Correlation between Quality and Geographical Origins of Poria cocos Revealed by Qualitative Fingerprint Profiling and Quantitative Determination of Triterpenoid Acids
Abstract
1. Introduction
2. Results and Discussion
2.1. Sample Extraction
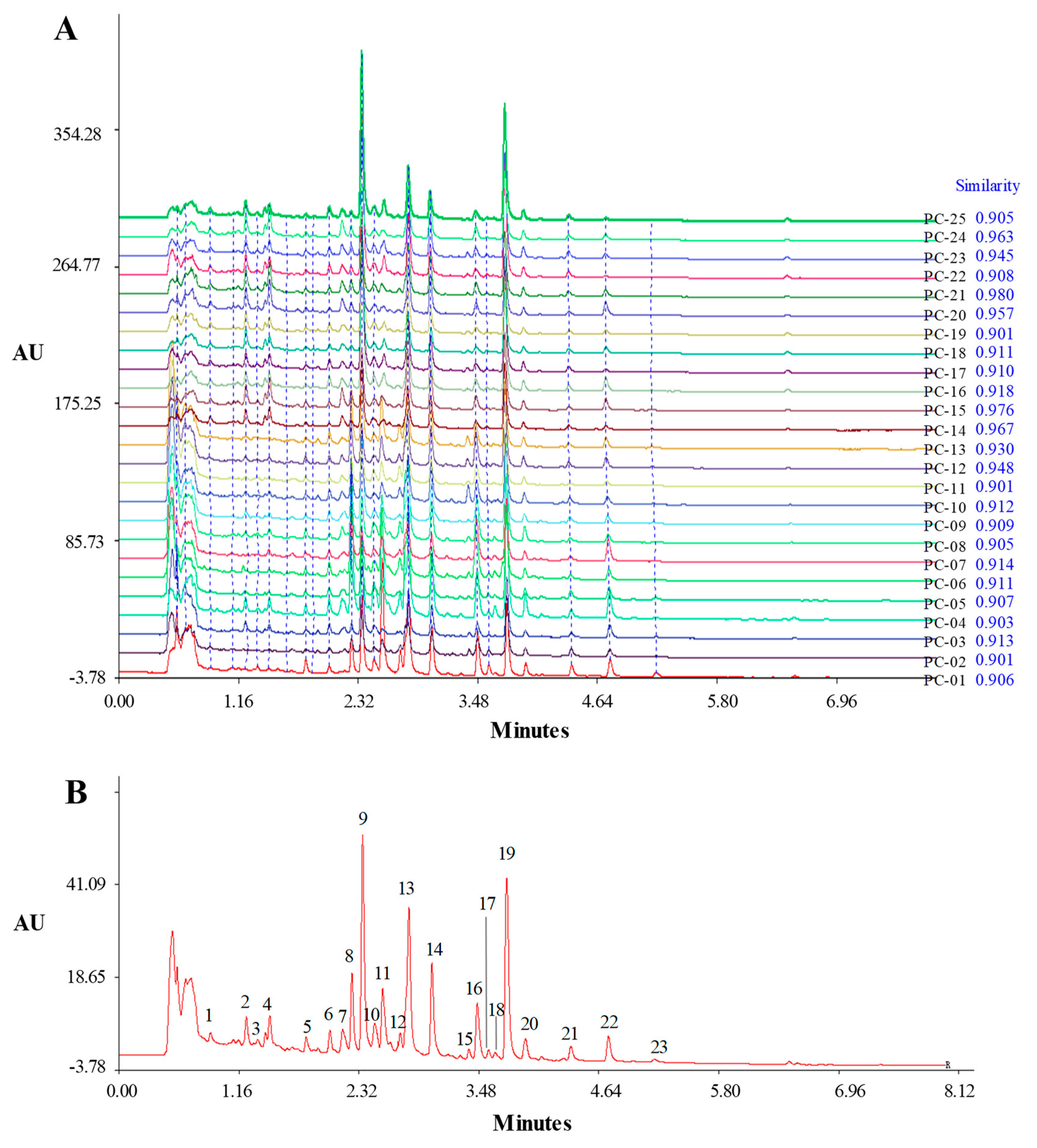
2.2. Establishment of UHPLC Fingerprint
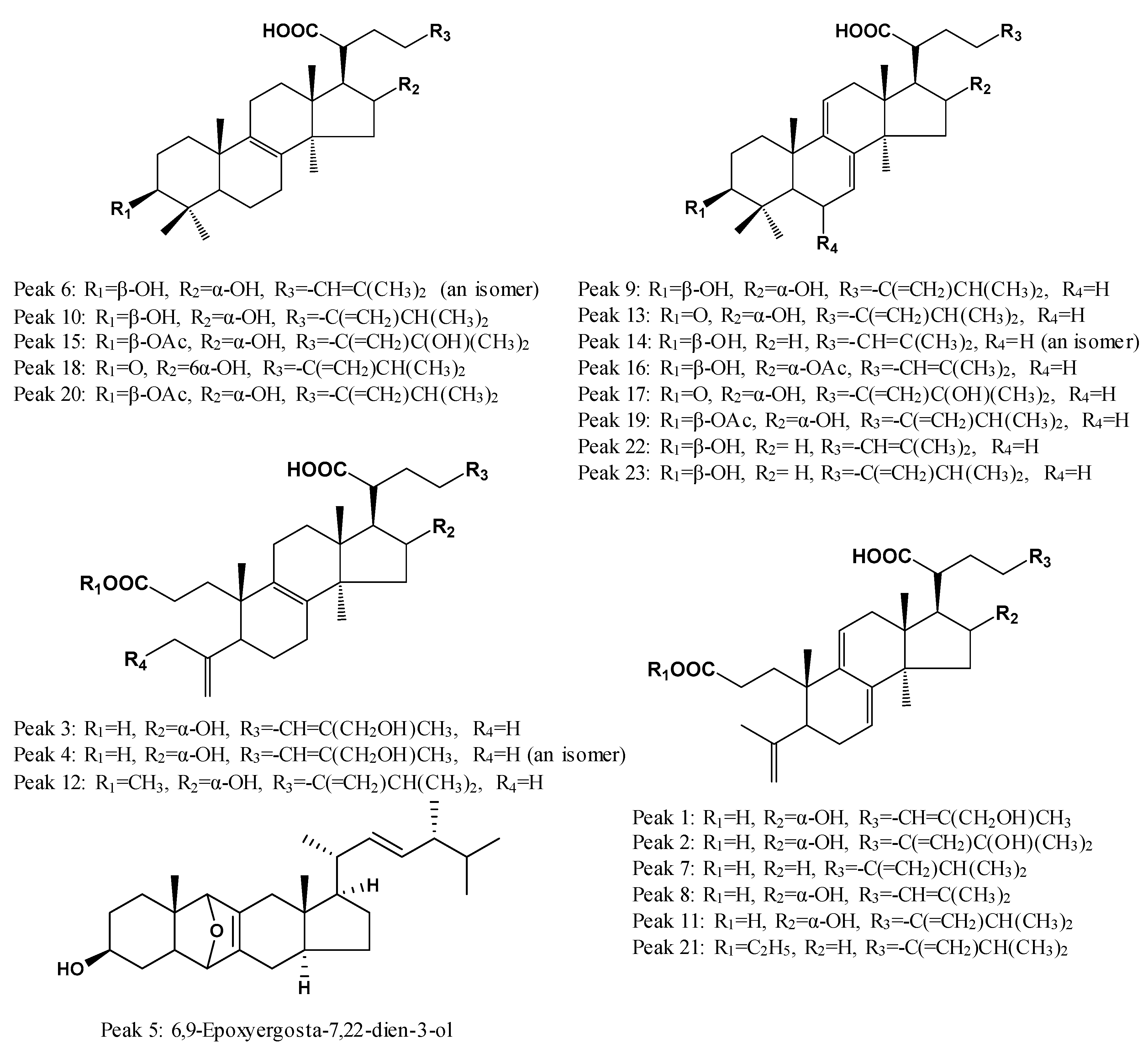
2.3. Identification of Common Peaks in the UHPLC Fingerprint
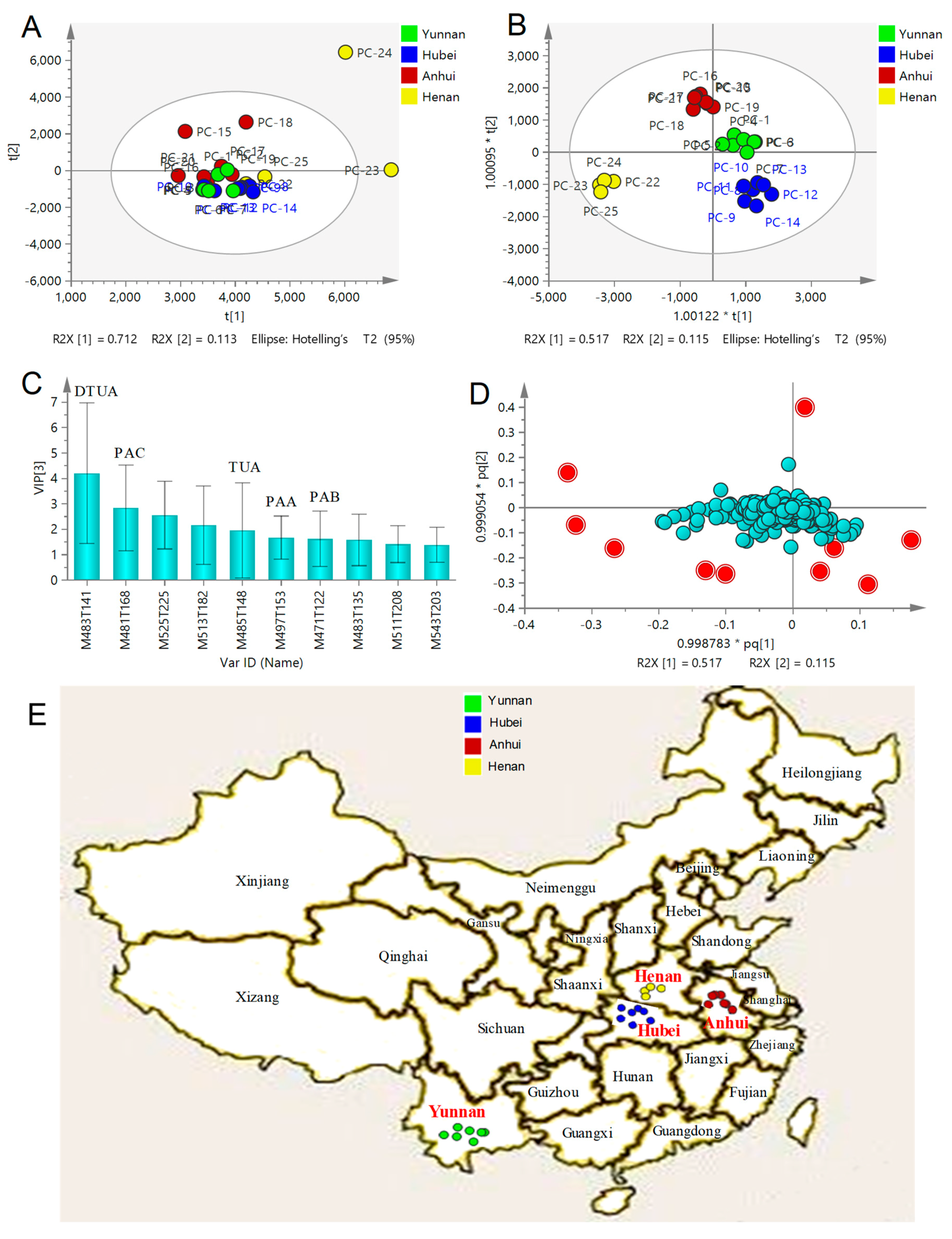
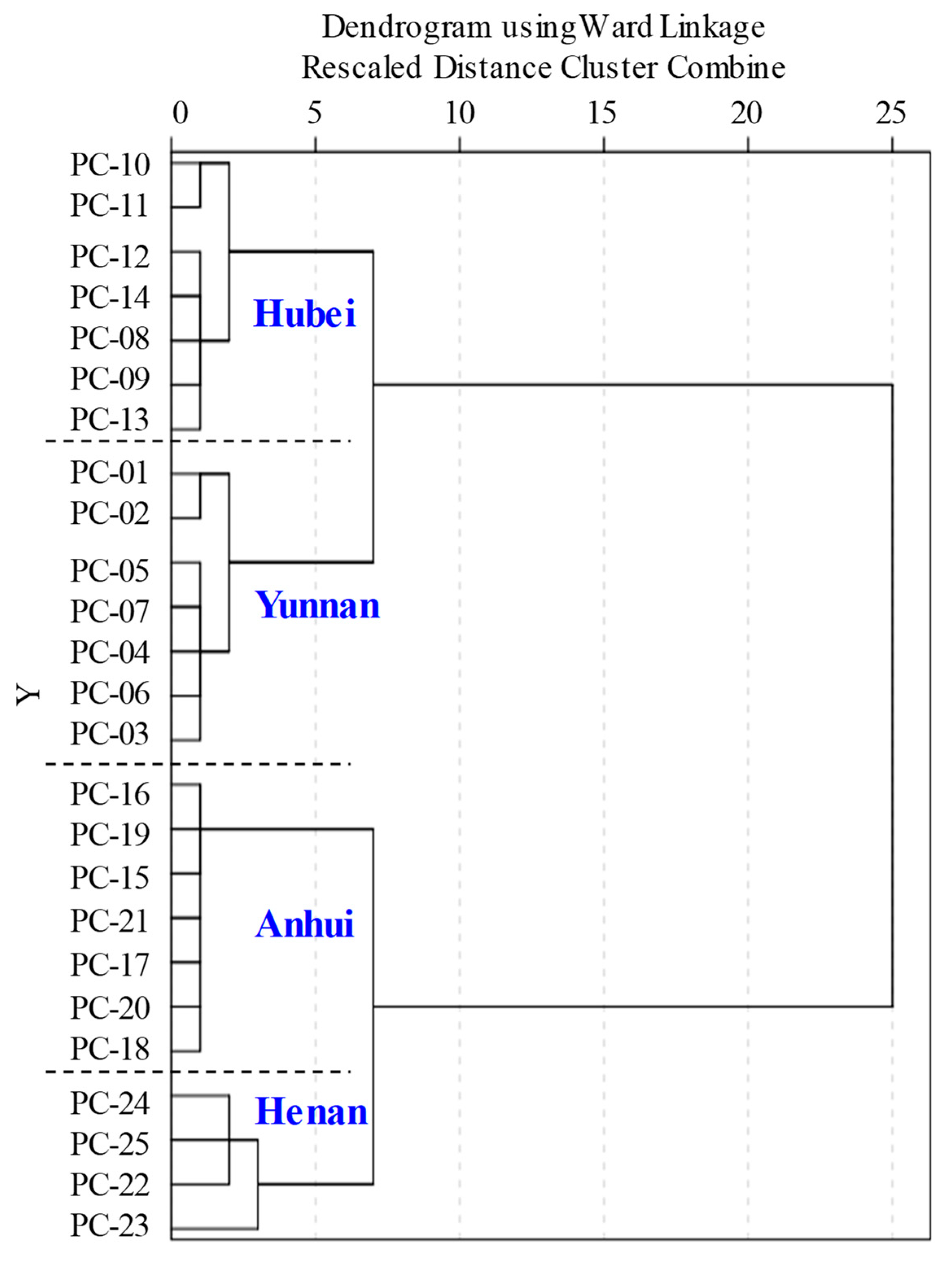
2.4. Multivariate Statistical Analysis of QTOF-MS/MS Data
2.5. Qualitative and Quantitative Method Validation
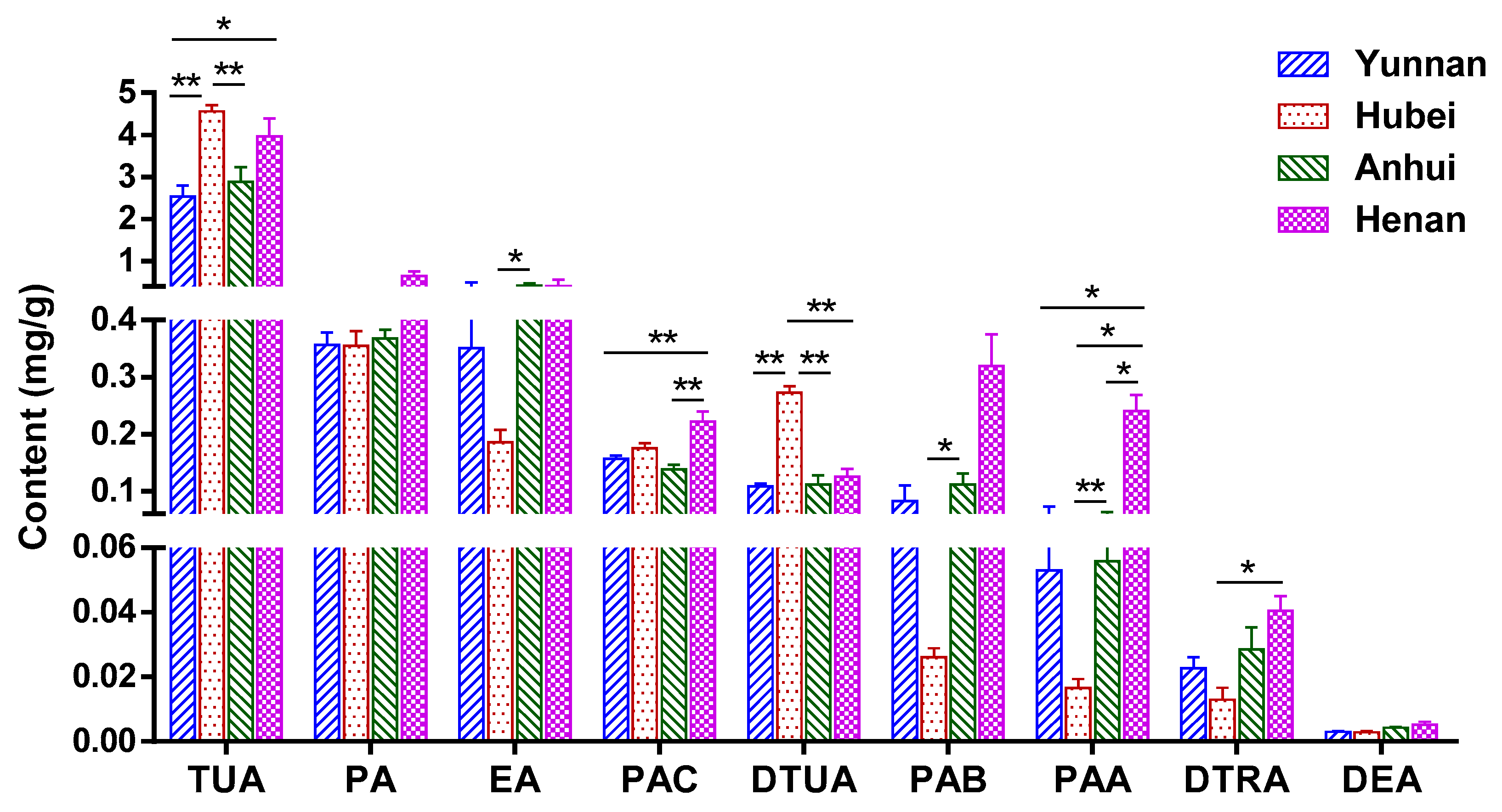
2.6. Quantitative Analysis of Triterpenoid Acids
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Samples Preparation
3.2.1. Sample Extraction for Qualitative Analysis
3.2.2. Sample Extraction for Quantitative Analysis
3.3. UHPLC Fingerprint Analysis
3.4. UHPLC-DAD-QTOF-MS/MS Analysis
3.5. UHPLC-QqQ-MS/MS Analysis
3.6. Qualitative and Quantitative Method Validation
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, L.X.; Xu, J.; Zhang, S.J.; Wang, R.J.; Huang, Q.; Chen, H.B.; Dong, X.P.; Zhao, Z.Z. Qualitatively and quantitatively comparing secondary metabolites in three medicinal parts derived from Poria cocos (Schw.) Wolf using UHPLC-QTOF-MS/MS-based chemical profiling. J. Pharm. Biomed. Anal. 2018, 150, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L. Chemical constituents and pharmacological properties of poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Lei, P.; Tian, T.; Yin, L.; Chen, D.Q.; Chen, H.; Mei, Q.B.; Zhao, Y.Y.; Lin, R.C. Diuretic activity of some fractions of the epidermis of Poria cocos. J. Ethnopharmacol. 2013, 150, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Feng, Y.L.; Du, X.; Xi, Z.H.; Cheng, X.L.; Wei, F. Diuretic activity of the ethanol and aqueous extracts of the surface layer of Poria cocos in rat. J. Ethnopharmacol. 2012, 144, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S. Antibacterial activity of the triterpenoid acid (polyporenic acid C) and of ungulinic acid, metabolic products of Polyporus benzoinus (Wahl.) Fr. Biochem. J. 1952, 50, 516–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Zhang, J.; Zhao, Y.L.; Li, T.; Shen, T.; Li, J.Q.; Li, W.Y.; Liu, H.G. Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb.: A review. J. Ethnopharmacol. 2013, 147, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Cheng, W.Y.; Huang, B.E.T.G.; Hsu, Y.H.; Huang, S.Y. Anti-inflammatory and hypoglycemic efficacy of Poria cocos and Dioscorea opposita in prediabetes mellitus rats. RSC Adv. 2014, 4, 55649–55657. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, H.H.; Han, M.H.; Kim, G.Y.; Hong, S.H.; Park, C.; Choi, Y.H. Ethanol extract of Poria cocos reduces the production of inflammatory mediators by suppressing the NF-kappaB signaling pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. BMC Complement. Altern. Med. 2014, 14, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.S.; Huang, S.S.; Lin, T.H.; Lee, M.M.; Kuo, C.C.; Sung, P.J.; Hou, W.C.; Huang, G.J.; Kuo, Y.H. Analgesic and anti-inflammatory bioactivities of eburicoic acid and dehydroeburicoic acid isolated from Antrodia camphorata on the inflammatory mediator expression in mice. J. Agric. Food Chem. 2013, 61, 5064–5071. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Ng, L.T.; Lin, C.C. Antioxidant activities of some common ingredients of traditional Chinese medicine, Angelica sinensis, Lycium barbarum and Poria cocos. Phytother. Res. 2004, 18, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Gapter, L.A.; Ling, H.; Agarwal, R.; Ng, K.Y. Cytotoxic and anti-oxidant activities of lanostane-type triterpenes isolated from Poria cocos. Chem. Pharm. Bull. 2008, 56, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Uchiyama, E.; Kikuchi, T.; Tokuda, H.; Suzuki, T.; Kimura, Y. Anti-tumor-promoting effects of 25-Methoxyporicoic Acid A and other triterpene acids from Poria cocos. J. Nat. Prod. 2009, 72, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Ukiya, M.; Akihisa, T.; Tokuda, H.; Hirano, M.; Oshikubo, M.; Nobukuni, Y.; Kimura, Y.; Tai, T.; Kondo, S.; Nishino, H. Inhibition of tumor-promoting effects by poricoic acids G and H and other lanostane-type triterpenes and cytotoxic activity of poricoic acids A and G from Poria cocos. J. Nat. Prod. 2002, 65, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Zhou, L.; Jia, X.B.; Gapter, L.A.; Agarwal, R.; Ng, K.Y. Polyporenic acid C induces caspase-8-mediated apoptosis in human lung cancer A549 cells. Mol. Carcinog. 2009, 48, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Li, T.H.; Hou, C.C.; Chang, C.L.; Yang, W.C. Anti-hyperglycemic properties of crude extract and triterpenes from Poria cocos. Evid.-Based Complement. Altern. Med. 2010, 2011, 128402. [Google Scholar]
- Tai, T.; Akita, Y.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Watanabe, K. Anti-emetic principles of Poria cocos. Planta Med. 1995, 61, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, X.F.; Wang, B.C.; Liu, H.Y.; Li, C.Y.; Liu, Z.H.; Zhang, G.W.; Lu, H.; Chi, C.; Wang, F. Pachymic acid, a novel compound for anti-rejection: Effect in rats following cardiac allograft transplantation. Chin. Med. J. 2009, 122, 2898–2902. [Google Scholar] [PubMed]
- Zeng, Y.L.; Lu, Y.; Chen, Z.; Tan, J.W.; Bai, J.; Li, P.Y.; Wang, Z.X.; Du, S.Y. Rapid Characterization of Components in Bolbostemma paniculatum by UPLC/LTQ-Orbitrap MS n Analysis and Multivariate Statistical Analysis for Herb Discrimination. Molecules 2018, 23, 1155. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.F.; Wang, K.F.; Mao, X.; Liang, W.Y.; Chen, W.J.; Li, S.; Qi, Q.; Cui, Y.P.; Zhang, L.Z. Screening and Analysis of the Potential Bioactive Components of Poria cocos (Schw.) Wolf by HPLC and HPLC-MS n with the Aid of Chemometrics. Molecules 2016, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Y.S.; Zhao, Y.; Gong, X.J.; Zhao, C.; Chen, H.G. An LC fingerprint study of Poria cocos (Schw.) Wolf. Chromatographia 2009, 69, 1283–1289. [Google Scholar] [CrossRef]
- Xia, B.; Zhou, Y.; Tan, H.S.; Ding, L.S.; Xu, H.X. Advanced ultra-performance liquid chromatography-photodiode array-quadrupole time-of-flight mass spectrometric methods for simultaneous screening and quantification of triterpenoids in Poria cocos. Food Chem. 2014, 152, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Thammana, M. A review on high performance liquid chromatography (HPLC). J. Pharm. Anal. 2016, 5, 22–28. [Google Scholar]
- Malviya, R.; Bansal, V.; Pal, O.P.; Sharma, P.K. High performance liquid chromatography: A short review. J. Glob. Pharma Technol. 2010, 2, 22–26. [Google Scholar]
- Huang, H.L.; Liu, M.; Chen, P. Recent Advances in Ultra-High Performance Liquid Chromatography for the Analysis of Traditional Chinese Medicine. Anal. Lett. 2014, 47, 1835–1851. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.T.; Chen, Y.J.; Xu, L.; Qin, M.J.; Yi, T.; Chen, H.B.; Zhao, Z.Z. Localization of ginsenosides in the rhizome and root of Panax ginseng by laser microdissection and liquid chromatography-quadrupole/time of flight-mass spectrometry. J. Pharm. Biomed. Anal. 2015, 105, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Jiang, X.L.; Zhang, S.X.; Dai, X.L.; Liu, Y.J.; Tan, H.R.; Gao, L.P.; Xia, T. Quantification of flavonol glycosides in Camellia sinensis by MRM mode of UPLC-QQQ-MS/MS. J. Chromatogr. B 2016, 1017–1018, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Meudec, E.; Verbaere, A.; Mazerolles, G.; Wirth, J.; Masson, G.; Cheynier, V.; Sommerer, N. A High-Throughput UHPLC-QqQ-MS Method for Polyphenol Profiling in Rosé Wines. Molecules 2015, 20, 7890–7914. [Google Scholar] [CrossRef] [PubMed]
- El Senousy, A.S.; Farag, M.A.; Al-Mandy, D.A.; Wessjohann, L.A. Developmental changes in leaf phenolics composition from three artichoke cvs. (Cynara scolymus) as determined via UHPLC-MS and chemometrics. Phytochemistry 2014, 108, 67–76. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the pachymic acid, polyporenic acid C, tumulosic acid, poricoic acid A, poricoic acid B, dehydrotumulosic acid, dehydrotrametenolic acid, dehydroeburicoic acid and eburicoic acid are available from the authors. |
| Peak No. | Precision (RSD, %) | Stability (RSD, %) | Repeatability (RSD, %) | |||||
|---|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | |||||||
| RRT | RPA | RRT | RPA | RRT | RPA | RRT | RPA | |
| 1 | 0.02 | 3.26 | 0.08 | 4.58 | 0.06 | 4.87 | 0.03 | 3.31 |
| 2 | 0.04 | 4.73 | 0.18 | 3.92 | 0.21 | 4.09 | 0.09 | 4.19 |
| 3 | 0.09 | 2.69 | 0.15 | 4.03 | 0.14 | 3.84 | 0.06 | 2.80 |
| 4 | 0.15 | 4.28 | 0.24 | 3.85 | 0.17 | 4.72 | 0.13 | 2.94 |
| 5 | 0.05 | 1.97 | 0.07 | 3.14 | 0.09 | 2.51 | 0.03 | 1.79 |
| 6 | 0.02 | 2.88 | 0.10 | 2.36 | 0.08 | 3.11 | 0.06 | 3.53 |
| 7 | 0.18 | 3.95 | 0.26 | 4.87 | 0.20 | 4.39 | 0.09 | 3.65 |
| 8 | 0.03 | 3.42 | 0.14 | 1.83 | 0.06 | 0.96 | 0.04 | 2.48 |
| 9 | 0.07 | 2.16 | 0.05 | 3.11 | 0.12 | 1.57 | 0.08 | 1.85 |
| 10 | 0.10 | 4.71 | 0.16 | 2.94 | 0.12 | 4.63 | 0.13 | 3.64 |
| 11 | 0.19 | 3.02 | 0.32 | 4.12 | 0.28 | 2.85 | 0.25 | 2.39 |
| 12 | 0.06 | 4.13 | 0.20 | 3.47 | 0.15 | 3.68 | 0.19 | 2.54 |
| 13 | 0.05 | 2.40 | 0.08 | 3.78 | 0.17 | 1.25 | 0.10 | 3.08 |
| 14 | 0.03 | 1.62 | 0.07 | 3.15 | 0.09 | 2.94 | 0.07 | 2.81 |
| 15 | 0.07 | 3.89 | 0.10 | 2.99 | 0.05 | 4.12 | 0.08 | 1.42 |
| 16 | 0.04 | 2.35 | 0.12 | 3.44 | 0.06 | 1.67 | 0.03 | 0.86 |
| 17 | 0.08 | 1.91 | 0.05 | 3.80 | 0.03 | 3.34 | 0.11 | 4.13 |
| 18 | 0.02 | 2.96 | 0.08 | 2.69 | 0.03 | 4.58 | 0.07 | 4.58 |
| 19 | 0.03 | 3.45 | 0.05 | 1.92 | 0.02 | 2.53 | 0.07 | 0.62 |
| 20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 21 | 0.04 | 2.67 | 0.10 | 3.15 | 0.02 | 1.80 | 0.06 | 1.56 |
| 22 | 0.06 | 3.46 | 0.17 | 2.74 | 0.03 | 2.36 | 0.12 | 2.24 |
| 23 | 0.11 | 3.82 | 0.16 | 4.07 | 0.07 | 4.74 | 0.06 | 3.50 |
| Reference Standards | Working Standard Curve | Precision (RSD, %, n = 6) | Stability (48 h, RSD, %) | Sensitivity (ng/mL) | Repeatability (RSD, %, n = 6) | Spike Recovery (Mean (RSD), %, n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equation | r | Linear Range (µg/mL) | Intra-Day | Inter-Day | LODs | LLOQs | Low | Middle | High | |||
| PAB | y = 139,887x + 104,903 | 0.9968 | 0.1–20 | 1.84 | 3.48 | 2.88 | 0.45 | 1.18 | 2.27 | 94.72 (7.38) | 101.65 (5.63) | 96.88 (6.24) |
| DTUA | y = 32,635x + 170,109 | 0.9967 | 0.125–25 | 3.25 | 5.49 | 3.35 | 0.74 | 2.23 | 5.35 | 93.59 (8.26) | 98.32 (4.08) | 105.16 (7.50) |
| TUA | y = 784x + 75,827 | 0.9959 | 0.5–100 | 3.62 | 3.71 | 2.92 | 1.83 | 4.86 | 3.91 | 104.35 (2.78) | 99.49 (1.82) | 96.95 (6.63) |
| PAA | y = 129,115x − 11,973 | 0.9946 | 0.05–10 | 4.45 | 4.13 | 4.47 | 0.37 | 1.01 | 4.18 | 97.86 (3.43) | 104.91 (4.85) | 92.64 (5.74) |
| PAC | y = 11,879x + 44,483 | 0.9957 | 0.125–25 | 2.14 | 4.77 | 3.75 | 0.49 | 1.54 | 3.64 | 102.48 (6.89) | 98.76 (3.91) | 107.19 (8.03) |
| PA | y = 63,136x + 576,258 | 0.9998 | 0.25–50 | 1.01 | 4.71 | 1.81 | 0.58 | 1.82 | 1.70 | 96.42 (4.07) | 100.54 (2.58) | 103.42 (3.98) |
| DTRA | y = 23,506x + 25,923 | 0.9956 | 0.05–10 | 2.67 | 0.84 | 3.36 | 3.04 | 8.79 | 6.39 | 86.75 (9.11) | 93.63 (6.87) | 105.38 (8.56) |
| DEA | y = 10,217x + 125 | 0.9996 | 0.05–10 | 3.29 | 6.60 | 4.85 | 0.97 | 2.65 | 7.12 | 82.98 (10.85) | 106.26 (8.36) | 108.55 (7.25) |
| EA | y = 283x − 4 | 0.9989 | 0.5–100 | 4.25 | 4.38 | 5.97 | 142 | 458 | 5.69 | 92.14 (6.75) | 96.93 (7.84) | 106.63 (5.27) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.-X.; Xu, J.; Wang, R.-J.; Li, H.-X.; Tan, Y.-Z.; Chen, H.-B.; Dong, X.-P.; Zhao, Z.-Z. Correlation between Quality and Geographical Origins of Poria cocos Revealed by Qualitative Fingerprint Profiling and Quantitative Determination of Triterpenoid Acids. Molecules 2018, 23, 2200. https://doi.org/10.3390/molecules23092200
Zhu L-X, Xu J, Wang R-J, Li H-X, Tan Y-Z, Chen H-B, Dong X-P, Zhao Z-Z. Correlation between Quality and Geographical Origins of Poria cocos Revealed by Qualitative Fingerprint Profiling and Quantitative Determination of Triterpenoid Acids. Molecules. 2018; 23(9):2200. https://doi.org/10.3390/molecules23092200
Chicago/Turabian StyleZhu, Li-Xia, Jun Xu, Ru-Jing Wang, Hong-Xiang Li, Yu-Zhu Tan, Hu-Biao Chen, Xiao-Ping Dong, and Zhong-Zhen Zhao. 2018. "Correlation between Quality and Geographical Origins of Poria cocos Revealed by Qualitative Fingerprint Profiling and Quantitative Determination of Triterpenoid Acids" Molecules 23, no. 9: 2200. https://doi.org/10.3390/molecules23092200
APA StyleZhu, L.-X., Xu, J., Wang, R.-J., Li, H.-X., Tan, Y.-Z., Chen, H.-B., Dong, X.-P., & Zhao, Z.-Z. (2018). Correlation between Quality and Geographical Origins of Poria cocos Revealed by Qualitative Fingerprint Profiling and Quantitative Determination of Triterpenoid Acids. Molecules, 23(9), 2200. https://doi.org/10.3390/molecules23092200





