Analysis of Contact Traces of Cannabis by In-Tube Solid-Phase Microextraction Coupled to Nanoliquid Chromatography
Abstract
1. Introduction
2. Results
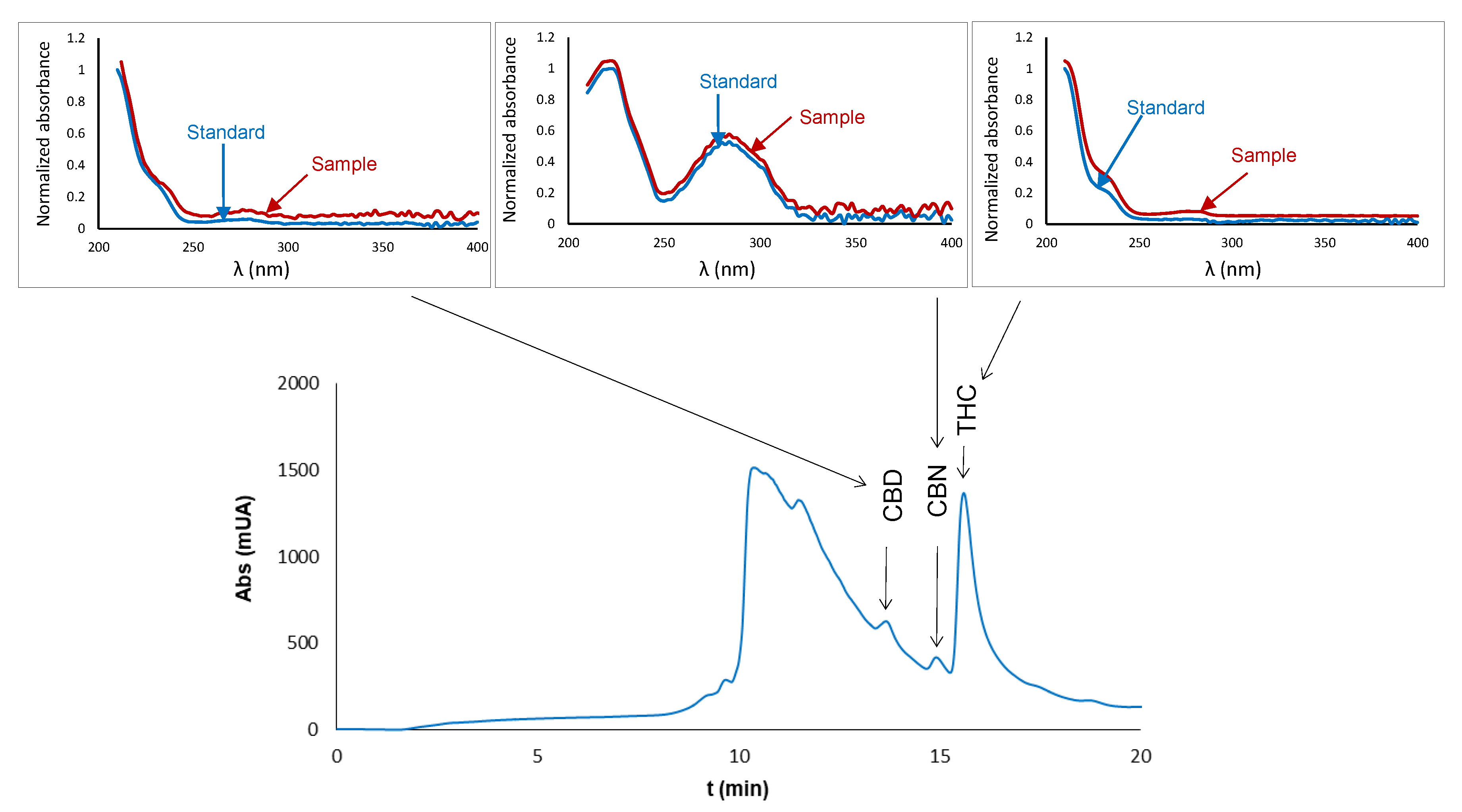
2.1. Chromatographic Analysis
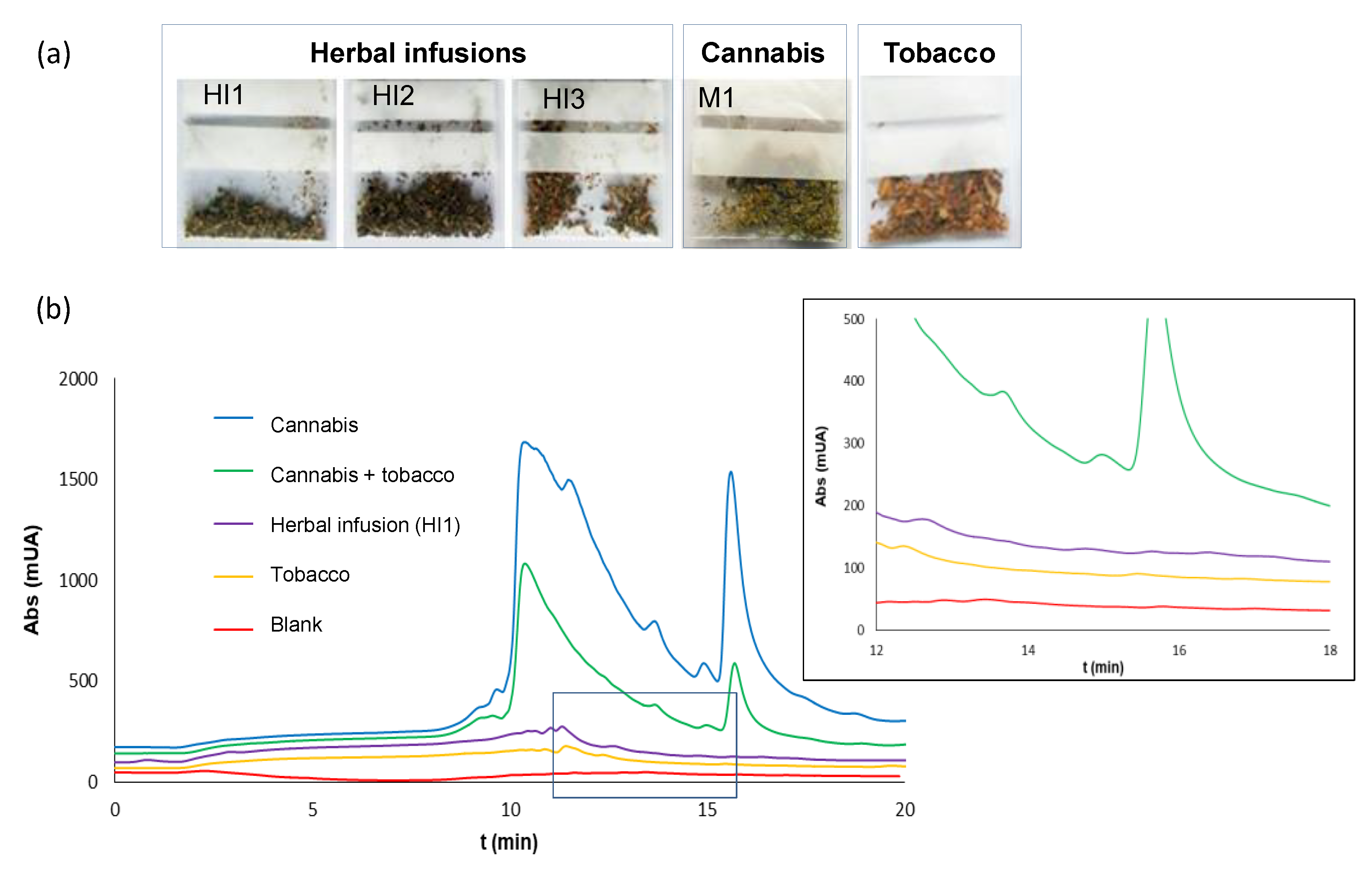
2.2. Analysis of Extracts of Cannabis Plants
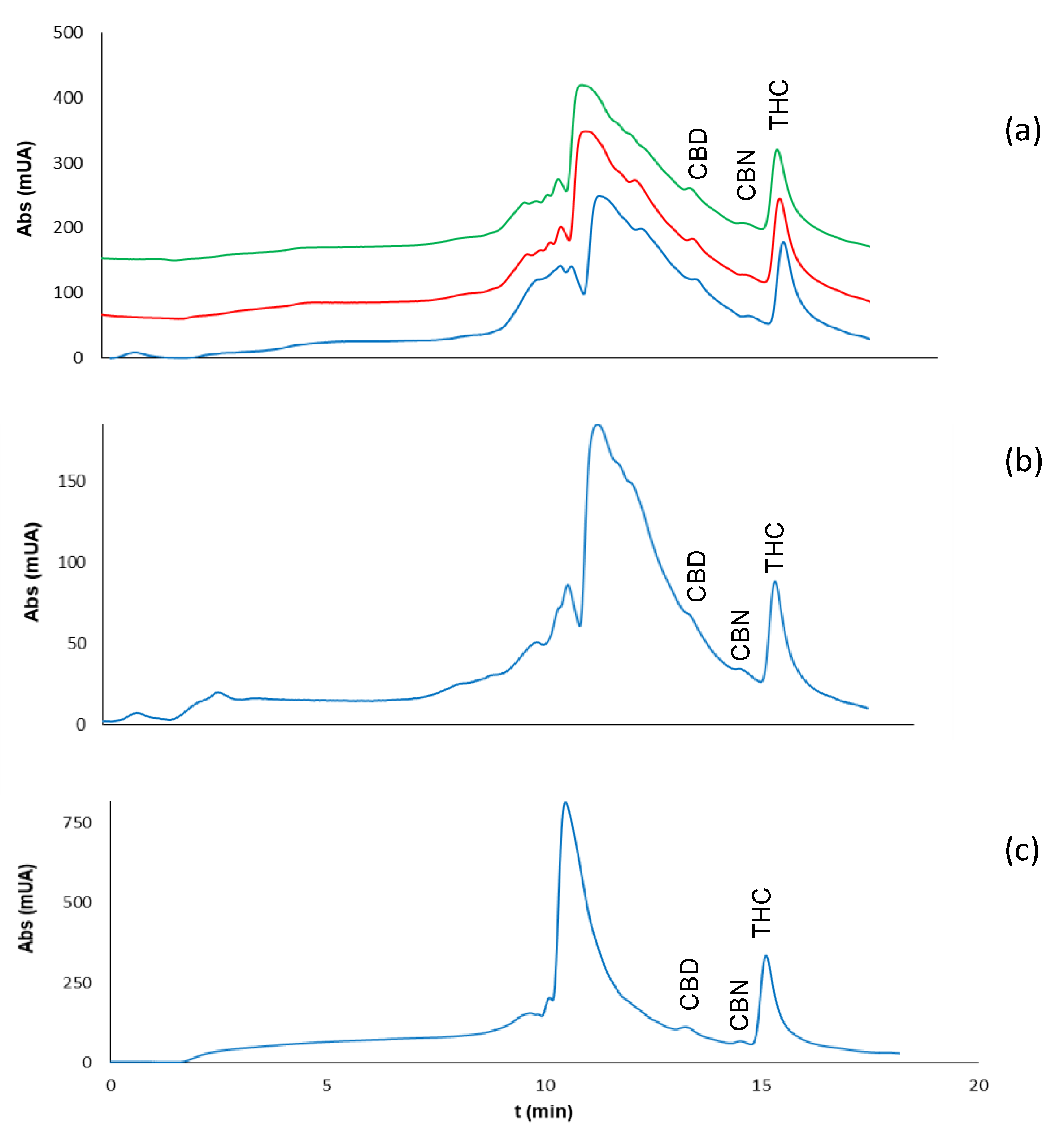
2.3. Analysis of Residues of Cannabis on Surfaces
2.3.1. Collection and Extraction Procedure
2.3.2. Type of Surface
2.3.3. Quantitation of THC
2.3.4. Selectivity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solutions
4.2. Chromatographic Conditions
4.3. IT-SPME Conditions
4.4. Analysis of Cannabis Plants
4.5. Analysis of Traces of Cannabis on Surfaces
Author Contributions
Funding
Conflicts of Interest
References
- Keller, T.; Keller, A.; Tutsch-Bauer, E.; Monticelli, F. Application of ion mobility spectrometry in cases of forensic interest. Forensic Sci. Int. 2006, 161, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Peinado, L.S.; de Castro, M.D.L. An overview on forensic analysis devoted to analytical chemists. Talanta 2017, 167, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, W.; Matyjasek, L.; Karpińska, M. Detection of contact traces of powdery substances. J. Forensic Sci. 2017, 62, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Klimer, B. New Developments in Cannabis Regulations. Available online: http://www.emcdda.europa.eu/document-library/new-developments-cannabis-regulation_en (accessed on 1 September 2018).
- Ali, E.M.A.; Edwards, G.M.; Hargreaves, M.D.; Scowen, I.J. In-situ detection of drugs-of-abuse on clothing using confocal Raman microscopy. Anal. Chim. Acta 2008, 615, 63–72. [Google Scholar] [CrossRef] [PubMed]
- West, M.J.; Went, M.J. The spectroscopic detection of drugs of abuse on textile fibers after recovery with adhesive lifters. Forensic Sci. Int. 2009, 189, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Talaty, N.; Mulligan, C.C.; Justes, D.R.; Jackson, A.U.; Noll, R.J.; Cooks, R.G. Fabric analysis by ambient mass spectrometry for explosive and drugs. Analyst 2008, 133, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Martyny, J.W.; Serrano, K.A.; Schaeffer, J.W.; Joshua, W.; Van Dyke, M.V. Potential exposures associated with indoor marijuana growing operations. J. Occup. Environ. Hyg. 2013, 10, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Doran, G.S.; Deans, R.; De Filippis, C.; Kostakis, C.; Howitt, J.A. Work place drug testing of police officers after THC exposure during large volume cannabis seizures. Forensic Sci. Int. 2017, 275, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Doran, G.S.; Deans, R.; De Filippis, C.; Kostakis, C.; Howitt, J.A. The presence of licit and illicit drugs in police stations and their implications for workplace drug testing. Forensic Sci. Int. 2017, 278, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Doran, G.S.; Deans, R.; De Filippis, C.; Kostakis, C.; Howitt, J.A. Quantification of licit and illicit drugs on typical police station work surface using LC-MS/MS. Anal. Methods 2017, 9, 198–210. [Google Scholar] [CrossRef]
- Nazario, C.E.D.; Silva, M.R.; Franco, M.S.; Lanças, F.M. Evolution in miniaturized column liquid chromatography instrumentation and applications: An overview. J. Chromatogr. A 2015, 1421, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Moliner-Martinez, Y.; Herráez-Hernández, R.; Verdú-Andrés, J.; Molins-Legua, C.; Campíns-Falcó, P. Recent advances of in-tube solid-phase microextraction. Trends Anal. Chem. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Serra-Mora, P.; Moliner-Martínez, Y.; Molins-Legua, C.; Herráez-Hernández, R.; Verdú-Andrés, J.; Campíns-Falcó, P. Trends in online in-tube solid phase microextraction. Compr. Anal. Chem. 2017, 76, 427–461. [Google Scholar] [CrossRef]
- Serra-Mora, P.; Rodríguez-Palma, C.E.; Verdú-Andrés, J.; Herráez-Hernández, R.; Campíns-Falcó, P. Improving the on-line extraction of polar compounds by IT-SPME with silica nanoparticles modified phases. Separations 2018, 5, 10. [Google Scholar] [CrossRef]
- Serra-Mora, P.; Jornet-Martínez, N.; Moliner-Martínez, Y.; Campíns-Falcó, P. In tube-solid phase microextraction-nano liquid chromatography: Application to the determination of intact and degraded polar triazines in waters and recovered struvite. J. Chromatogr. A 2017, 1513, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ambach, L.; Penitschka, F.; Broillet, A.; König, S.; Weinmann, W.; Bernhard, W. Simultaneous quantification of delta-9-THC, THC-acid, CBN and CBD in seized drugs using HPLC-DAD. Forensic Sci. Int. 2014, 243, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Gul, W.; Gul, S.W.; Radwan, M.M.; Wanas, A.S.; Mehmedic, Z.; Khan, I.I.; Sharaf, M.H.M.; ElSohly, M.A. Determination of 11 cannabinoids in biomass and extracts of different varieties of cannabis using high-performance liquid chromatography. J. AOAC Int. 2015, 98, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Wene, D.; Fan, Z. Qualitative and quantitative measurement of cannabinoids in cannabis using modified HPLC/DAD method. J. Pharm. Biomed. Anal. 2017, 146, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Campíns-Falcó, P.; Verdú-Andrés, J.; Sevillano-Cabeza, A.; Molins-Legua, C.; Herráez-Hernández, R. New micromethod combining miniaturized matrix solid-phase dispersion and in-tube-in-valve solid phase microextraction for estimating polycyclic aromatic hydrocarbons in bivalves. J. Chromatogr. A 2008, 1211, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Molnar, A.; Lewis, J.; Fu, S. Recovery of spiked Δ9-tetrahydrocannabinol in oral fluid from polypropilene containers. Forensic Sci. Int. 2013, 227, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Philp, M.; Fu, S. A review of chemical ‘spot’ tests: A presumptive illicit drug identification technique. Drug Test. Anal. 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stoney, D.A.; Stoney, P.L. Critical review of forensic trace evidence analysis and the need for a new approach. Forensic Sci. Int. 2015, 251, 159–170. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Compound | Linearity 1, y = ax + b | n | LOD (ng/mL) | LOQ (ng/mL) | ||
|---|---|---|---|---|---|---|
| a ± sa (mL/ng) | b ± sb | r2 | ||||
| THC | 24 ± 1 | −270 ± 80 | 0.98 | 7 | 2 | 8 |
| CBD | 28 ± 2 | −300 ± 100 | 0.990 | 7 | 5 | 15 |
| CBN | 7.8 ± 0.5 | −10 ± 30 | 0.990 | 6 | 5 | 15 |
| Storage Conditions | Percentage of THC (%) 1 | |||
|---|---|---|---|---|
| M1 | M2 | M3 | M4 | |
| Ambient | 0.4, 0.8, 0.8 | 0.4, 0.5 | 0.4, 0.8 | 0.6, 0.7 |
| 50 °C | 1.0, 1.0, 0.8 | 3.2, 2.8 | 4.8, 4.7 | 3.2, 2.6 |
| Item | Cannabis Sample | Amount of Sample Collected (mg) | Dilution Factor | Concentration of THC Measured (ng/mL) | Estimated Amount of THC Collected (µg) | Percentage of THC in the Sample (%) |
|---|---|---|---|---|---|---|
| Plastic bag | M1 | 0.82 | 1:100 | 79 | 8.2 | 1.0 |
| Plastic bag | M2 | 0.42 | 1:50 | 78 | 3.8 | 0.9 |
| Plastic bag | M3 | 0.87 | 1:400 | 61 | 24.4 | 2.8 |
| Plastic bag | M4 | 0.38 | 1:100 | 99 | 9.9 | 2.6 |
| Plastic bag | Mixture of plants | 0.10 | 1:20 | 58 | 1.2 | 1.2 |
| Plastic bag | Mixture of plants | 0.08 | 1:100 | 41 | 4.1 | 5.1 |
| Aluminum foil | M1 | 0.27 | 1:20 | 82 | 1.6 | 0.6 |
| Office paper | M1 | 0.10 | 1:20 | 75 | 1.5 | 1.5 |
| Piece of cotton cloth | M1 | 4.28 | 1:250 | 71 | 34.2 | 0.4 |
| Skin | M1 | 0.20 | 1:20 | 94 | 1.9 | 0.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jornet-Martínez, N.; Ortega-Sierra, A.; Verdú-Andrés, J.; Herráez-Hernández, R.; Campíns-Falcó, P. Analysis of Contact Traces of Cannabis by In-Tube Solid-Phase Microextraction Coupled to Nanoliquid Chromatography. Molecules 2018, 23, 2359. https://doi.org/10.3390/molecules23092359
Jornet-Martínez N, Ortega-Sierra A, Verdú-Andrés J, Herráez-Hernández R, Campíns-Falcó P. Analysis of Contact Traces of Cannabis by In-Tube Solid-Phase Microextraction Coupled to Nanoliquid Chromatography. Molecules. 2018; 23(9):2359. https://doi.org/10.3390/molecules23092359
Chicago/Turabian StyleJornet-Martínez, Neus, Adrián Ortega-Sierra, Jorge Verdú-Andrés, Rosa Herráez-Hernández, and Pilar Campíns-Falcó. 2018. "Analysis of Contact Traces of Cannabis by In-Tube Solid-Phase Microextraction Coupled to Nanoliquid Chromatography" Molecules 23, no. 9: 2359. https://doi.org/10.3390/molecules23092359
APA StyleJornet-Martínez, N., Ortega-Sierra, A., Verdú-Andrés, J., Herráez-Hernández, R., & Campíns-Falcó, P. (2018). Analysis of Contact Traces of Cannabis by In-Tube Solid-Phase Microextraction Coupled to Nanoliquid Chromatography. Molecules, 23(9), 2359. https://doi.org/10.3390/molecules23092359






