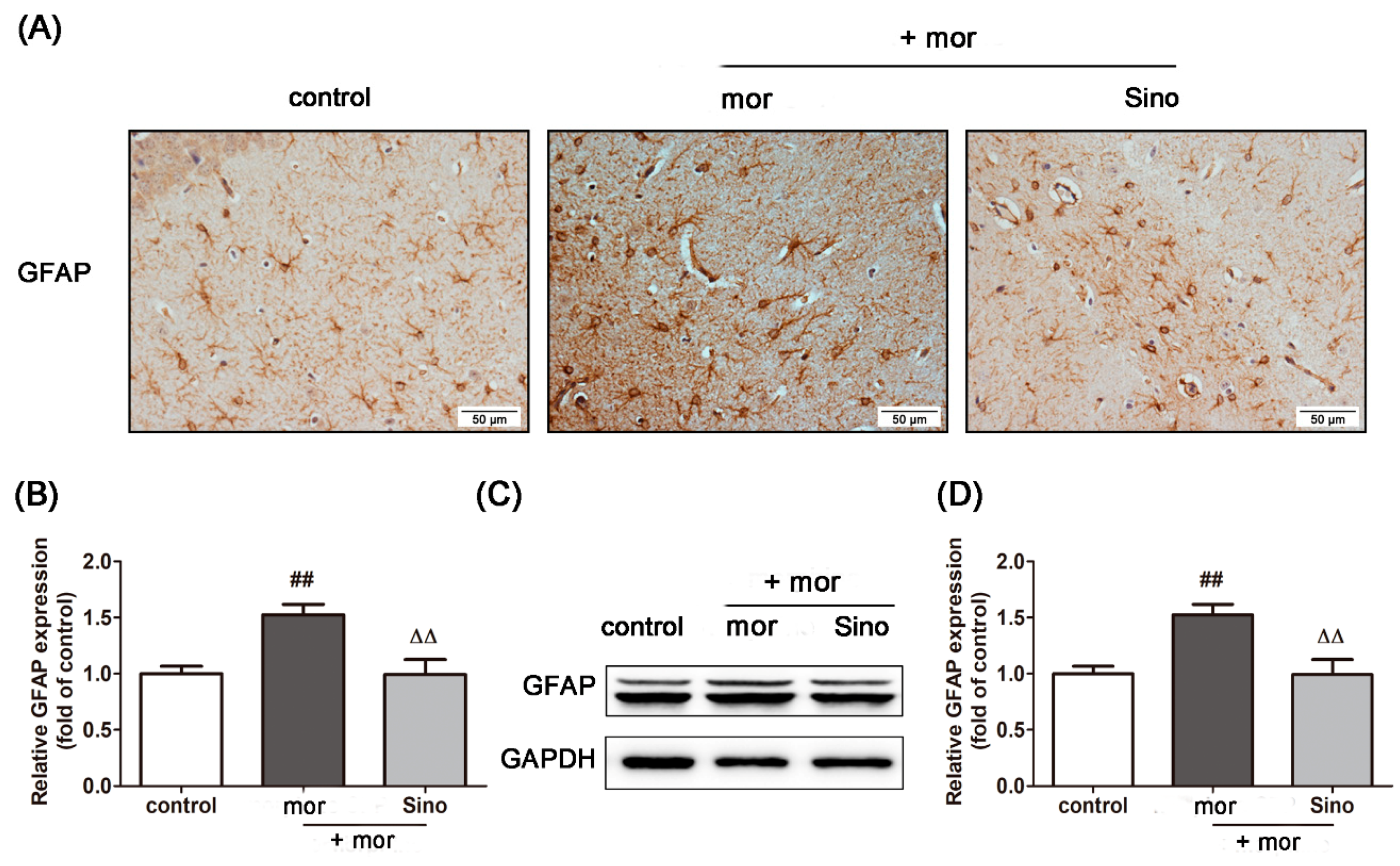
2.6. Sinomenine Inhibited the Activation of Astrocytes Induced by Morphine
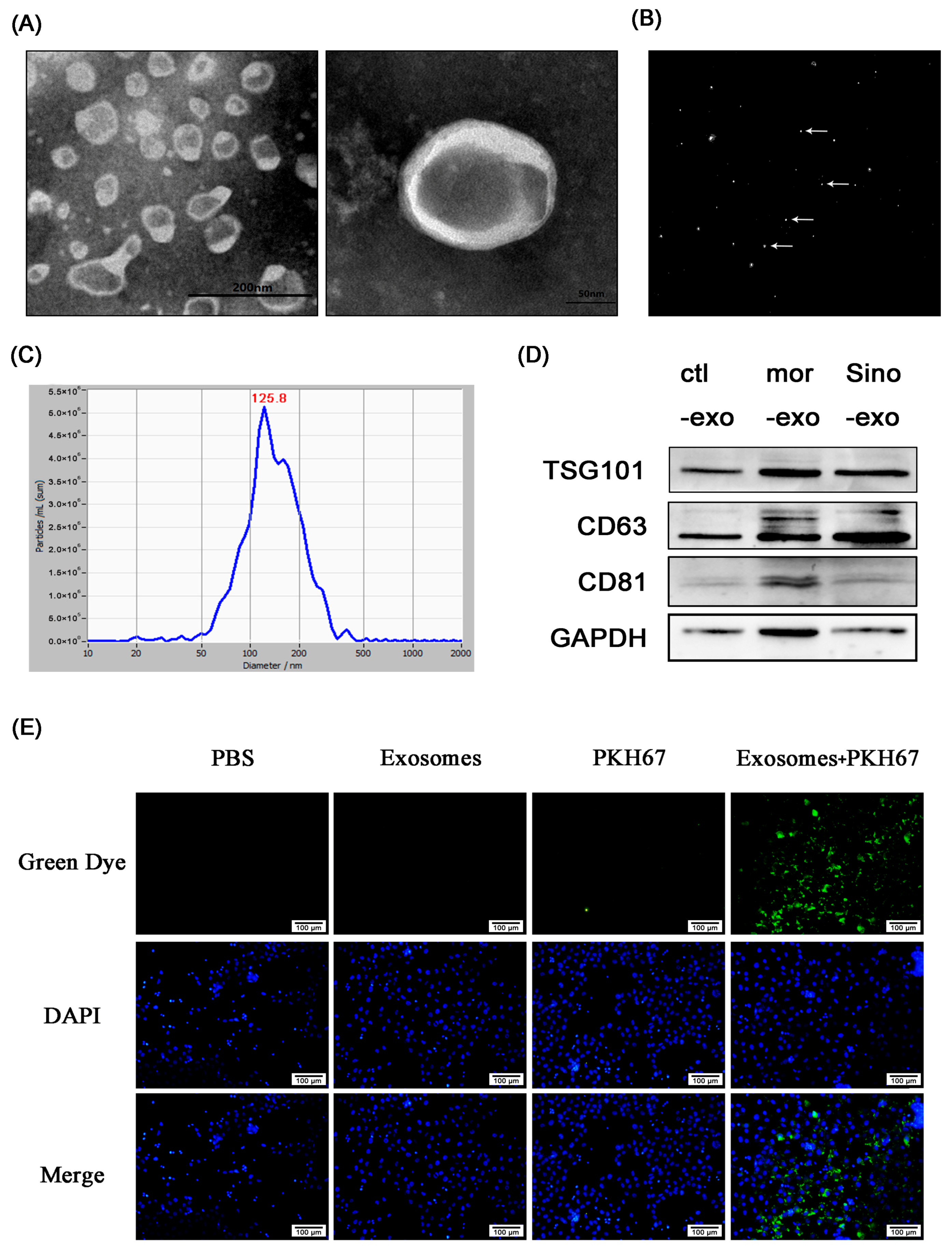
2.7. Astrocyte-Derived Exosomes were Identified byTransmission Electron Microscope, Nanoparticle Size Analysis, and Marker Protein
2.8. Cultured Primary Astrocyte-Derived Exosomes Can Be TakenUp by Neuronal SH-SY5Y Cells
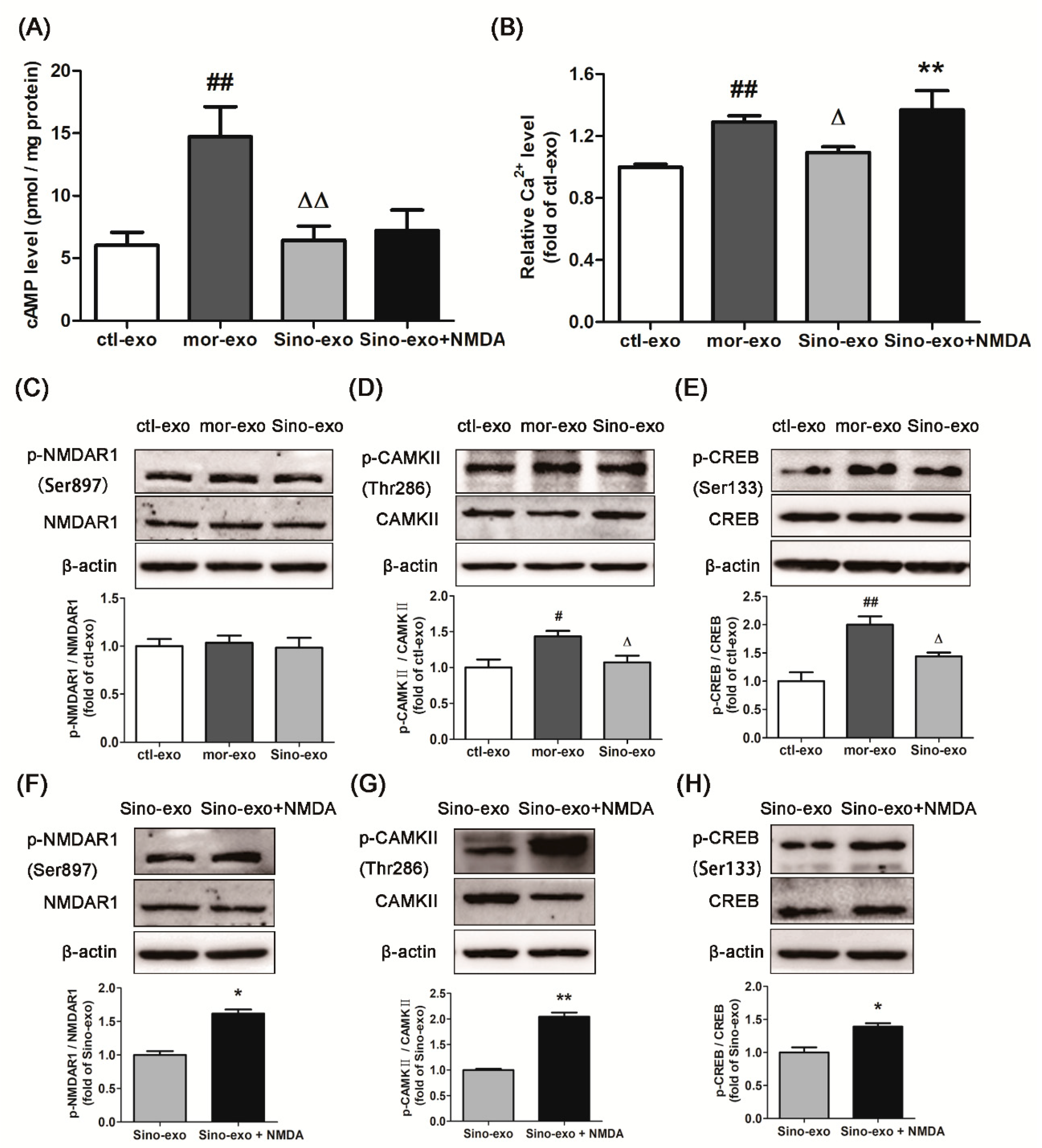
2.9. Effect of Astrocyte-Derived Exosomes on cAMP Level in Morphine-Treated SH-SY5Y Cells and Subsequent Intervention by Sinomenine
2.10. Effects of Astrocyte-Derived Exosomes on the Level of Intracellular Ca2+ in Morphine-Treated SH-SY5Y Cells and Subsequent Intervention by Sinomenine
2.11. Effects of Astrocyte-Derived Exosomes on the Expression of p-NMDAR1/NMDAR1, p-CAMKII/CAMKII, and p-CREB/CREB in Morphine-Treated SH-SY5Y Cells and Subsequent Intervention by Sinomenine
3. Discussion
4. Materials and Methods
4.1. Reagents and Assay Kits
4.2. Animals
4.3. Establishment of a Morphine-Dependence Model by Conditioned Place Preference (CPP), and Treatment Details
4.3.1. Apparatus
4.3.2. CPP Phases
4.3.3. Preconditioning Phase
4.3.4. Conditioning Phase
4.3.5. Postconditioning Phase
4.4. Immunohistochemistry Staining
4.5. Western Blotting
4.6. Cell Culture
4.7. Determination of cAMP Level in SH-SY5YCells
4.8. Determination of Intracellular Calcium ([Ca2+]) in SH-SY5Y Cells
4.9. Extraction of Astrocyte-Derived Exosomes by Differential Centrifugation
4.10. Transmission Electron Microscopy (TEM)
4.11. Nanoparticle Tracking Analysis
4.12. Exosome Labeling by Fluorescent Dye PKH67
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Sino | sinomenine |
| mor | morphine |
| nal | naloxone |
| exo | exosomes |
| NMDAR1 | N-methyl-d-aspartate receptor 1 |
| CAMKII | Ca2+/calmodulin-dependent protein kinase II |
| CREB | cyclic adenosine monophosphate response element-binding protein |
References
- Milton, A.L.; Everitt, B.J. The persistence of maladaptive memory: Addiction, drug memories and anti-relapse treatments. Neurosci. Biobehav. Rev. 2012, 36, 1119–1139. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, L.; Balleine, B.W.; Corbit, LH.; Killcross, S. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann. N. Y. Acad. Sci. 2013, 1282, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, M.G.; Gould, T.J. Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: Contributions to development and maintenance of addiction. Learn. Mem. 2016, 23, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Glanzman, D. Mediation of classical conditioning in Aply-siacalifornica by LTP of sensorimotor synapses. Science 1997, 278, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Volianskis, A.; France, G.; Jensen, M.S.; Bortolotto, Z.A.; Jane, D.E.; Collingridge, G.L. Long-term potentiation and the role of N-methyl-d-aspartate receptors. Brain. Res. 2015, 1621, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, F.; Guo, H.; Zhang, J.; Yan, P.; Lai, J. The Role of Dopamine D1 and D3 Receptors in N-Methyl-d-Aspartate (NMDA)/GlycineB Site-Regulated Complex Cognitive Behaviors following Repeated Morphine Administration. Int. J. Neuropsychopharmacol. 2017, 20, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Guitart, X.; Thompson, M.A.; Mirante, C.K.; Greenberg, M.E.; Nestler, E.J. Regulation of cyclic AMP response element-binding protein (CREB) phosphorylation by acute and chronic morphine in the rat locus coeruleus. J. Neurochem. 1992, 58, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, Y.; Huang, Y.; Lin, L. Chinese Herbal Medicine for the Treatment of Drug Addiction. Int. Rev. Neurobiol. 2017, 135, 279–295. [Google Scholar] [PubMed]
- Mo, Z.; Zhou, J.; Wang, C. An experimental study on physical and psychological dependences of Sinomenine. Chin. Mag. Drug Abuse Prev. Treat. 2004, 10, 190–193. [Google Scholar]
- Wang, C.; Mo, Z.; Zhu, G. Effect of sinomenine on morphine dependence in isolated guinea pig ileum. J. Fourth Milit. Med. Univ. 2003, 23, 421–423. [Google Scholar]
- Mo, Z.; Xu, D.; Wang, C. Effect of Caulis Sinomenii and sinomenine on naloxone-precipitated withdrawal response in morphine-dependent models in vitro and in vivo. Chin. J. Clin. Rehabil. 2004, 8, 7879–7881. [Google Scholar]
- Mo, Z.; An, S.; Zhou, J. Effects of Caulis Sinomenii and sinomenine on morphine-induced place preference and brain histamine level. J. South Med. Univ. 2006, 26, 1709–1713. [Google Scholar]
- Scofield, M.D. Exploring the Role of Astroglial Glutamate Release and Association with Synapses in Neuronal Function and Behavior. Biol. Psychiatry 2017. [Google Scholar] [CrossRef] [PubMed]
- Boury-Jamot, B.; Carrard, A.; Martin, J.L.; Halfon, O.; Magistretti, P.J.; Boutrel, B. Disrupting astrocyte-neuron lactate transfer persistently reduces conditioned responses to cocaine. Mol. Psychiatry 2016, 21, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, R.; Guo, L.; Zhen, X. Morphine-induced inhibition of Ca (2+) -dependent d-serine release from astrocytes suppresses excitability of GABAergic neurons in the nucleus accumbens. Addict. Biol. 2017, 22, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Santello, M.; Cali, C.; Bezzi, P. Gliotransmission and the tripartite synapse. In Synaptic Plasticity; Kreutz, M., Sala, C., Eds.; Springer: Vienna, Austria, 2012; Volume 970, pp. 307–331. ISBN 9783709109311. [Google Scholar]
- Ridet, J.L.; Malhotra, S.K.; Privat, A.; Gage, F.H. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 1997, 20, 570–577. [Google Scholar] [CrossRef]
- Cossetti, C.; Smith, J.A.; Iraci, N.; Leonardi, T.; Alfaro-Cervello, C.; Pluchino, S. Extracellular membrane vesicles and immune regulation in the brain. Front. Physiol. 2012, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Lachenal, G.; Pernetgallay, K.; Chivet, M.; Hemming, F.J.; Belly, A.; Bodon, G.; Blot, B.; Haase, G.; Goldberg, Y.; Sadoul, R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 2011, 46, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Yao, H.; Chaudhuri, A.D.; Duan, M.; Yelamanchili, S.V.; Wen, H.; Cheney, P.D.; Fox, H.S.; Buch, S. Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated Neuronal dysfunction. Cell Death Dis. 2012, 3, e381. [Google Scholar] [CrossRef] [PubMed]
- Carr, G.; Fibiger, H.; Phillips, A. Conditioned place preference as a measure of drug reward. In Topics in Experimental Psychopharmacology, 1st ed.; Liebman, J.M., Cooper, S.J., Eds.; Clarendon Press/Oxford University Press: New York, NY, USA, 1989; pp. 264–319. ISBN 0198521766. [Google Scholar]
- Koob, G.F.; Bloom, F.E. Cellular and molecular mechanisms of drug dependence. Science 1988, 242, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Greg, H. Methadone and buprenorphine added to the WHO list of essential medicines. HIV AIDS Policy Law Rev. 2006, 10, 23–24. [Google Scholar]
- Gowing, L.; Farrell, M.; Ali, R.; White, J.M. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst. Rev. 2014, 3, Cd002024. [Google Scholar]
- Lu, L.; Liu, Y.; Zhu, W.; Shi, J.; Liu, Y.; Ling, W.; Kosten, T.R. Traditional Medicine in the Treatment of Drug Addiction. Am. J. Drug Alcohol Abuse 2009, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hopf, F.W. Do specific NMDA receptor subunits act as gateways for addictive behaviors? Genes Brain Behav. 2017, 16, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.P.; Quednow, B.B.; Lourdusamy, A.; Kornhuber, J.; Schumann, G.; Giese, K.P. CaM Kinases: From Memories to Addiction. Trends Pharmacol. Sci. 2016, 37, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Siahposhtkhachaki, A.; Ezzatpanah, S.; Razavi, Y.; Haghparast, A. NMDA receptor dependent changes in c-fos and p-CREB signaling following extinction and reinstatement of morphine place preference. Neurosci. Lett. 2017, 662, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carmona, J.A.; Camejo, D.M.; Almela, P.; Jimenez, A.; Milanes, M.V.; Sevilla, F.; Laorden, M.L. CP-154,526 Modifies CREB Phosphorylation and Thioredoxin-1 Expression in the Dentate Gyrus following Morphine-Induced Conditioned Place Preference. PLoS ONE 2015, 10, e0136164. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.S.; Chen, Z.G.; Liu, W.T.; Chi, Z.Q.; He, L.; Liu, J.G. Dorsal hippocampal NMDA receptor blockade impairs extinction of naloxone-precipitated conditioned place aversion in acute morphine-treated rats by suppressing ERK and CREB phosphorylation in the basolateral amygdala. Br. J. Pharmacol. 2015, 172, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.F.; Sun, L.L.; Cui, C.L.; Han, J.S. NAc Shell Arc/Arg3.1 Protein Mediates Reconsolidation of Morphine CPP by Increased GluR1 Cell Surface Expression: Activation of ERK-Coupled CREB is Required. Int. J. Neuropsychopharmacol. 2015, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Haghparast, A.; Taslimi, Z.; Ramin, M.; Azizi, P.; Khodagholi, F.; Hassanpourezatti, M. Changes in phosphorylation of CREB, ERK, and c-fos induction in rat ventral tegmental area, hippocampus and prefrontal cortex after conditioned place preference induced by chemical stimulation of lateral hypothalamus. Behav. Brain Res. 2011, 220, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.H.; Wang, Y.H.; Wang, J.J.; Tao, P.L.; Tung, C.S.; Wong, C.S. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain 2006, 124, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Miyatake, M.; Narita, M.; Shibasaki, M.; Shindo, K.; Nakamura, A.; Kuzumaki, N.; Nagumo, Y.; Suzuki, T. Direct Evidence of Astrocytic Modulation in the Development of Rewarding Effects Induced by Drugs of Abuse. Neuropsychopharmacology 2006, 31, 2476–2488. [Google Scholar] [CrossRef] [PubMed]
- Arezoomandan, R.; Moradi, M.; Attarzadehyazdi, G.; Tomaz, C.; Haghparast, A. Administration of activated glial condition medium in the nucleus accumbens extended extinction and intensified reinstatement of methamphetamine-induced conditioned place preference. Brain Res. Bull. 2016, 125, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tu, G.; Luo, C.; Guo, Y.; Fang, M.; Zhu, C.; Li, H.; Ou, J.; Zhou, Y.; Liu, W.; et al. Effects of rhynchophylline on the hippocampal miRNA expression profile in ketamine-addicted rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Li, J.; Zhu, D.; Luo, C.; Li, C.; Zhu, C.; Fan, M.; Yung, K.K.-L.; Mo, Z. Effect of Sinomenine on the Morphine-Dependence and Related Neural Mechanisms in Mice. Neurochem. Res. 2017, 42, 3587–3596. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.H.; Ding, J.H.; Zhou, F.; Wang, F.; Hu, L.F.; Sun, T.; Hu, G. Enhancement of glutamate uptake mediates the neuroprotection exerted by activating group II or III metabotropic glutamate receptors on astrocytes. J. Neurochem. 2005, 92, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the sinomenine are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, J.; Zhou, Y.; Li, C.; Chen, Z.; Li, H.; Fang, M.; Zhu, C.; Huo, C.; Yung, K.K.-L.; Li, J.; et al. Sinomenine Protects Against Morphine Dependence through the NMDAR1/CAMKII/CREB Pathway: A Possible Role of Astrocyte-Derived Exosomes. Molecules 2018, 23, 2370. https://doi.org/10.3390/molecules23092370
Ou J, Zhou Y, Li C, Chen Z, Li H, Fang M, Zhu C, Huo C, Yung KK-L, Li J, et al. Sinomenine Protects Against Morphine Dependence through the NMDAR1/CAMKII/CREB Pathway: A Possible Role of Astrocyte-Derived Exosomes. Molecules. 2018; 23(9):2370. https://doi.org/10.3390/molecules23092370
Chicago/Turabian StyleOu, Jinying, Yuting Zhou, Chan Li, Zhijie Chen, Hancheng Li, Miao Fang, Chen Zhu, Chuying Huo, Ken Kin-Lam Yung, Jing Li, and et al. 2018. "Sinomenine Protects Against Morphine Dependence through the NMDAR1/CAMKII/CREB Pathway: A Possible Role of Astrocyte-Derived Exosomes" Molecules 23, no. 9: 2370. https://doi.org/10.3390/molecules23092370
APA StyleOu, J., Zhou, Y., Li, C., Chen, Z., Li, H., Fang, M., Zhu, C., Huo, C., Yung, K. K.-L., Li, J., Luo, C., & Mo, Z. (2018). Sinomenine Protects Against Morphine Dependence through the NMDAR1/CAMKII/CREB Pathway: A Possible Role of Astrocyte-Derived Exosomes. Molecules, 23(9), 2370. https://doi.org/10.3390/molecules23092370



