Use of Polyamidoamine Dendrimers in Brain Diseases
Abstract
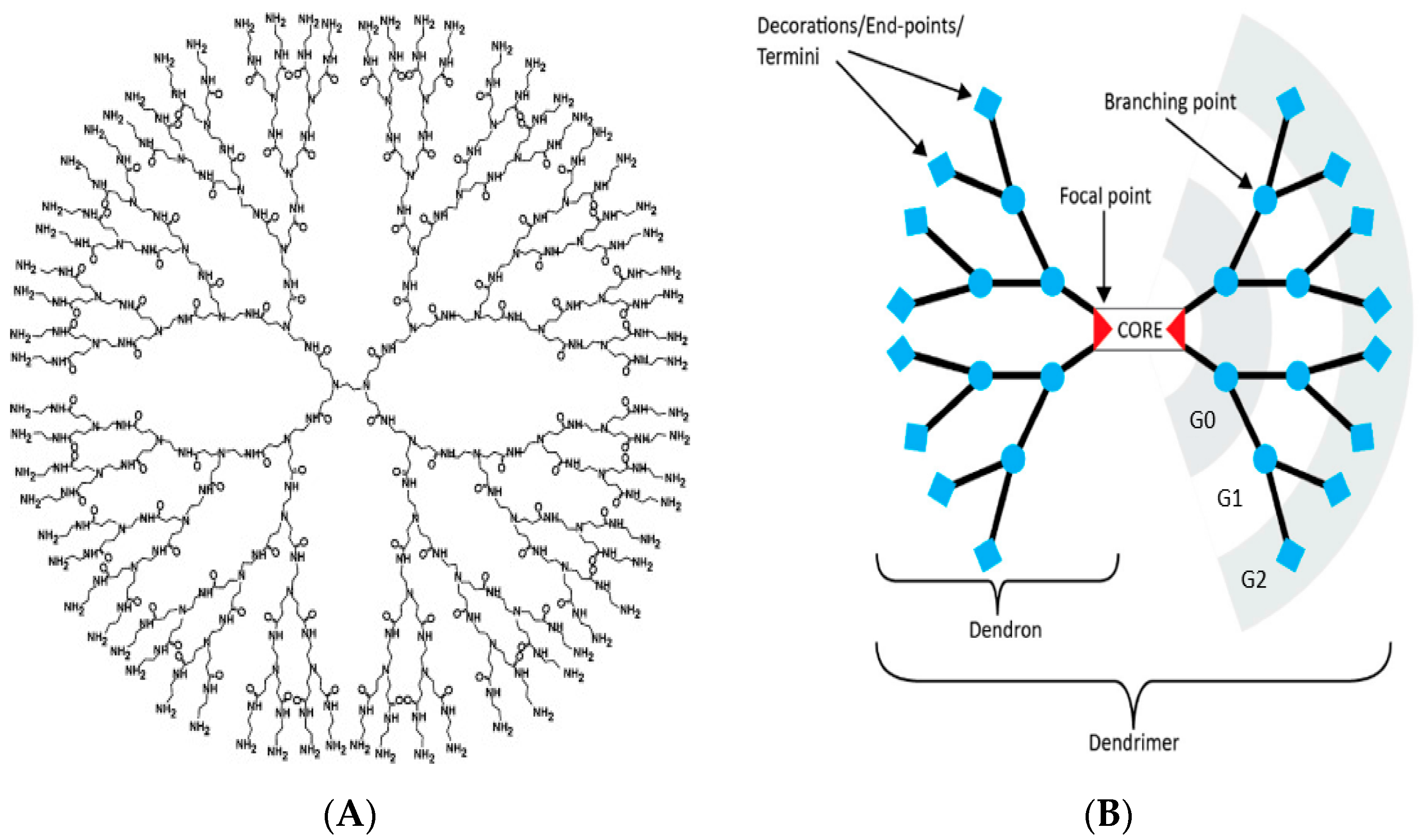
1. Introduction
- Safety of the dendrimer formulation
- Reproducibility in preparing the dendrimer formulation
2. Features of PAMAM Dendrimers Useful for Neuroscience Applications
- High stability
- High water-solubility
- Small size
- Precision
- Presence of cavities
- Surfaces that can be readily modified
3. PAMAM Dendrimers and the Delivery of Small Molecule Drugs and Genes across the Blood Brain Barrier
4. Delivery of Small Molecules
- Carbamazepine (CBZ), an anti-epileptic drug, was shown to enhance autophagy and protect against neurodegeneration in vivo. However, it is poorly soluble in water and shows unpredictable pharmacokinetic profiles. Generation 4.5 carboxyl-terminated dendrimers were loaded with CBZ to yield stable, soluble and safe (as tested on RBCs and zebrafish) formulations for potential applications in the treatment of neurodegenerative diseases associated with toxicity of aggregated proteins such as Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease and Parkinson’s disease [30]. No further studies were performed to see if the CBZ-dendrimer formulations were effective for the treatment of these diseases.
- He and co-workers encapsulated doxorubicin into G4 PAMAM dendrimers with surface PEG (PEGylated dendrimers) and the targeting ligands wheat germ agglutinin (WGA) and transferrin (Tf). The BBB-targeting ligands WGA and Tf increased the BBB permeability of the dendrimers. These nanoparticles were < 20 nm in size as measured by electron microscopy and dynamic light scattering. The PAMAM-PEG-WGA-Tf delivered more payload (doxorubicin) at brain tumor sites compared to free drug or dendrimers without Tf and WGA [52].
- Swami and colleagues loaded docetaxel (DTX) into G4 PAMAM dendrimers and covalently attached p-hydroxyl benzoic acid to the surface (pHBA). The pHBA has high affinity to sigma receptors that are predominant in the central nervous system. The G4-pHBA-DTX dendrimers were more effective in killing glioblastoma cells and delivered more DTX to the brain compared to free drug [53].
- Microtubule inhibitors (estramustine and podophyllotoxin), covalently attached to PAMAM dendrimers, were found to be more effective in killing glioma cells compared to free drug [56].
- Teow and colleagues conjugated paclitaxel and lauryl chains on the surface of a G3 PAMAM dendrimer. The conjugates showed increased cytotoxicity and permeability across porcine brain endothelial cells [57].
- Sharma and colleagues found that minocycline, conjugated to G6 hydroxyl-terminated PAMAM dendrimers via amide linkages, reduced neuroinflammation in vivo when compared to free minocycline and did so at lower dosages, thus reducing potential drug toxicity [58].
- Kannan and colleagues conjugated N-acetyl-l-cysteine to a G4 hydroxylated PAMAM dendrimer via disulfide linkages, which could be cleaved by intracellular glutathione (GSH). This formulation was shown to reduce motor dysfunction in rabbit models with cerebral palsy when administered postnatally [59].
- Yang and Lopina showed that attaching venlafaxine, a SNRI antidepressant, to PAMAM dendrimer-PEG hydrogels via ester linkages provided an extended release formulation that may help patients with poor drug compliance [60].
- Gamage and colleagues showed that curcumin was conjugated onto a G3-succinamic acid surface dendrimer via ester bonds. This formulation was administered to rats implanted with human glioma cells. The G3-curcumin showed tumor specific distribution, suggesting a potential use for the treatment of brain cancer [61].
5. Delivery of Genes
- One method of attenuating neurodegenerative disease progression is by slowing down the rate of neuronal death. This can be achieved by providing growth factors to the regions of neuronal degeneration, such as brain-derived neurotrophic factor (BDNF), glial-derived neurotropic factor (GDNF) and nerve growth factor (NGF) which are needed for the survival of particular neurons in the brain [72,73]. In HD, lack of BDNF results in the loss of medium spiny neurons of the striatum, a brain region involved in motor, cognitive, and emotional functions. As a result, HD patients present with deterioration in all three of these domains. Examples of this include chorea (an involuntary dance like movement), learning, and memory impairments as well as psychiatric problems. Since the lack of BDNF in the striatum results in the death of these cells, our laboratory has been involved in developing methods to increase BDNF production in the brains of HD rodent models. We have shown that increasing BDNF in HD mice (such as YAC128 and R6/2 models) resulted in the attenuation of motor and cognitive loss [73,74]. Shakhbazau and colleagues found that PAMAM G4 complexed with a plasmid for BDNF significantly increased the secretion of BDNF protein in human bone marrow mesenchymal stem cells (hMSCs), which can be implanted into diseased brains [75]. Although most researchers use charge ratios greater than one, it is interesting that these authors used a charge ratio of 1:1, giving a dendriplex size of about 150–200 nm.
- Similar complexes, formed between G4 and a plasmid for neurotrophin, were successfully used for transfecting human and rodent stem cells [75].
- Huang and colleagues have shown that transferrin conjugated to PAMAM dendrimer-DNA dendriplexes increases gene expression approximately two-fold in the brain compared to dendriplexes that were not conjugated to transferrin [76].
- Additionally, the same researchers have shown in BALB/c mice that conjugating lactoferrin to PAMAM dendrimers with PEG spacers increased brain uptake of the dendrimer 4.6-fold, compared to non-conjugated PAMAM dendrimers and by a 2.2-fold increase compared to dendrimers conjugated to transferrin [77].
- LRP receptors have also been shown to be abundantly expressed in mammalian neuronal cells [78]. Angiopep has been used to target LRP receptors with some specificity [79]. Researchers have shown that gene expression was significantly increased in the cortex, caudate putamen, hippocampus and substantia nigra of BALB/c mice when Angiopep-PEG-PAMAM loaded with DNA was administered, as compared to unconjugated PAMAM loaded with DNA [80].
- Another receptor found on the BBB, the mannose 6-phosphate/insulin-like growth factor II receptor (M6P/IGFR2R), binds to M6P and IGFR2 at distinct sites. After the binding of M6P-tagged proteins, as well as IGF-2 on those receptors, the molecules may be internalized and sent to lysosomes for degradation [81]. Urayama and colleagues found that this receptor is highly expressed in neonatal mice compared to adult mice. Their findings showed increased uptake of radiolabeled β-glucuronidase in neonatal mice compared to adults, and that this uptake was inhibited by M6P via competitive inhibition [82]. Potentially, conjugating a dendrimer to one of the ligands of the M6P/IGFR2R could be therapeutic for neonatal diseases of the CNS.
- Another targeting ligand for CNS-enhanced drug delivery is the 29 amino-acid rabies virus glycoprotein (RVG29), which allows viral entry into the CNS by binding to nicotinic acetylcholine receptors on neurons [83]. Liu and colleagues have successfully exploited this interaction when RVG29 was conjugated to a PAMAM dendrimer loaded with DNA. They found that this conjugation crossed the BBB more efficiency in vitro, and had a preferential brain accumulation in vivo [84].
- Serramía and colleagues delivered a small siRNA systemically using carbosilane dendrimers targeting astrocytes in BALB/c mice and detected the presence of the dendrimers and the dendriplexes in the brain one hour and 24 hours following injection [85].
6. Fate of PAMAM Dendrimers in Cells
7. Routes of Dendrimer Administration
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huebner, E.A.; Strittmatter, S.M. Axon regeneration in the peripheral and central nervous systems. Results Probl. Cell Differ. 2009, 48, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Young, A.B. Four decades of neurodegenerative disease research: How far we have come! J. Neurosci. 2009, 29, 12722–12728. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.C. Glioblastoma multiforme: The terminator. Proc. Natl. Acad. Sci. USA 2000, 97, 6242–6244. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Otto, D.P.; de Villiers, M.M. Poly(amidoamine) Dendrimers as a Pharmaceutical Excipient. Are We There yet? J. Pharm. Sci. 2018, 107, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Mol. Basel Switz. 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, Y.-A.; Kannan, S.; Kannan, R.M. Targeting specific cells in the brain with nanomedicines for CNS therapies. J. Control. Release 2016, 240, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Somani, S.; Dufès, C. Applications of dendrimers for brain delivery and cancer therapy. Nanomedicine 2014, 9, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Kaminskas, L.M.; Karellas, P.; Krippner, G.; Lessene, R.; Porter, C.J.H. Cationic poly-l-lysine dendrimers: Pharmacokinetics, biodistribution, and evidence for metabolism and bioresorption after intravenous administration to rats. Mol. Pharm. 2006, 3, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak-Baranska, J.; Pietkiewicz, A.; Janicka, M.; Wei, Y.; Turrin, C.-O.; Majoral, J.-P.; Nawrot, B.; Caminade, A.-M. Synthesis of a fluorescent cationic phosphorus dendrimer and preliminary biological studies of its interaction with DNA. Nucleosides Nucleotides Nucleic Acids 2010, 29, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, D.; Kubikova, R.; Müllerová, M.; Strašák, T.; RůŽička, K.; Fulem, M.; Maly, J. Phosphonium carbosilane dendrimers—Interaction with a simple biological membrane model. Phys. Chem. Chem. Phys. 2018, 20, 14753–14764. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Nwe, K.; Milenic, D.E.; Ray, G.L.; Kim, Y.-S.; Brechbiel, M.W. Preparation of cystamine core dendrimer and antibody-dendrimer conjugates for MRI angiography. Mol. Pharm. 2012, 9, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M.; Laurent, R.; Majoral, J.-P. Characterization of dendrimers. Adv. Drug Deliv. Rev. 2005, 57, 2130–2146. [Google Scholar] [CrossRef] [PubMed]
- Dendrimers and Other Dendritic Polymers. Available online: https://www.wiley.com/en-us/Dendrimers+and+Other+Dendritic+Polymers-p-9780471638506 (accessed on 18 July 2018).
- Dougherty, C.A.; Vaidyanathan, S.; Orr, B.G.; Banaszak Holl, M.M. Fluorophore:dendrimer ratio impacts cellular uptake and intracellular fluorescence lifetime. Bioconjug. Chem. 2015, 26, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Albertazzi, L.; Gherardini, L.; Brondi, M.; Sulis Sato, S.; Bifone, A.; Pizzorusso, T.; Ratto, G.M.; Bardi, G. In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol. Pharm. 2013, 10, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zheng, J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Medical Biochemistry: Molecules to Disease, Understanding & Applying Biochemistry in Healthcare; Educational Life Designs, 2016, 1st ed. Available online: https://www.pinterest.com/pin/183943966009628878/ (accessed on 18 June 2018).
- Lesniak, W.G.; Mishra, M.K.; Jyoti, A.; Balakrishnan, B.; Zhang, F.; Nance, E.; Romero, R.; Kannan, S.; Kannan, R.M. Biodistribution of fluorescently labeled PAMAM dendrimers in neonatal rabbits: Effect of neuroinflammation. Mol. Pharm. 2013, 10, 4560–4571. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.K.; Beaty, C.A.; Lesniak, W.G.; Kambhampati, S.P.; Zhang, F.; Wilson, M.A.; Blue, M.E.; Troncoso, J.C.; Kannan, S.; Johnston, M.V.; et al. Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. ACS Nano 2014, 8, 2134–2147. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; Monteagudo, S.; Ceña, V. Nanoparticles for brain-specific drug and genetic material delivery, imaging and diagnosis. Nanomedicine 2016, 11, 833–849. [Google Scholar] [CrossRef] [PubMed]
- Sarin, H.; Kanevsky, A.S.; Wu, H.; Brimacombe, K.R.; Fung, S.H.; Sousa, A.A.; Auh, S.; Wilson, C.M.; Sharma, K.; Aronova, M.A.; et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J. Transl. Med. 2008, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Nance, E.; Zhang, F.; Mishra, M.K.; Zhang, Z.; Kambhampati, S.P.; Kannan, R.M.; Kannan, S. Nanoscale effects in dendrimer-mediated targeting of neuroinflammation. Biomaterials 2016, 101, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Cho, H.; Kim, S.W.; Na, D.H. Chromatographic methods for characterization of poly(ethylene glycol)-modified polyamidoamine dendrimers. Anal. Biochem. 2014, 449, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Na, D.H. Difference in microchip electrophoretic mobility between partially and fully PEGylated poly(amidoamine) dendrimers. Anal. Biochem. 2015, 488, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Desai, A.; Ali, R.; Tomalia, D. Polyacrylamide gel electrophoresis separation and detection of polyamidoamine dendrimers possessing various cores and terminal groups. J. Chromatogr. A 2005, 1081, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Igartúa, D.E.; Martinez, C.S.; Temprana, C.F.; Alonso, S.D.V.; Prieto, M.J. PAMAM dendrimers as a carbamazepine delivery system for neurodegenerative diseases: A biophysical and nanotoxicological characterization. Int. J. Pharm. 2018, 544, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of Dendrimers on Solubility of Hydrophobic Drug Molecules. Front. Pharmacol. 2017, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Motoyama, K.; Higashi, T. Cyclodextrin/Dendrimer conjugates as DNA and oligonucleotide carriers. Curr. Top. Med. Chem. 2014, 14, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Guzman, L. Dendrimer nanocarriers drug action: Perspective for neuronal pharmacology. Neural Regen. Res. 2015, 10, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release 2016, 244, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.C.; Bhalgat, M.K.; Zera, R.T. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst dendrimers. J. Biomed. Mater. Res. 1996, 30, 53–65. [Google Scholar] [CrossRef]
- Srinageshwar, B.; Peruzzaro, S.; Andrews, M.; Johnson, K.; Hietpas, A.; Clark, B.; McGuire, C.; Petersen, E.; Kippe, J.; Stewart, A.; et al. PAMAM Dendrimers Cross the Blood–Brain Barrier When Administered through the Carotid Artery in C57BL/6J Mice. Int. J. Mol. Sci. 2017, 18, 628. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Banerjee, S.; Gupta, U.; Mohd Amin, M.C.I.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. PAMAM dendrimers as promising nanocarriers for RNAi therapeutics. Mater. Today 2015, 18, 565–572. [Google Scholar] [CrossRef]
- Arseneault, M.; Wafer, C.; Morin, J.-F. Recent advances in click chemistry applied to dendrimer synthesis. Mol. Basel Switz. 2015, 20, 9263–9294. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.; Wu, Y. Dendrimer Advances for the Central Nervous System Delivery of Therapeutics. ACS Chem. Neurosci. 2013, 5, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Coomber, B.L.; Stewart, P.A. Morphometric analysis of CNS microvascular endothelium. Microvasc. Res. 1985, 30, 99–115. [Google Scholar] [CrossRef]
- Simpson, I.A.; Carruthers, A.; Vannucci, S.J. Supply and demand in cerebral energy metabolism: The role of nutrient transporters. J. Cereb. Blood Flow Metab. 2007, 27, 1766–1791. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.M.; Shusta, E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-brain barrier drug targeting: The future of brain drug development. Mol. Interv. 2003, 3, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Hervé, F.; Ghinea, N.; Scherrmann, J.-M. CNS delivery via adsorptive transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, J.; Pardridge, W.M. Drug targeting of a peptide radiopharmaceutical through the primate blood-brain barrier in vivo with a monoclonal antibody to the human insulin receptor. J. Clin. Investig. 1997, 100, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, R.R.; Bondy, C.A. Insulin-like growth factors cross the blood-brain barrier. Endocrinology 1994, 135, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Ramge, P.; Petrov, V.; Hamm, S.; Gelperina, S.E.; Engelhardt, B.; Alyautdin, R.; von Briesen, H.; Begley, D.J. Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm. Res. 2003, 20, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xue, Y.; Shang, X.; Liu, Y. Diphtheria toxin mutant CRM197-mediated transcytosis across blood-brain barrier in vitro. Cell. Mol. Neurobiol. 2010, 30, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug and gene delivery to the brain: The vascular route. Neuron 2002, 36, 555–558. [Google Scholar] [CrossRef]
- Kim, Y.; Park, E.J.; Na, D.H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 2018, 41, 571–582. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, Y.; Jia, X.-R.; Du, J.; Ying, X.; Lu, W.-L.; Lou, J.-N.; Wei, Y. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials 2011, 32, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Swami, R.; Singh, I.; Kulhari, H.; Jeengar, M.K.; Khan, W.; Sistla, R. Correction to: p-Hydroxy benzoic acid-conjugated dendrimer nanotherapeutics as potential carriers for targeted drug delivery to brain: An in vitro and in vivo evaluation. J. Nanopart. Res. 2017, 19, 358. [Google Scholar] [CrossRef]
- Patri, A.K.; Kukowska-Latallo, J.F.; Baker, J.R. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 2005, 57, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.F.; de Brabander-van den Berg, E.M.; Meijer, E.W. Encapsulation of guest molecules into a dendritic box. Science 1994, 266, 1226–1229. [Google Scholar] [CrossRef] [PubMed]
- Sk, U.H.; Dixit, D.; Sen, E. Comparative study of microtubule inhibitors—Estramustine and natural podophyllotoxin conjugated PAMAM dendrimer on glioma cell proliferation. Eur. J. Med. Chem. 2013, 68, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Teow, H.M.; Zhou, Z.; Najlah, M.; Yusof, S.R.; Abbott, N.J.; D’Emanuele, A. Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier. Int. J. Pharm. 2013, 441, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kim, S.-Y.; Sharma, A.; Zhang, Z.; Kambhampati, S.P.; Kannan, S.; Kannan, R.M. Activated Microglia Targeting Dendrimer-Minocycline Conjugate as Therapeutics for Neuroinflammation. Bioconjug. Chem. 2017, 28, 2874–2886. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Dai, H.; Navath, R.S.; Balakrishnan, B.; Jyoti, A.; Janisse, J.; Romero, R.; Kannan, R.M. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci. Transl. Med. 2012, 4, 130ra46. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lopina, S.T. Extended release of a novel antidepressant, venlafaxine, based on anionic polyamidoamine dendrimers and poly(ethylene glycol)-containing semi-interpenetrating networks. J. Biomed. Mater. Res. A 2005, 72, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Gamage, N.; Jing, L.; Worsham, M.; Ali, M. Targeted Theranostic Approach for Glioma Using Dendrimer-Based Curcumin Nanoparticle. J. Nanomed. Nanotechnol. 2016, 7, 393. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, H.; Jia, X.; Lu, W.-L.; Lou, J.; Wei, Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials 2012, 33, 3899–3908. [Google Scholar] [CrossRef] [PubMed]
- Milowska, K.; Malachowska, M.; Gabryelak, T. PAMAM G4 dendrimers affect the aggregation of α-synuclein. Int. J. Biol. Macromol. 2011, 48, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Rekas, A.; Lo, V.; Gadd, G.E.; Cappai, R.; Yun, S.I. PAMAM dendrimers as potential agents against fibrillation of alpha-synuclein, a Parkinson’s disease-related protein. Macromol. Biosci. 2009, 9, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Klajnert, B.; Cladera, J.; Bryszewska, M. Molecular interactions of dendrimers with amyloid peptides: pH dependence. Biomacromolecules 2006, 7, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Cordes, H.; Boas, U.; Olsen, P.; Heegaard, P.M.H. Guanidino- and urea-modified dendrimers as potent solubilizers of misfolded prion protein aggregates under non-cytotoxic conditions. dependence on dendrimer generation and surface charge. Biomacromolecules 2007, 8, 3578–3583. [Google Scholar] [CrossRef] [PubMed]
- Katare, Y.K.; Daya, R.P.; Sookram Gray, C.; Luckham, R.E.; Bhandari, J.; Chauhan, A.S.; Mishra, R.K. Brain Targeting of a Water Insoluble Antipsychotic Drug Haloperidol via the Intranasal Route Using PAMAM Dendrimer. Mol. Pharm. 2015, 12, 3380–3388. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.J.; Temprana, C.F.; del Río Zabala, N.E.; Marotta, C.H.; del Valle Alonso, S. Optimization and in vitro toxicity evaluation of G4 PAMAM dendrimer-risperidone complexes. Eur. J. Med. Chem. 2011, 46, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Braun, C.S.; Vetro, J.A.; Tomalia, D.A.; Koe, G.S.; Koe, J.G.; Middaugh, C.R. Structure/function relationships of polyamidoamine/DNA dendrimers as gene delivery vehicles. J. Pharm. Sci. 2005, 94, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Dufès, C.; Uchegbu, I.F.; Schätzlein, A.G. Dendrimers in gene delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-C.; Zhou, J.; Liu, X.; Wu, J.; Qu, F.; Zhang, Z.-L.; Pang, D.-W.; Quéléver, G.; Zhang, C.-C.; Peng, L. Importance of size-to-charge ratio in construction of stable and uniform nanoscale RNA/dendrimer complexes. Org. Biomol. Chem. 2007, 5, 3674–3681. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liang, Y.; Lu, H.; Yang, Z.; Liu, R.; Wang, J.; Song, X.; Long, J.; Li, Y.; Lei, D.; et al. Co-transplantation of GDNF-overexpressing neural stem cells and fetal dopaminergic neurons mitigates motor symptoms in a rat model of Parkinson’s disease. PLoS ONE 2013, 8, e80880. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.D.; Bombard, M.C.; Roland, B.P.; Davidson, S.; Lu, M.; Rossignol, J.; Sandstrom, M.I.; Skeel, R.L.; Lescaudron, L.; Dunbar, G.L. Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington’s disease. Behav. Brain Res. 2010, 214, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.; Fink, K.D.; Crane, A.T.; Davis, K.K.; Bombard, M.C.; Clerc, S.; Bavar, A.M.; Lowrance, S.A.; Song, C.; Witte, S.; et al. Reductions in behavioral deficits and neuropathology in the R6/2 mouse model of Huntington’s disease following transplantation of bone-marrow-derived mesenchymal stem cells is dependent on passage number. Stem Cell Res. Ther. 2015, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Shakhbazau, A.; Shcharbin, D.; Seviaryn, I.; Goncharova, N.; Kosmacheva, S.; Potapnev, M.; Gabara, B.; Ionov, M.; Bryszewska, M. Use of polyamidoamine dendrimers to engineer BDNF-producing human mesenchymal stem cells. Mol. Biol. Rep. 2010, 37, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Q.; Qu, Y.-H.; Ke, W.-L.; Zhu, J.-H.; Pei, Y.-Y.; Jiang, C. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. FASEB J. 2007, 21, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ke, W.; Liu, Y.; Jiang, C.; Pei, Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials 2008, 29, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Bu, G.; Maksymovitch, E.A.; Nerbonne, J.M.; Schwartz, A.L. Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neurons. J. Biol. Chem. 1994, 269, 18521–18528. [Google Scholar] [PubMed]
- Demeule, M.; Currie, J.-C.; Bertrand, Y.; Ché, C.; Nguyen, T.; Régina, A.; Gabathuler, R.; Castaigne, J.-P.; Béliveau, R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J. Neurochem. 2008, 106, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Shao, K.; Huang, R.; Han, L.; Liu, Y.; Li, J.; Kuang, Y.; Ye, L.; Lou, J.; Jiang, C. Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials 2009, 30, 6976–6985. [Google Scholar] [CrossRef] [PubMed]
- Auletta, M.; Nielsen, F.C.; Gammeltoft, S. Receptor-mediated endocytosis and degradation of insulin-like growth factor I and II in neonatal rat astrocytes. J. Neurosci. Res. 1992, 31, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Urayama, A.; Grubb, J.H.; Sly, W.S.; Banks, W.A. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc. Natl. Acad. Sci. USA 2004, 101, 12658–12663. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Wu, H.; McBride, J.L.; Jung, K.-E.; Kim, M.H.; Davidson, B.L.; Lee, S.K.; Shankar, P.; Manjunath, N. Transvascular delivery of small interfering RNA to the central nervous system. Nature 2007, 448, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bryantsev, V.S.; Diallo, M.S.; Goddard, W.A. PAMAM dendrimers undergo pH responsive conformational changes without swelling. J. Am. Chem. Soc. 2009, 131, 2798–2799. [Google Scholar] [CrossRef] [PubMed]
- Serramía, M.J.; Álvarez, S.; Fuentes-Paniagua, E.; Clemente, M.I.; Sánchez-Nieves, J.; Gómez, R.; de la Mata, J.; Muñoz-Fernández, M.Á. In vivo delivery of siRNA to the brain by carbosilane dendrimer. J. Control. Release 2015, 200, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Miranda, V.; Peñaloza, J.P.; Araya-Durán, I.; Reyes, R.; Vidaurre, S.; Romero, V.; Fuentes, J.; Céric, F.; Velásquez, L.; González-Nilo, F.D.; Otero, C. Effect of Terminal Groups of Dendrimers in the Complexation with Antisense Oligonucleotides and Cell Uptake. Nanoscale Res. Lett. 2016, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Vásquez, P.; Díaz, C.; Nova, D.; Alderete, J.; Guzmán, L. Mechanism of PAMAM Dendrimers Internalization in Hippocampal Neurons. Mol. Pharm. 2016, 13, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Sone, H.; Win-Shwe, T.-T.; Zeng, Y.; Kimura, H.; Koyama, Y.; Yagi, Y.; Matsui, Y.; Yamazaki, M.; Hirano, S. Aggregation is a critical cause of poor transfer into the brain tissue of intravenously administered cationic PAMAM dendrimer nanoparticles. Int. J. Nanomed. 2017, 12, 3967–3975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Trent Magruder, J.; Lin, Y.-A.; Crawford, T.C.; Grimm, J.C.; Sciortino, C.M.; Wilson, M.A.; Blue, M.E.; Kannan, S.; Johnston, M.V.; et al. Generation-6 hydroxyl PAMAM dendrimers improve CNS penetration from intravenous administration in a large animal brain injury model. J. Control. Release 2017, 249, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Zhao, Y.; Huang, R.; Jiang, C.; Pei, Y. Enhanced oral bioavailability of doxorubicin in a dendrimer drug delivery system. J. Pharm. Sci. 2008, 97, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Sadekar, S.; Ghandehari, H. Transepithelial transport and toxicity of PAMAM dendrimers: Implications for oral drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Wiwattanapatapee, R.; Carreño-Gómez, B.; Malik, N.; Duncan, R. Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: A potential oral delivery system? Pharm. Res. 2000, 17, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, D.; Enda, M.; Bond, T.; Moghaddam, S.P.H.; Conarton, J.; Scaife, C.; Volckmann, E.; Ghandehari, H. Transepithelial Transport of PAMAM Dendrimers Across Isolated Human Intestinal Tissue. Mol. Pharm. 2015, 12, 4099–4107. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-T.; Zhao, Y.-Z.; Wong, H.L.; Cai, J.; Peng, L.; Tian, X.-Q. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomed. 2014, 9, 2241–2257. [Google Scholar] [CrossRef] [PubMed]
- Win-Shwe, T.-T.; Sone, H.; Kurokawa, Y.; Zeng, Y.; Zeng, Q.; Nitta, H.; Hirano, S. Effects of PAMAM dendrimers in the mouse brain after a single intranasal instillation. Toxicol. Lett. 2014, 228, 207–215. [Google Scholar] [CrossRef] [PubMed]
| Drug(s) | Loading Method | Application | Results |
|---|---|---|---|
| Carbamazepine | Encapsulation within a G4.5 carboxyl-terminated dendrimer | Neurodegenerative diseases | Decreased neurodegeneration in vivo, decreased protein aggregation, enhanced autophagy, and increased drug solubility [30] |
| Curcumin | Covalent linkage to a G3-succinamic acid surface dendrimer via ester bonds | Glioma | Increased delivery in a tumor-specific distribution [61] |
| Docetaxel | Encapsulation within G4 PAMAM dendrimers with covalently attached pHBA | Glioblastoma | Increased glioblastoma-cell death, and increased drug delivery to the brain [53] |
| Doxorubicin | Encapsulation within PEGylated G4 PAMAM dendrimers with WGA and Tf targeting ligands | Brain tumors | Increased doxorubicin payload at tumor sites [52] |
| Estramustine and podophyllotoxin | Covalent linkage to PAMAM dendrimers | Glioma | More effective killing of glioma cells [56] |
| Haloperidol | Encapsulation within a G5 PAMAM dendrimer with 1,4-diaminobutane core | Psychiatric | Increased brain and plasma concentrations of haloperidol compared to control formulation in a rat model [67] |
| Minocycline | Covalent linkage to G6 hydroxyl-terminated PAMAM dendrimers via amide linkages | Stroke | Reduced neuroinflammation in vivo at lower doses [58] |
| N-acetyl-l-cysteine | Covalent linkage to a G4 hydroxylated PAMAM dendrimer via disulfide linkages | Cerebral palsy | Reduced motor dysfunction in rabbit models [59] |
| Paclitaxel | Covalent linkage to G3 PAMAM dendrimers with added lauryl chains | Brain tumors | Increased cytotoxicity and permeability across porcine brain endothelial cells [57] |
| Risperidone | Encapsulation within a G4 PAMAM dendrimer | Psychiatric | Increased aqueous solubility of risperidone without significant hemolysis or morphological changes to human red blood cells [68] |
| Tamoxifen and doxorubicin | Combination encapsulation (tamoxifen) and covalent linkage (doxorubicin) to G4 PAMAM dendrimers with added PEG and Tf | Glioma | Increased accumulation within glioma cells [62] |
| Venlafaxine | Covalent linkage to PAMAM dendrimers-PEG hydrogels via ester linkages | Psychiatric | Extended release [60] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florendo, M.; Figacz, A.; Srinageshwar, B.; Sharma, A.; Swanson, D.; Dunbar, G.L.; Rossignol, J. Use of Polyamidoamine Dendrimers in Brain Diseases. Molecules 2018, 23, 2238. https://doi.org/10.3390/molecules23092238
Florendo M, Figacz A, Srinageshwar B, Sharma A, Swanson D, Dunbar GL, Rossignol J. Use of Polyamidoamine Dendrimers in Brain Diseases. Molecules. 2018; 23(9):2238. https://doi.org/10.3390/molecules23092238
Chicago/Turabian StyleFlorendo, Maria, Alexander Figacz, Bhairavi Srinageshwar, Ajit Sharma, Douglas Swanson, Gary L. Dunbar, and Julien Rossignol. 2018. "Use of Polyamidoamine Dendrimers in Brain Diseases" Molecules 23, no. 9: 2238. https://doi.org/10.3390/molecules23092238
APA StyleFlorendo, M., Figacz, A., Srinageshwar, B., Sharma, A., Swanson, D., Dunbar, G. L., & Rossignol, J. (2018). Use of Polyamidoamine Dendrimers in Brain Diseases. Molecules, 23(9), 2238. https://doi.org/10.3390/molecules23092238








