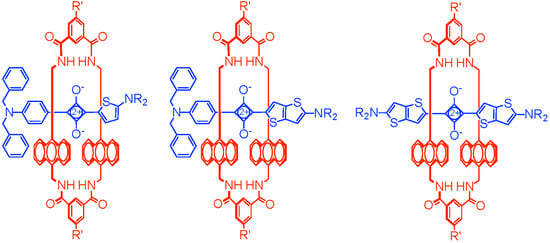
Fluorescent Thienothiophene-Containing Squaraine Dyes and Threaded Supramolecular Complexes with Tunable Wavelengths between 600–800 nm
Abstract
1. Introduction
2. Results and Discussion
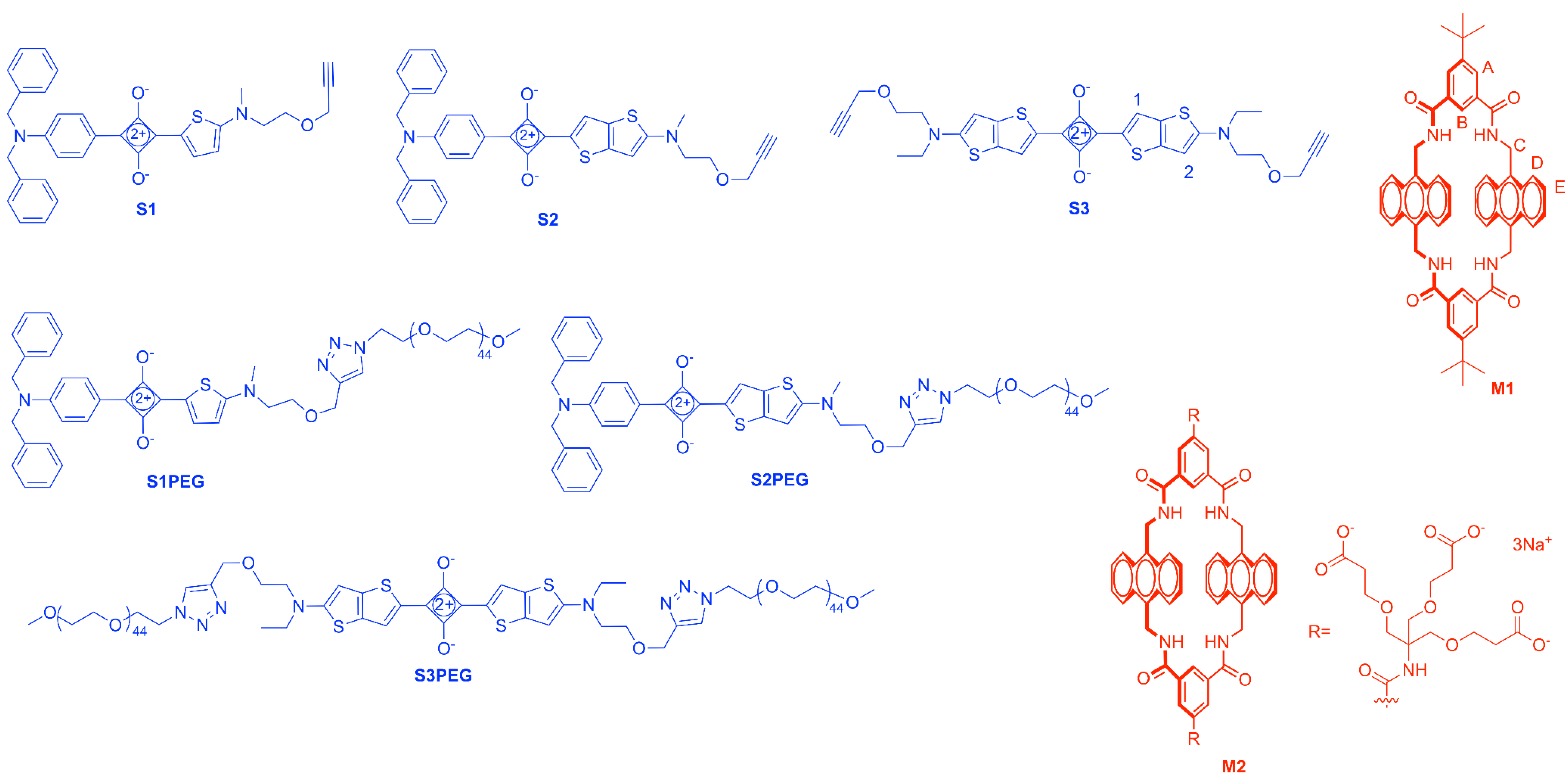
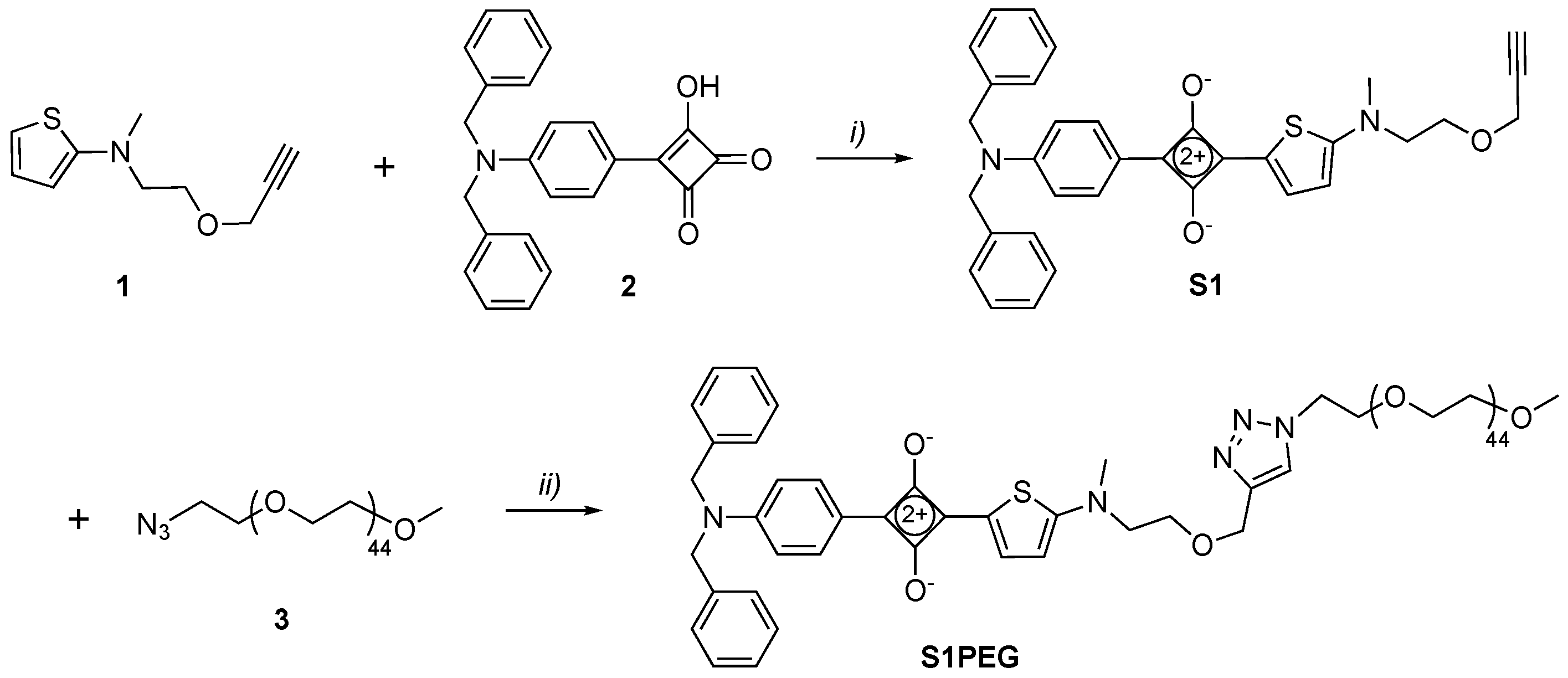
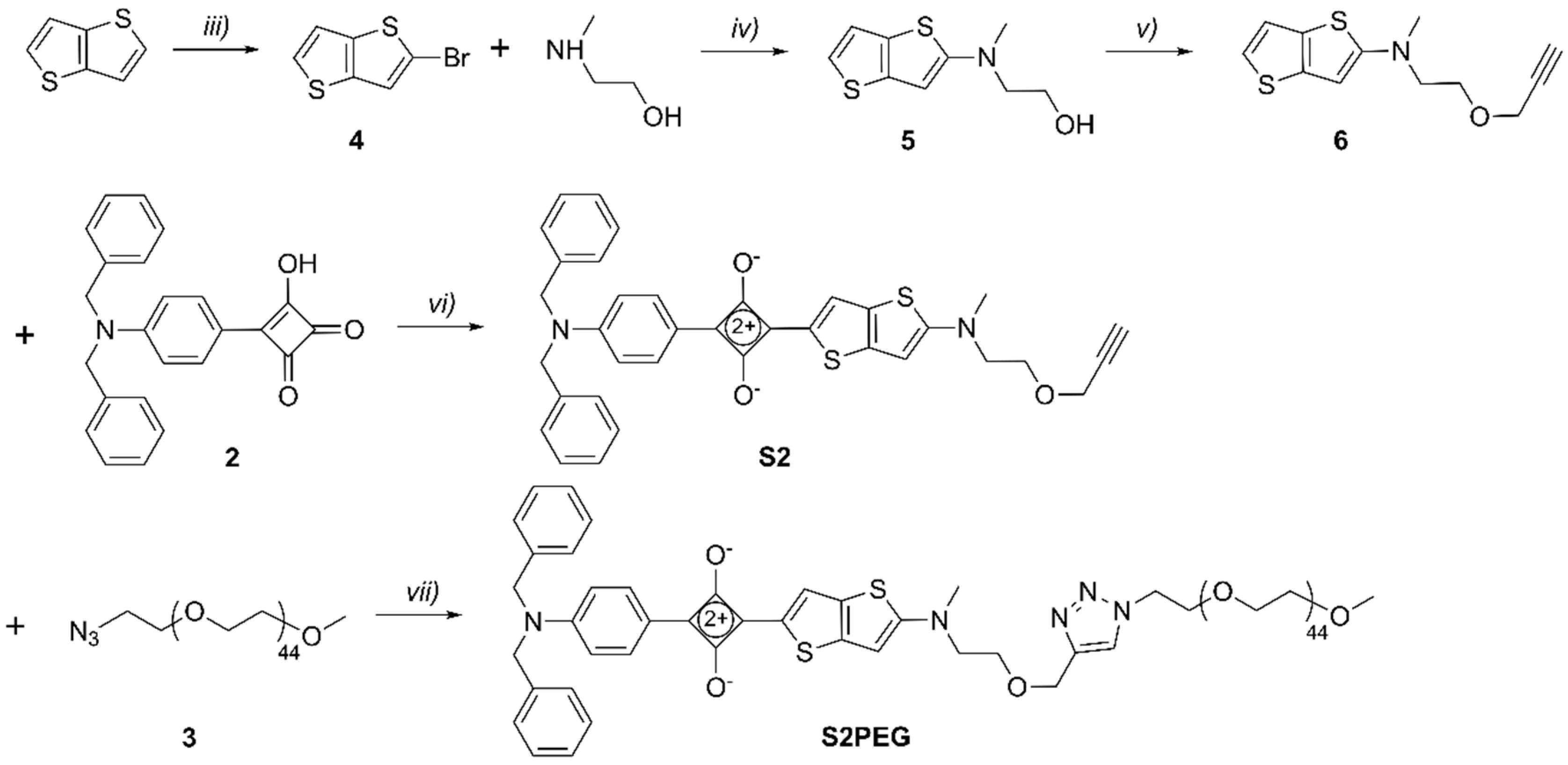
2.1. Synthesis
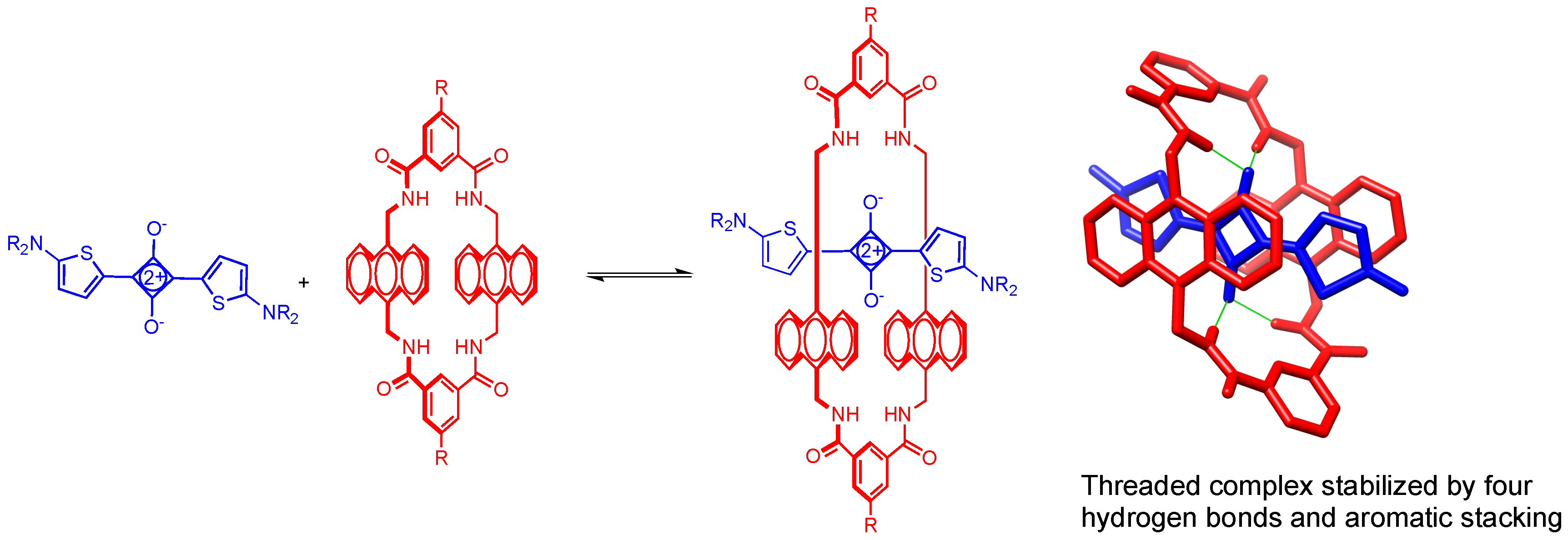
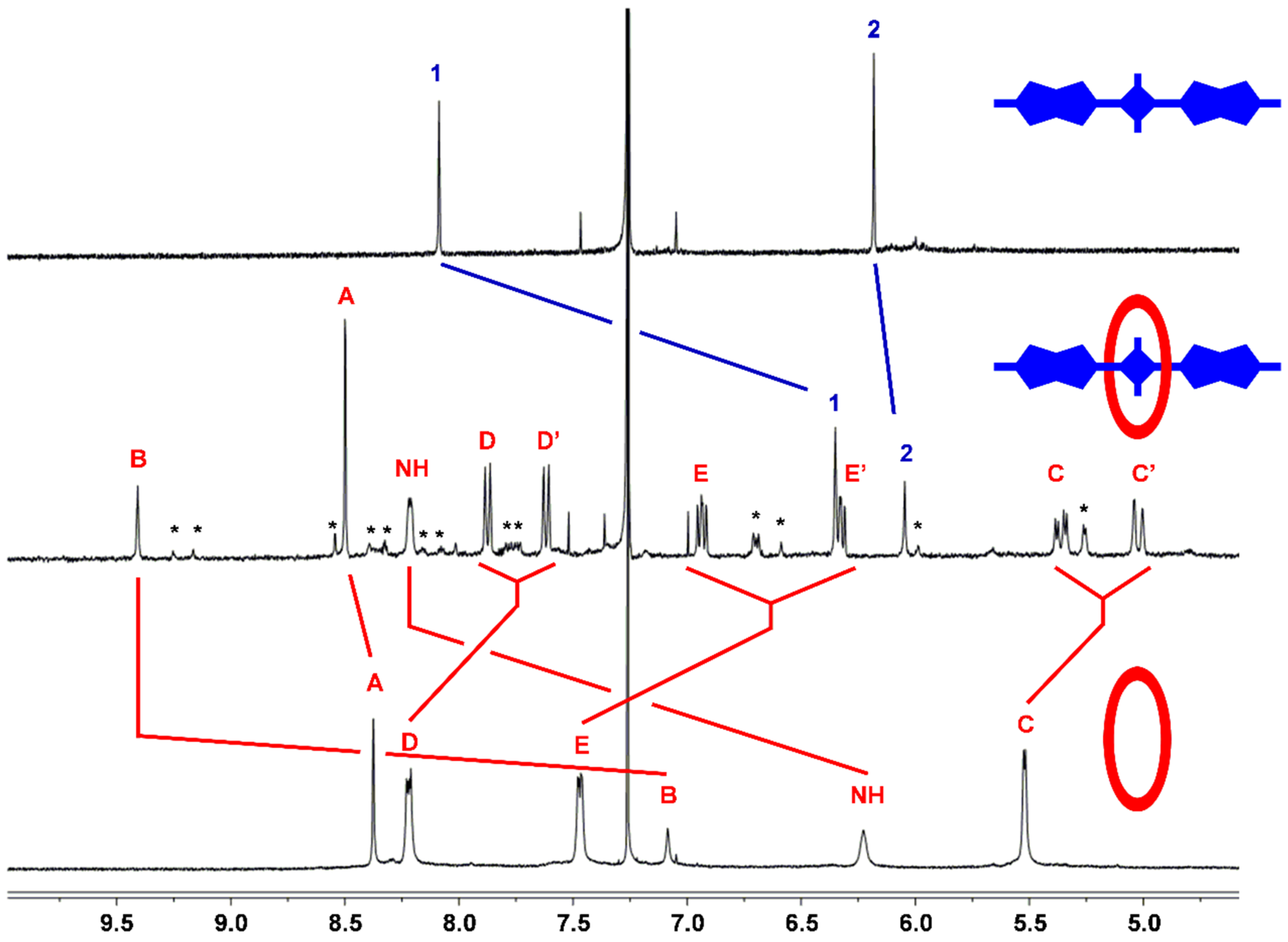
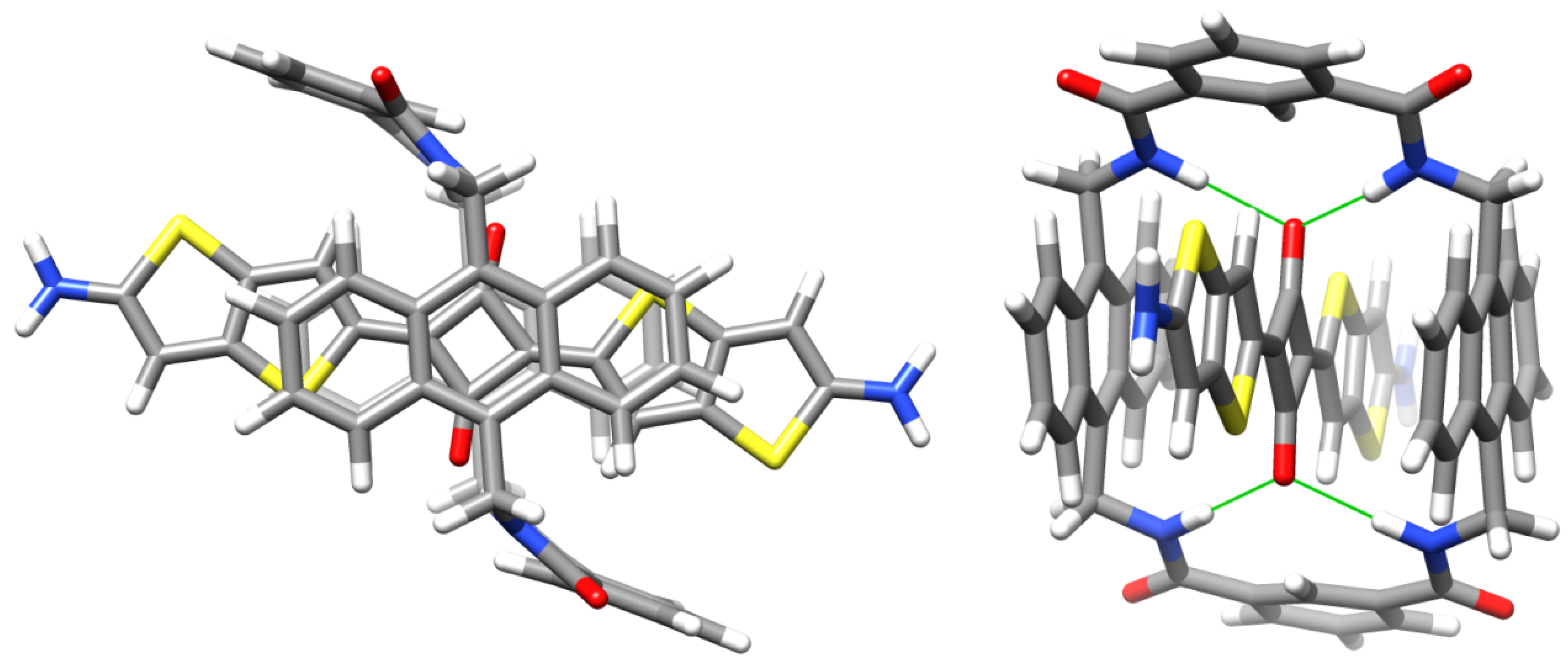
2.2. Macrocycle Threading in Chloroform
2.3. Macrocycle Threading in Water
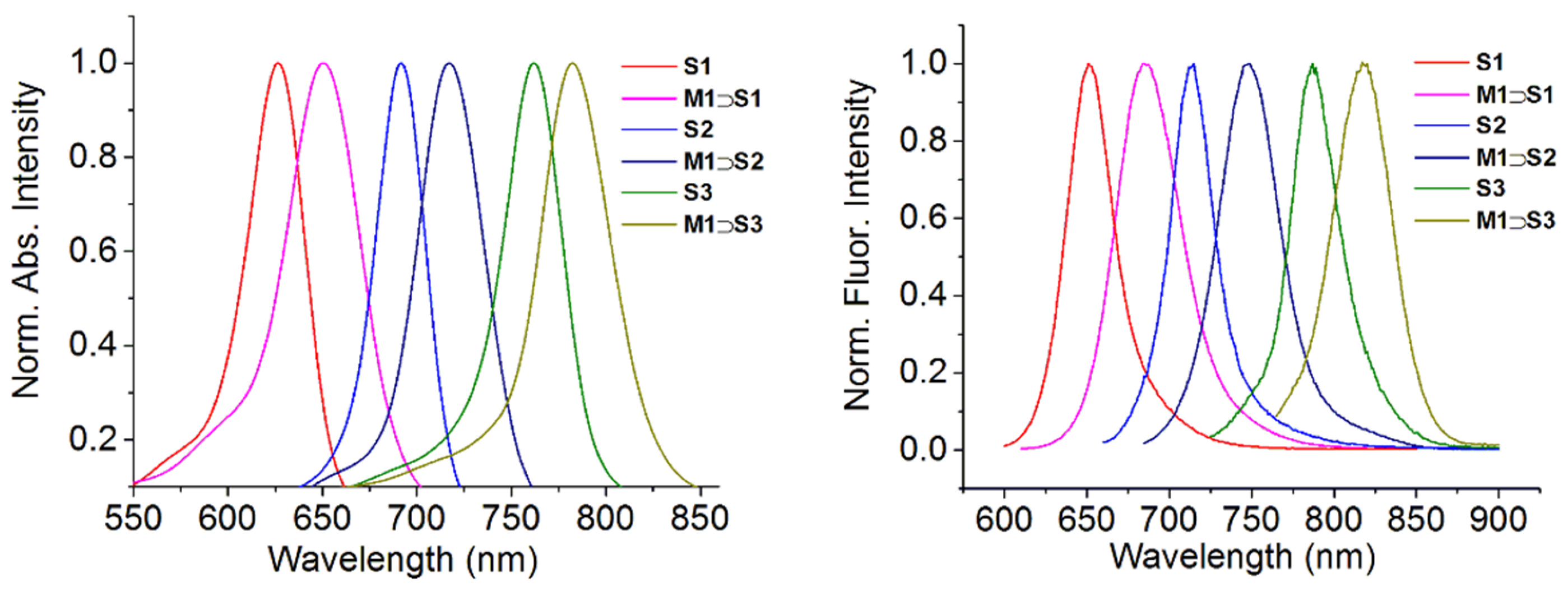
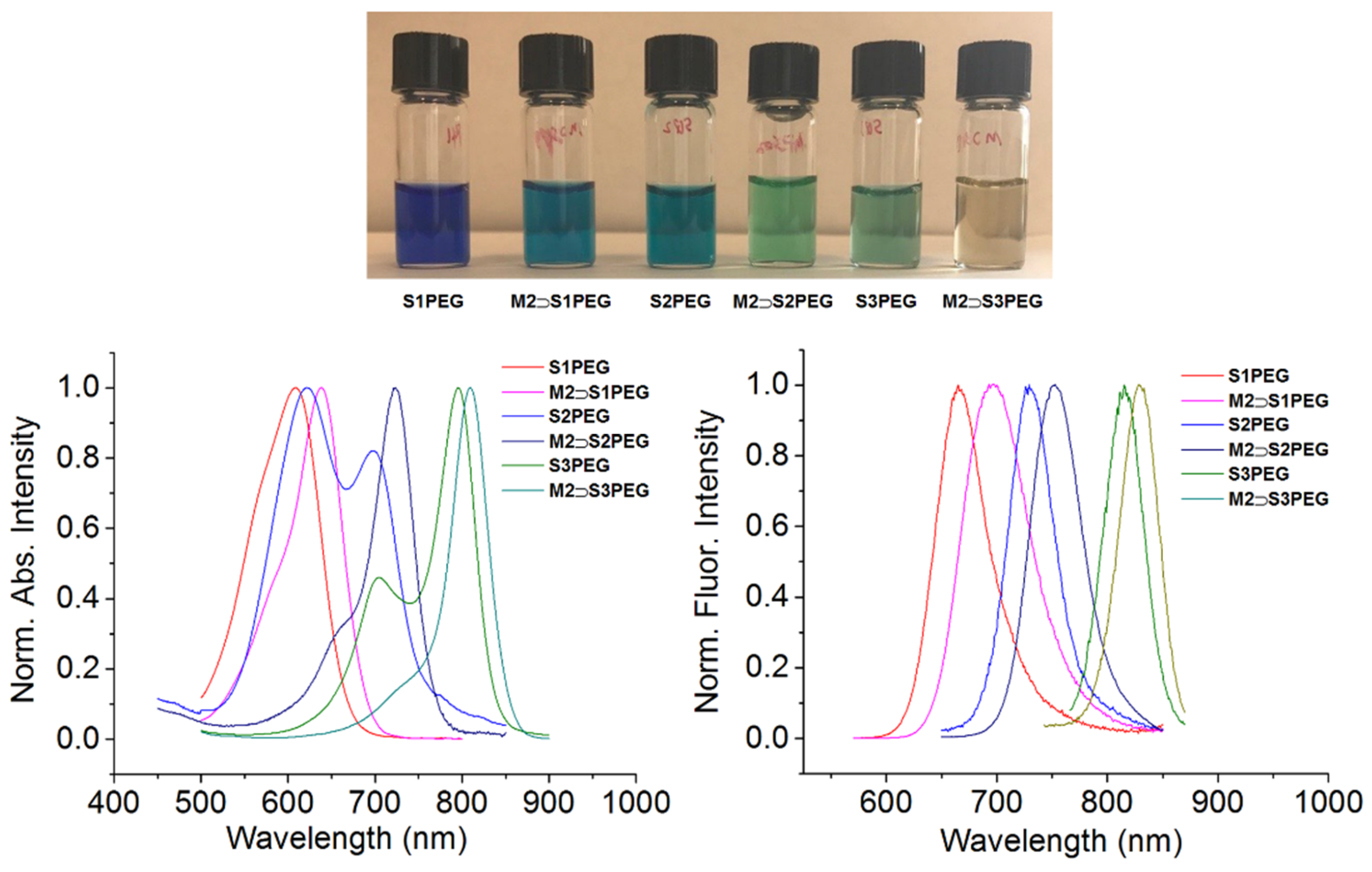
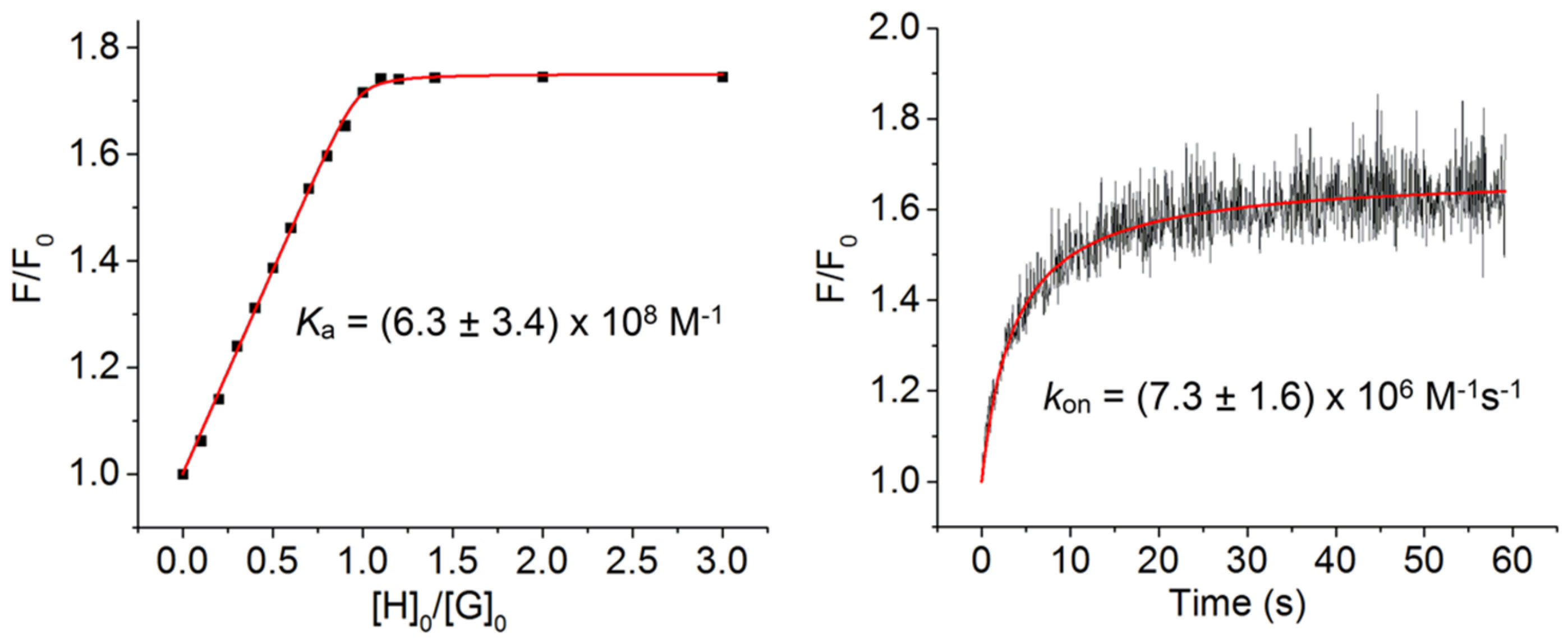
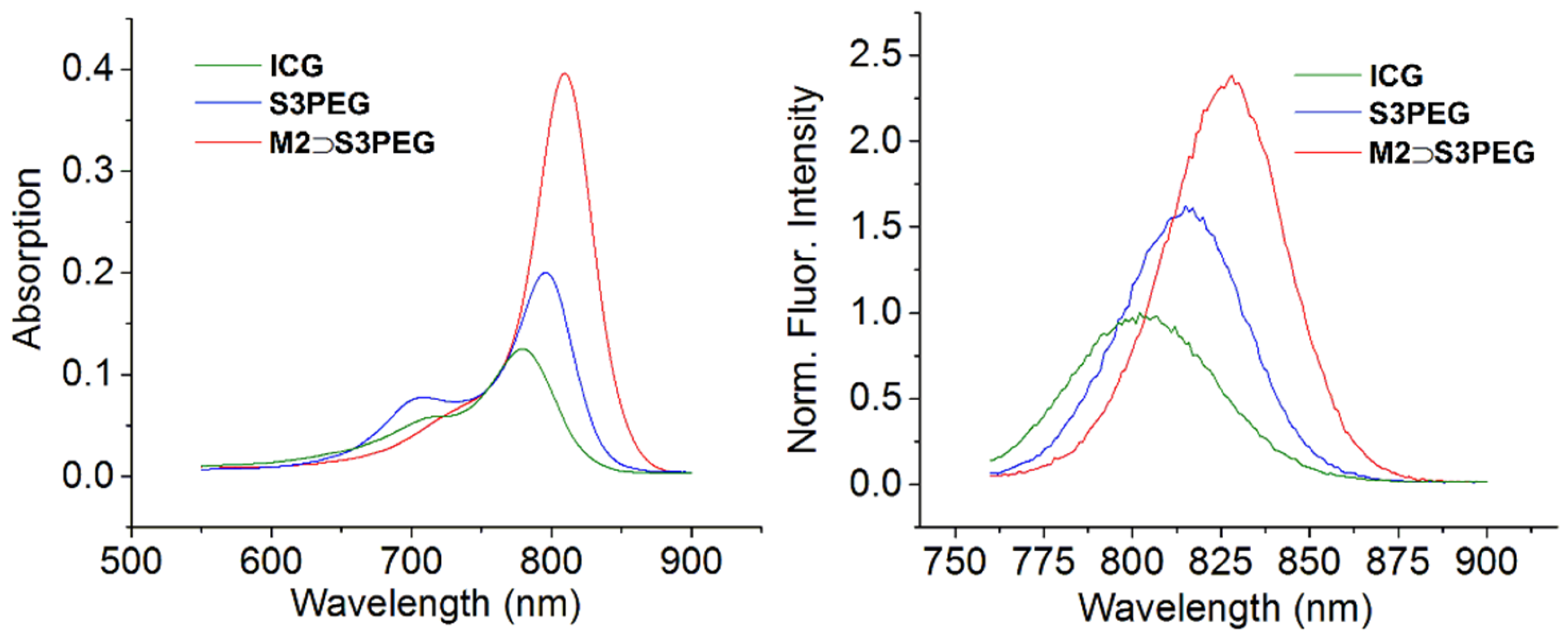
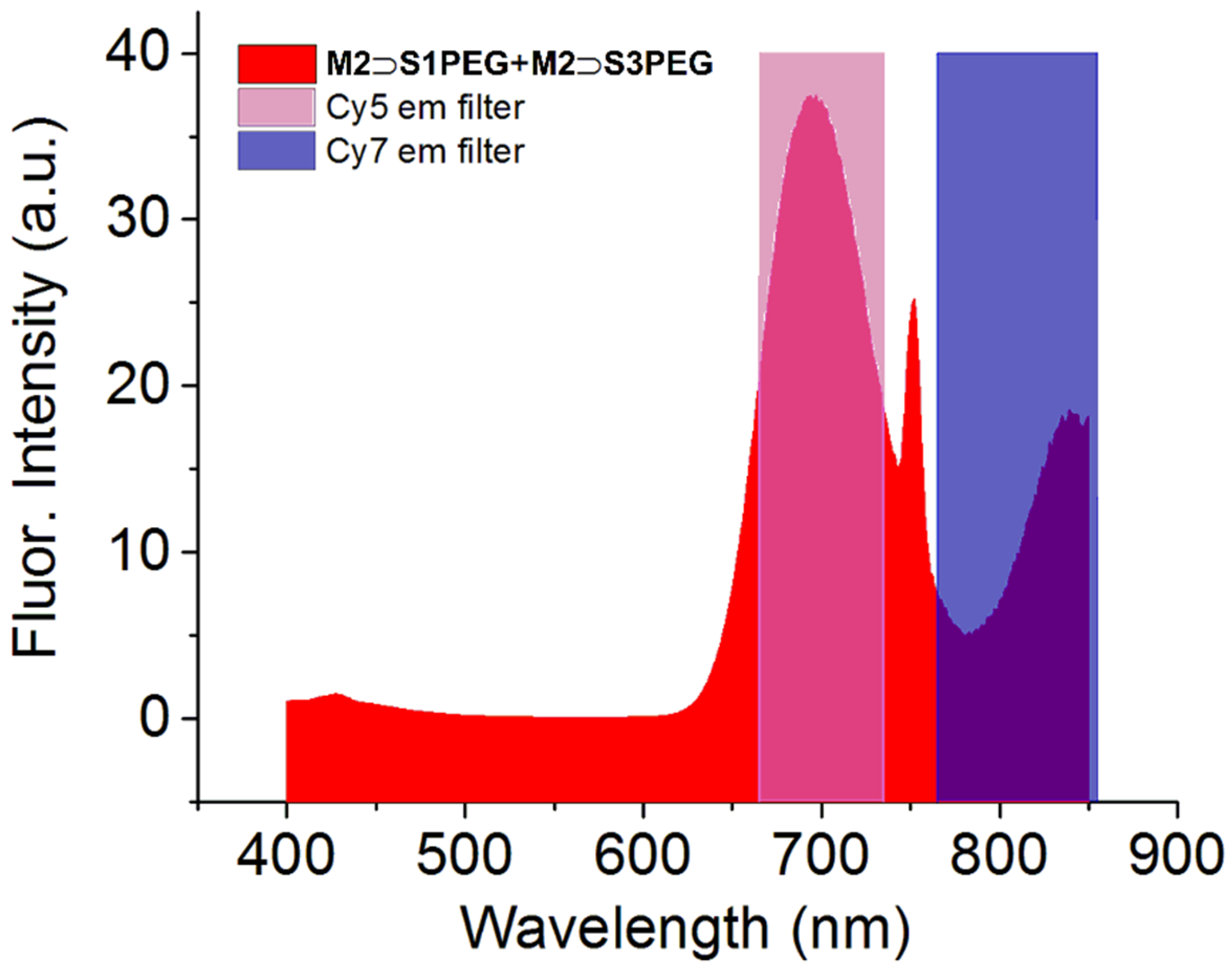
2.4. Photophysical Properties
3. Materials and Methods
3.1. General
3.2. Synthesis
3.3. Association and Kinetic Measurements
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guo, Z.Q.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.; Kim, H.J. Near-infrared fluorescent probes for peptidases. Coord. Chem. Rev. 2018, 354, 169–181. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent Development of chemosensors based on cyanine platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef] [PubMed]
- Bricks, J.L.; Kachkovskii, A.D.; Slominskii, Y.L.; Gerasov, A.O.; Popov, S.V. Molecular design of near infrared polymethine dyes: A review. Dye Pigment. 2015, 121, 238–255. [Google Scholar] [CrossRef]
- Beverina, L.; Salice, P. Squaraine compounds: Tailored design and synthesis towards a variety of material science applications. Eur. J. Org. Chem. 2010, 2010, 1207–1225. [Google Scholar] [CrossRef]
- Karpenko, I.A.; Collot, M.; Richert, L.; Valencia, C.; Villa, P.; Mély, Y.; Hibert, M.; Bonnet, D.; Klymchenko, A.S. Fluorogenic squaraine dimers with polarity-sensitive folding as bright far-red probes for background-free bioimaging. J. Am. Chem. Soc. 2015, 137, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Maltese, V.; Cospito, S.; Beneduci, A.; De Simone, B.C.; Russo, N.; Chidichimo, G.; Janssen, R.A.J. Electro-optical properties of neutral and radical ion thienosquaraines. Chem. A Eur. J. 2016, 22, 10179–10186. [Google Scholar] [CrossRef] [PubMed]
- Stokes, R.J.; Ingram, A.; Gallagher, J.; Armstrong, D.R.; Smith, W.E.; Graham, D. Squaraines as unique reporters for SERRS multiplexing. Chem. Commun. 2008, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Shafeekh, K.M.; Soumya, M.S.; Rahim, M.A.; Abraham, A.; Das, S. Synthesis and characterization of near-Infrared absorbing water soluble squaraines and study of their photodynamic effects in DLA live cells. Photochem. Photobiol. 2014, 90, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Yum, J.; Walter, P.; Huber, S.; Rentsch, D.; Geiger, T.; Nüesch, F.; De Angelis, F.; Grätzel, M.; Nazeeruddin, M.K. Efficient near-IR sensitization of nanocrystalline TiO2 films by an asymmetrical squaraine dye. J. Am. Chem. Soc. 2007, 129, 10320–10321. [Google Scholar] [CrossRef] [PubMed]
- Gassensmith, J.J.; Baumes, J.M.; Smith, B.D. Discovery and early development of squaraine rotaxanes. Chem. Commun. 2009, 6329–6338. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, E.; Forbes, C.C.; Noll, B.C.; Smith, B.D. Squaraine-derived rotaxanes: Sterically protected fluorescent near-IR dyes. J. Am. Chem. Soc. 2005, 127, 3288–3289. [Google Scholar] [CrossRef] [PubMed]
- Peck, E.M.; Liu, W.; Spence, G.T.; Shaw, S.K.; Davis, A.P.; Destecroix, H.; Smith, B.D. Rapid macrocycle threading by a fluorescent dye-polymer conjugate in water with nanomolar affinity. J. Am. Chem. Soc. 2015, 137, 8668–8671. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, E.; Fu, N.; Smith, B.D. Squaraine-derived rotaxanes: Highly stable, fluorescent near-IR dyes. Chem. A Eur. J. 2006, 12, 4684–4690. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Durán, C.F.A.; Liu, W.; Lourdes, D.; Smith, B.D. Structural control of kinetics for macrocycle threading by fluorescent squaraine dye in water. J. Org. Chem. 2017, 82, 8334–8341. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Peck, E.M.; Hendzel, K.D.; Smith, B.D. Sensitive structural control of macrocycle threading by a fluorescent squaraine dye flanked by polymer chains. Org. Lett. 2015, 17, 5268–5271. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Fu, N.; Arunkumar, E.; Leevy, W.M.; Gammon, S.T.; Piwnica-Worms, D.; Smith, B.D. Squaraine rotaxanes: Superior substitutes for Cy-5 in molecular probes for near-infrared Fluorescence cell imaging. Angew. Chem. Int. Ed. 2007, 46, 5528–5531. [Google Scholar] [CrossRef] [PubMed]
- Roland, F.M.; Peck, E.M.; Rice, D.R.; Smith, B.D. Preassembled fluorescent multivalent probes for the imaging of anionic membranes. Bioconjugate Chem. 2017, 28, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gómez-Durán, C.F.A.; Smith, B.D. Fluorescent neuraminidase assay based on supramolecular dye capture after enzymatic cleavage. J. Am. Chem. Soc. 2017, 139, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.S.K.; Liu, W.; Brennan, S.; Smith, B.D.; Shaw, S.K.; Liu, W.; Brennan, S.P.; Betancourt-mendiola, M.D.L.; Smith, D. Non-covalent assembly method that simultaneously endows a liposome surface with targeting ligands, protective PEG chains, and deep-red fluorescence reporter groups. Chem. Eur. J. 2017, 23, 12646–12654. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-J.; Zheng, S.; Odani, T.; Beverina, L.; Fu, J.; Padilha, L.A.; Biesso, A.; Hales, J.M.; Zhan, X.; Schmidt, K.; et al. Extended squaraine dyes with large two-photon absorption cross-sections. J. Am. Chem. Soc. 2006, 128, 14444–14445. [Google Scholar] [CrossRef] [PubMed]
- Meier, H.; Petermann, R.; Gerold, J. Bathochromic or hypsochromic effects via the extension of conjugation: A study of stilbenoid squaraines. Chem. Commun. 1999, 977–978. [Google Scholar] [CrossRef]
- Büschel, M.; Ajayaghosh, A.; Arunkumar, E.; Daub, J. Redox-switchable squaraines with extended conjugation. Org. Lett. 2003, 5, 2975–2978. [Google Scholar] [CrossRef] [PubMed]
- Meier, H.; Petermann, R. NIR absorbing squaraines by extension of the conjugation with (aminothiazolyl)ethenyl groups. Helv. Chim. Acta 2004, 87, 1109–1118. [Google Scholar] [CrossRef]
- Basheer, M.C.; Santhosh, U.; Alex, S.; Thomas, K.G.; Suresh, C.H.; Das, S. Design and synthesis of squaraine based near infrared fluorescent probes. Tetrahedron 2007, 63, 1617–1623. [Google Scholar] [CrossRef]
- Bassal, F.; Laurent, A.D.; Le Guennic, B.; Jacquemin, D. Exploring the excited-states of squaraine dyes with TD-DFT, SOS-CIS(D) and ADC(2). Dye Pigment. 2017, 138, 169–175. [Google Scholar] [CrossRef]
- Lu, Z.; Twieg, R.J. A mild and practical copper catalyzed amination of halothiophenes. Tetrahedron 2005, 61, 903–918. [Google Scholar] [CrossRef]
- Liu, W.; Johnson, A.; Smith, B.D. Guest back-folding: A molecular design strategy that produces a deep-red fluorescent host/guest pair with picomolar affinity in water. J. Am. Chem. Soc. 2018, 140, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Rurack, K.; Spieles, M. Fluorescence quantum yields of a series of red and near-infrared dyes emitting at 600–1000 nm. Anal. Chem. 2011, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Tsien, R.Y. Fluorescence-guided surgery with live molecular navigation—A new cutting edge. Nat. Rev. Cancer 2013, 13, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Schroeder, A.B.; Grudzinski, J.J.; Rosenthal, E.L.; Warram, J.M.; Pinchuk, A.N.; Eliceiri, K.W.; Kuo, J.S.; Weichert, J.P. Beyond the margins: Real-time detection of cancer using targeted fluorophores. Nat. Rev. Clin. Oncol. 2017, 14, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Gassensmith, J.J.; Arunkumar, E.; Barr, L.; Baumes, J.M.; DiVittorio, K.M.; Johnson, J.R.; Noll, B.C.; Smith, B.D. Self-assembly of fluorescent inclusion complexes in competitive media including the interior of living cells. J. Am. Chem. Soc. 2007, 129, 15054–15059. [Google Scholar] [CrossRef] [PubMed]
- Mahou, R.; Wandrey, C. Versatile route to synthesize heterobifunctional poly(ethylene glycol) of variable functionality for subsequent pegylation. Polymers 2012, 4, 561–589. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds S1–S3, S1PEG–S3PEG, M1, and M2 are available from the authors. |

| Ka (M−1) | kon (M−1s−1) | |
|---|---|---|
| S1PEG | (4.9 ± 2.0) × 109 | (1.9 ± 0.2) × 108 |
| S2PEG | (3.4 ± 1.5) × 109 | (7.0 ± 1.0) × 108 |
| S3PEG | (6.3 ± 3.4) × 108 | (7.3 ± 1.6) × 106 |
| S1PEG | M2 ⊃ S1PEG | S2PEG | M2 ⊃ S2PEG | S3PEG | M2 ⊃ S3PEG | |
|---|---|---|---|---|---|---|
| Abs (nm) | 608 | 638 | 695 | 722 | 795 | 809 |
| Em (nm) | 665 | 697 | 731 | 753 | 815 | 829 |
| Φfa | 6% | 25% | 3% | 19% | 7% | 11% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; McGarraugh, H.H.; Smith, B.D. Fluorescent Thienothiophene-Containing Squaraine Dyes and Threaded Supramolecular Complexes with Tunable Wavelengths between 600–800 nm. Molecules 2018, 23, 2229. https://doi.org/10.3390/molecules23092229
Liu W, McGarraugh HH, Smith BD. Fluorescent Thienothiophene-Containing Squaraine Dyes and Threaded Supramolecular Complexes with Tunable Wavelengths between 600–800 nm. Molecules. 2018; 23(9):2229. https://doi.org/10.3390/molecules23092229
Chicago/Turabian StyleLiu, Wenqi, Hannah H. McGarraugh, and Bradley D. Smith. 2018. "Fluorescent Thienothiophene-Containing Squaraine Dyes and Threaded Supramolecular Complexes with Tunable Wavelengths between 600–800 nm" Molecules 23, no. 9: 2229. https://doi.org/10.3390/molecules23092229
APA StyleLiu, W., McGarraugh, H. H., & Smith, B. D. (2018). Fluorescent Thienothiophene-Containing Squaraine Dyes and Threaded Supramolecular Complexes with Tunable Wavelengths between 600–800 nm. Molecules, 23(9), 2229. https://doi.org/10.3390/molecules23092229