Euphosantianane A–D: Antiproliferative Premyrsinane Diterpenoids from the Endemic Egyptian Plant Euphorbia Sanctae-Catharinae
Abstract
1. Introduction
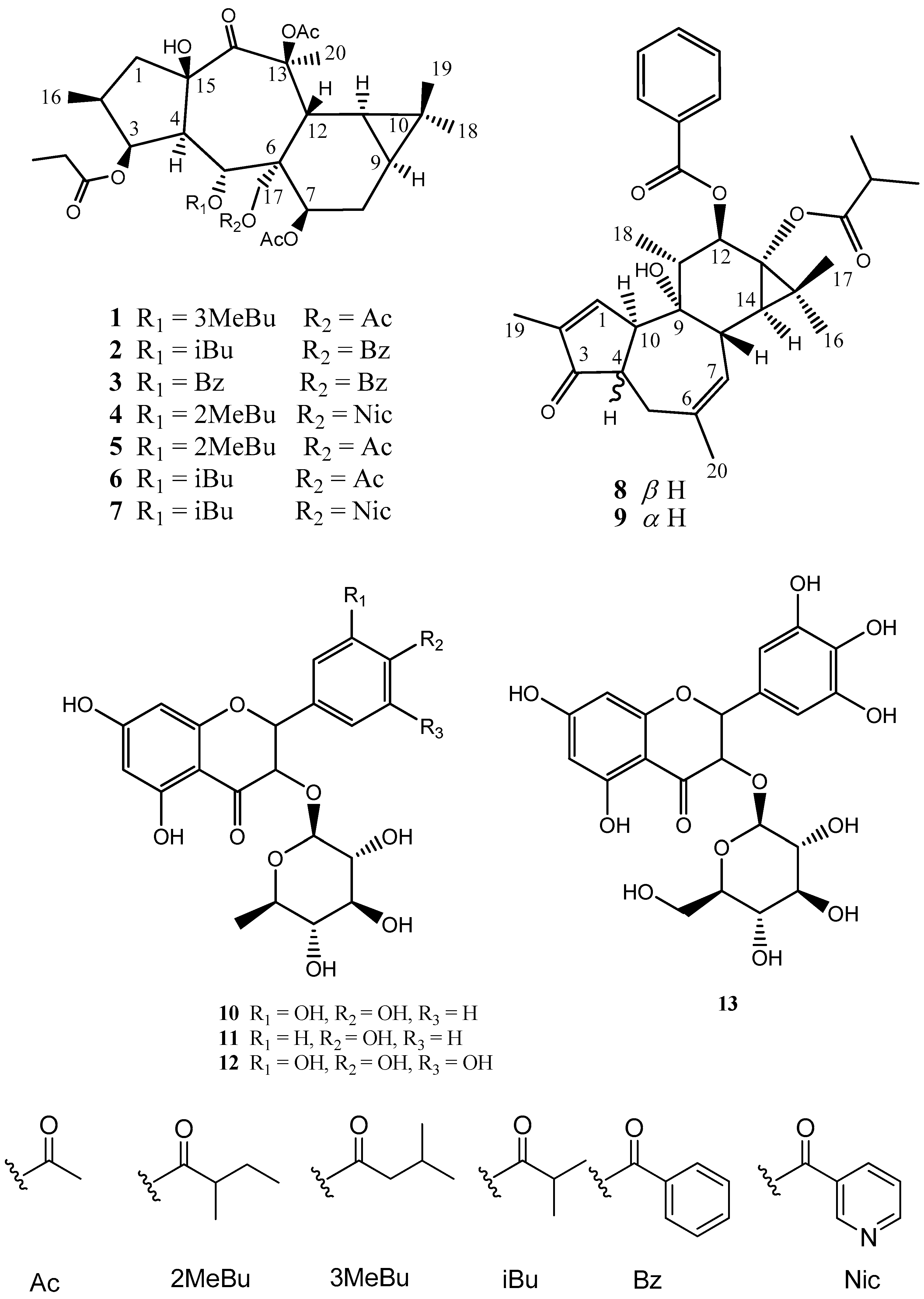
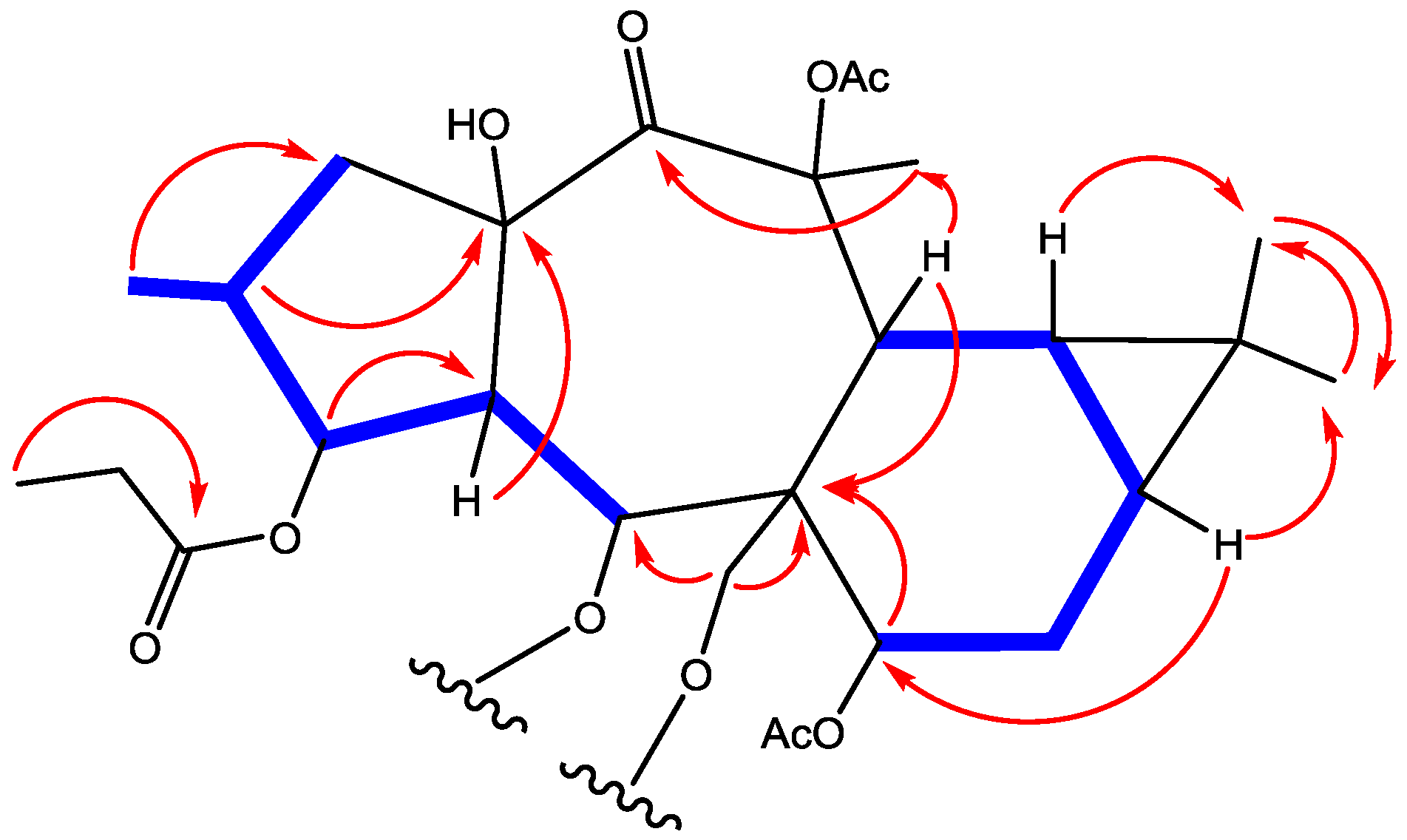
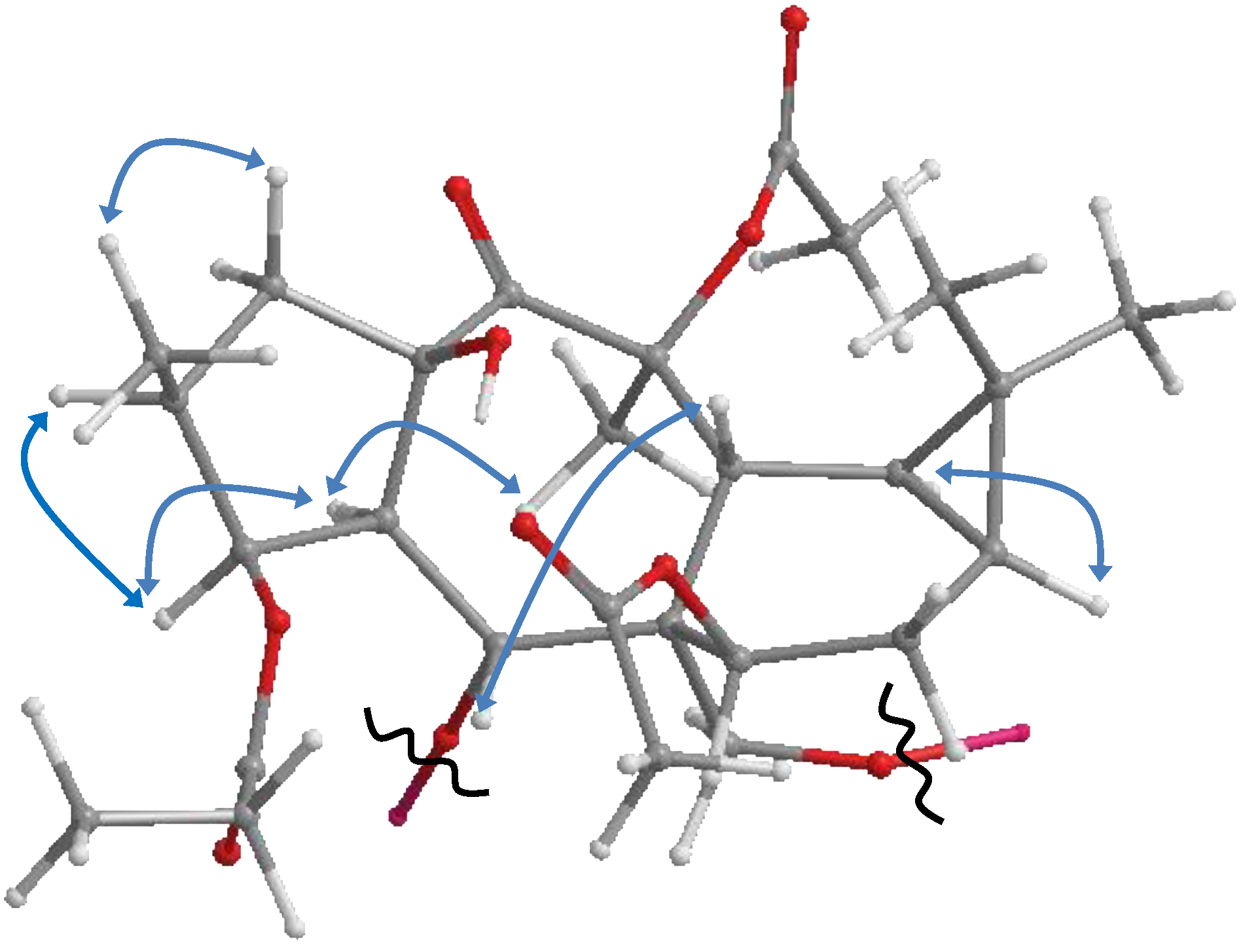
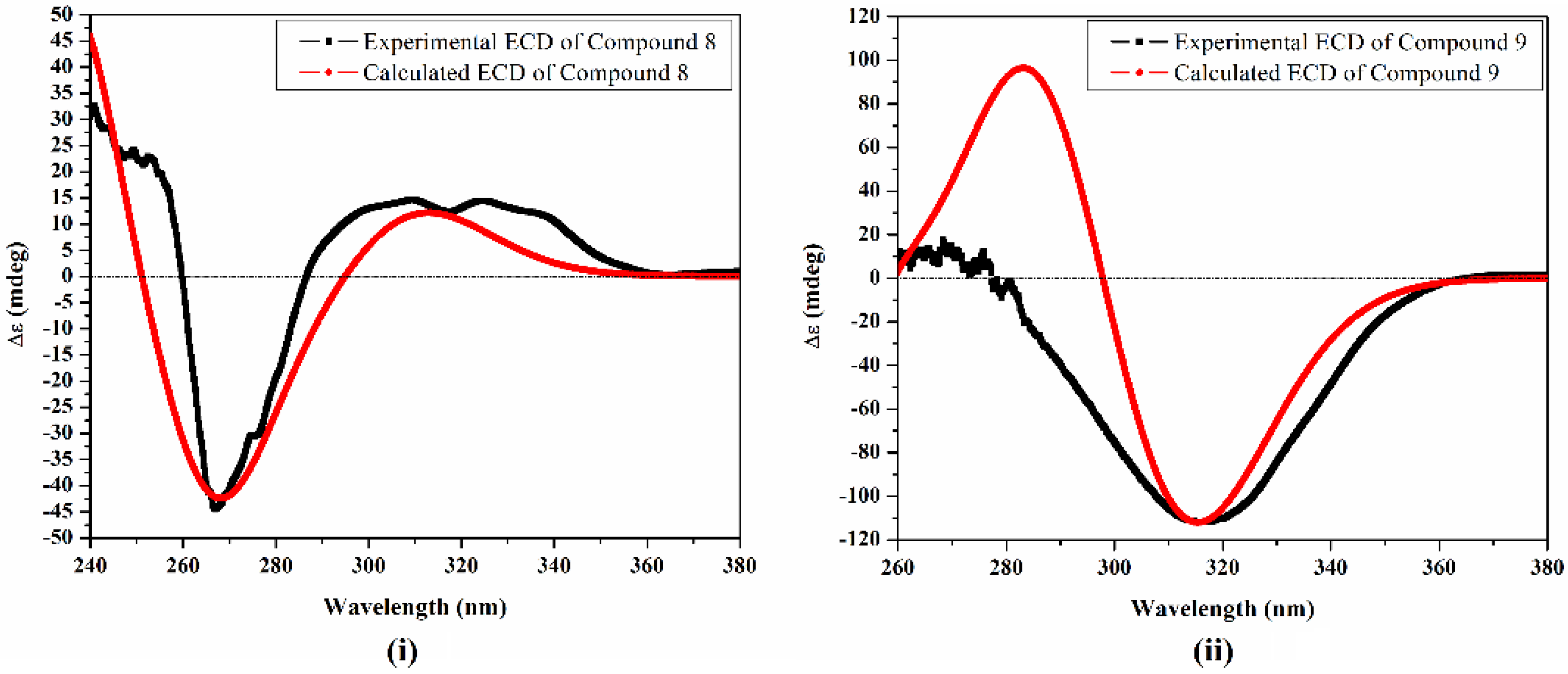
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Biological Activity
3.4.1. Cell Culture
3.4.2. Cell Proliferation Assay
3.4.3. Anti-Proliferation Quantitative Analysis
3.5. Computational Calculations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barla, A.; Bİrman, H.; Kültür, Ş.; Öksüz, S. Secondary metabolites from Euphorbia helioscopia and their vasodepressor activity. Turk. J. Chem. 2006, 30, 325–332. [Google Scholar]
- Batanouny, K.H.; Stichler, W.; Ziegler, H. Phytosynthetic pathways and ecological distribution of Euphorbia species in Egypt. Oecologia 1991, 87, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, K.; Akihisa, T.; Yoshida, Z.Y.; Takido, M. Inhibitory effect of euphol, a triterpene alcohol from the roots of Euphorbia kansui, on tumour promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. J. Pharm. Pharmacol. 2000, 52, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Delgado, I.F.; De-Carvalho, R.R.; De-Oliveira, A.C.; Kuriyama, S.N.; Oliveira-Filho, E.C.; Souza, C.A.; Paumgartten, F.J. Absence of tumor promoting activity of Euphorbia milii latex on the mouse back skin. Toxicol. Lett. 2003, 145, 175–180. [Google Scholar] [CrossRef]
- King, A.R.; Dotsey, E.Y.; Lodola, A.; Jung, K.M.; Ghomian, A.; Qiu, Y. Discovery of potent and reversible monoacylglycerol lipase inhibitors. Chem. Biol. 2009, 16, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.J. Discovery of potent and reversible monoacylglycerol lipase inhibitors. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 199–203. [Google Scholar] [PubMed]
- Pracheta, S.V.; Veena, S.; Ritu, P.; Sadhana, S. Preliminary Phytochemical Screening and in vitro Antioxidant Potential of Hydro-Ethanolic extract of Euphorbia neriifolia Linn. Int. J. Pharm. Tech. Res. 2011, 3, 124–132. [Google Scholar]
- Jie, C.; Xin, Y.; Ai-jun, D.; Da-you, C.; Jing, W.; Hai-tian, Z. Chemical composition and antioxidant activity of Euphorbia fischeriana essential oil from China. J. Med. Plants Res. 2011, 5, 4794–4798. [Google Scholar]
- Tanaka, R.; Kasubuchi, K.; Kita, S.; Matsunaga, S. Obtusifoliol and related steroids from the whole herb of Euphorbia chamaesyce. Phytochemistry 1999, 51, 457–463. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, A.U.; Chaudhary, B.A.; Janbaz, K.H.; Uzair, M.; Akhtar, M.; Gilani, A.H. Antifungal and antispasmodic activities of the extracts of Euphorbia granulata. J. Med. Plants Res. 2012, 6, 19–32. [Google Scholar]
- Shu, X.; Yu, L.; Tang, Y.; Zhang, L.; Ding, A.; Luo, D. Bioassay-guided separation of the proinflamatory constituents from the roots of Euphorbia kansui. J. Nat. Med. 2010, 64, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Peijian, Z.; Xiaofang, W. The Application of Antibacterial Components of Euphorbia Humifusa Willd on Silk Fabrics. Adv. Mater. Res. 2012, 441, 315–319. [Google Scholar]
- Lirio, L.G.; Hermano, M.L.; Fontanilla, M.Q. Note antibacterial activity of medicinal plants from the Philippines. Pharm. Biol. 1998, 36, 357–359. [Google Scholar] [CrossRef]
- Ibraheim, Z.Z.; Ahmed, A.S.; Abdel-Mageed, W.M. Chemical and biological studies of Euphorbia aphylla. J. Nat. Prod. 2013, 13, 35–45. [Google Scholar]
- Esposito, M.; Nothias, L.-E.; Retailleau, P.; Costa, J.; Roussi, F.; Neyts, J.; Leyssen, P.; Touboul, D.; Litaudon, M.; Paolini, J. Isolation of Premyrsinane, Myrsinane, and Tigliane Diterpenoids from Euphorbia pithyusa Using a Chikungunya Virus Cell-Based Assay and Analogue Annotation by Molecular Networking. J. Nat. Prod. 2017, 80, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Kawashty, S.A.; Abdalla, M.F.; El-Hadidi, M.N.; Saleh, N.A.M. The chemosystematics of Egyptian Euphorbia species. Biochem. Syst. Ecol. 1990, 18, 487–490. [Google Scholar] [CrossRef]
- Saleh, N.A. Flavonol glycosides of Euphorbia retusa and E. sanctae-catharinae. Phytochemistry 1985, 24, 371–372. [Google Scholar] [CrossRef]
- Hegazy, M.E.F.; Abd El-Razek, M.H.; Nagashima, F.; Asakawa, Y.; Paré, P.W. Rare prenylated flavonoids from Tephrosia purpurea. Phytochemistry 2009, 70, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.-E.F.; Matsuda, H.; Nakamura, S.; Yabe, M.; Matsumoto, T.; Yoshikawa, M. Sesquiterpenes from an Egyptian Herbal Medicine, Pulicaria undulate, with Inhibitory Effects on Nitric Oxide Production in RAW264.7 Macrophage Cells. Chem. Pharm. Bull. 2012, 60, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Elmasri, W.A.; Hegazy, M.E.F.; Aziz, M.; Koksal, E.; Amor, W.; Mechref, Y.; Hamood, A.N.; Cordes, D.B.; Paré, P.W. Biofilm blocking sesquiterpenes from Teucrium polium. Phytochemistry 2014, 103, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.F.; Matsuda, H.; Nakamura, S.; Hussein, T.A.; Yoshikawa, M.; Paré, P.W. Chemical constituents and their antibacterial and antifungal activity from the Egyptian herbal medicine Chiliadenus montanus. Phytochemistry 2014, 103, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Elmasri, W.A.; Yang, T.; Tran, P.; Hegazy, M.E.F.; Hamood, A.N.; Mechref, Y.; Paré, P.W. Teucrium polium phenylethanol and iridoid glycoside characterization and flavonoid inhibition of biofilm-forming staphylococcus aureus. J. Nat. Prod. 2015, 78, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Elmasri, W.A.; Yang, T.; Hegazy, M.E.F.; Mechref, Y.; Paré, P.W. Iridoid glycoside permethylation enhances chromatographic separation and chemical ionization. Rapid Commun. Mass Spectrom. 2016, 30, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.R.; Hegazy, M.E.F.; Higgins, M.; Mohamed, T.A.; Abdel-Azim, N.S.; Pare, P.W.; Dinkova-Kostova, A.T. Potency of extracts from selected Egyptian plants as inducers of the Nrf2-dependent chemopreventive enzyme NQO1. J. Nat. Med. 2016, 70, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.F.; Ibrahim, A.Y.; Mohamed, T.A.; Shahat, A.A.; El Halawany, A.M.; Abdel-Azim, N.S.; Alsaid, M.S.; Paré, P.W. Sesquiterpene Lactones from Cynara cornigera: Acetyl Cholinesterase Inhibition and in Silico Ligand Docking. Planta Med. 2016, 82, 138–146. [Google Scholar] [PubMed]
- Appendino, G.; Belloro, E.; Tron, G.C.; Jakupovic, J.; Ballero, M. Diterpenoids from Euphorbia pithyusa subsp. cupanii. J. Nat. Prod. 1999, 62, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Chen, D.F.; Hou, A.J. New myrsinol diterpenes from Euphorbia prolifera. Chin. J. Chem. 2004, 22, 103–108. [Google Scholar] [CrossRef]
- Shokoohinia, Y.; Sajjadi, S.E.; Zolfaghari, B.; Chianese, G.; Appendino, G.; Taglialatela-Scafati, O. Diterpenoid (poly)esters and a ring A-seco-phorboid from the aerial parts of Euphorbia macroclada Boiss. Fitoterapia 2010, 8, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Aichour, S.; Haba, H.; Benkhaled, M.; Harakat, D.; Lavaud, C. Terpenoids and other constituents from Euphorbia bupleuroides. Phytochem. Lett. 2014, 10, 198–203. [Google Scholar] [CrossRef]
- Dagang, W.; Sorg, B.; Hecker, E. Oligo and macrocyclic diterpenes in thymelaeaceae and Euphorbiaceae occurring and utilized in Yunnan (Southwest China). 6. Tigliane type diterpene esters from latex of Euphorbia prolifera. Phytother. Res. 1994, 8, 95–99. [Google Scholar] [CrossRef]
- Jung, M.J.; Kang, S.S.; Jung, H.A.; Kim, G.J.; Choi, J.S. Isolation of flavonoids and a cerebroside from the stem bark of Albizzia julibrissin. Arch. Pharm. Res. 2004, 27, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Diantini, A.; Subarnas, A.; Lestari, K.; Halimah, E.; Susilawati, Y.; Supriyatna, S.; Kobayashi, K. Kaempferol-3-O-rhamnoside isolated from the leaves of Schimawallichii Korth. inhibits MCF-7 breast cancer cell proliferation through activation of the caspase cascade pathway. Oncol. Lett. 2012, 3, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Cruz, F.G.; Guedes, M.L.S.; Chávez, J.P. Flavonol glycosides from Davillaflexuosa. J. Braz. Chem. Soc. 1996, 7, 115–118. [Google Scholar] [CrossRef]
- Agrawal, P.K. Carbon-13 NMR of Flavonoids; Elsevier Science: Amsterdam, The Netherlands, 1989; pp. 283–364. ISBN 9781483290744. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Dutta, A.; Bandyopadhyay, S.; Mandal, C.; Chatterjee, M. Development of a modified MTT assay for screening antimonial resistant field isolates of Indian visceral leishmaniasis. Parasitol. Int. 2005, 54, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.F.; El-Beih, A.A.; Hamed, A.R.; Abd El Aty, A.A.; Mohamed, N.S.; Paré, P.W. 3-Oxo-γ-costic acid fungal-transformation generates eudesmane sesquiterpenes with in vitro tumor-inhibitory activity. Bioorg. Med. Chem. Lett. 2017, 27, 3825–3828. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; El-Naggar, D.H.; Hamed, A.R.; Ibrahim, H.S.; Ghabbour, H.A.; Abdel-Aziz, H.A. One-pot three-component synthesis of novel spirooxindoles with potential cytotoxic activity against triple-negative breast cancer MDA-MB-231 cells. J. Enzyme Inhib. Med. Chem. 2018, 33, 309–318. [Google Scholar] [CrossRef] [PubMed]
- OMEGA 2.5.1.4; OpenEye Scientific Software: Santa Fe, NM, USA, 2017.
- Hawkins, P.C.D.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer Generation with OMEGA: Algorithm and Validation Using High Quality Structures from the Protein Databank and Cambridge Structural Database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Babin, V.; Berryman, J.T.; Betz, R.M.; Cai, Q.; Cerutti, D.S.; Cheatham, T.E.; Darden, T.A.; Duke, R.E.; Gohlke, H.; et al. AMBER 14; University of California: San Francisco, CA, USA, 2014. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09; Revision E.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Pescitelli, G. SpecDis version 1.71; Berlin, Germany. 2017. Available online: https:/specdis-software.jimdo.com (accessed on 18 August 2018).
Sample Availability: Samples of the compounds 1–13 are not available from the authors. |
| No. | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| dH (J in Hz) | δC | dH (J in Hz) | δC | dH (J in Hz) | δC | dH (J in Hz) | δC | |
| 1α | 3.12 dd (8.4, 13.8) | 42.9 | 3.14 dd (8.4, 13.8) | 42.9 | 3.15 dd (7.2, 13.8) | 42.9 | 3.14 dd (7.8, 13.2) | 42.9 |
| 1β | 1.59 dd (12.6, 13.2) | 1.60 dd (13.2) | 1.61 dd (13.8, 13.2) | 1.61 dd | ||||
| 2 | 1.80 m | 37.5 | 1.86 m | 37.5 | 1.87 m | 37.3 | 1.79 m | 37.5 |
| 3 | 5.24 dd (3.6, 6.0) | 78.4 | 5.23 t | 78.4 | 5.36 t (3.6) | 78.3 | 5.21 t | 77.3 |
| 4 | 2.32 m | 50.4 | 2.32 m | 50.5 | 2.34 m | 50.4 | 2.36 dd (3.0) | 50.6 |
| 5 | 6.18 d (11.4) | 68.8 | 6.21 d (12.0) | 69.1 | 6.46 d (11.4) | 69.9 | 6.23 d (12.0) | 69 |
| 6 | ---- | 47.4 | ---- | 47.8 | ---- | 48.2 | ---- | 47.7 |
| 7 | 4.48 d (6.6) | 70.7 | 4.72 d (6.6) | 71 | 4.97 d (6.6) | 70.7 | 4.85 d (12.6) | 70.8 |
| 8α | 2.09 m | 23.9 | 2.14 m | 21.4 | 2.05 m | 22.2 | 2.09 m | 23.9 |
| 8β | 1.80 brd (17.0) | 23.9 | 1.77 brd (17.0) | 21.4 | 1.87 brd (17.0) | 22.2 | 1.90 d (13.2) | 23.9 |
| 9 | 0.72 m | 18.9 | 0.77 m | 18.4 | 0.72 m | 19.1 | 0.62 m | 19 |
| 10 | ---- | 18.2 | ---- | 18.3 | ---- | 18.4 | ---- | 18.3 |
| 11 | 0.72 m | 21.4 | 0.77 m | 18.5 | 0.72 m | 21.5 | 0.62 m | 21.3 |
| 12 | 3.37 d (6) | 34.8 | 3.46 d (5.4) | 33.9 | 3.55 d (6.6) | 35.3 | 3.46 d (3.6) | 35 |
| 13 | ---- | 86.0 | ---- | 85.9 | ---- | 85.9 | ---- | 85.8 |
| 14 | ---- | 204.5 | ---- | 204.5 | ---- | 204.4 | ---- | 204.3 |
| 15-OH | 4.44 s | 84.1 | 4.44 s | 84.1 | 4.45 s | 84.2 | 4.48 s | 84.1 |
| 16 | 0.87 d (6.0) | 14.1 | 0.86 d (1.8) | 14.2 | 0.86 d (6.0) | 14 | 0.87 d (14.4) | 14.7 |
| 17α | 4.39 d (12.0) | 63.6 | 4.81 d (12.0) | 64 | 4.58 d (11.4) | 63.4 | 4.67 (d, J = 11.4 Hz) | 64.5 |
| 17β | 4.31 d (12.0) | 4.46 d (12.0) | 4.91 d (10.8) | 4.46 brd (11.4) | ||||
| 18 | 1.04 s | 29.5 | 1.05 s | 29.5 | 1.06 s | 29.5 | 1.05 s | 29.5 |
| 19 | 0.90 s | 14.9 | 0.94 s | 14.9 | 0.95 s | 15 | 0.93 s | 14.9 |
| 20 | 1.68 s | 24.6 | 1.73 s | 24.6 | 1.66 s | 25 | 1.71 s | 25.8 |
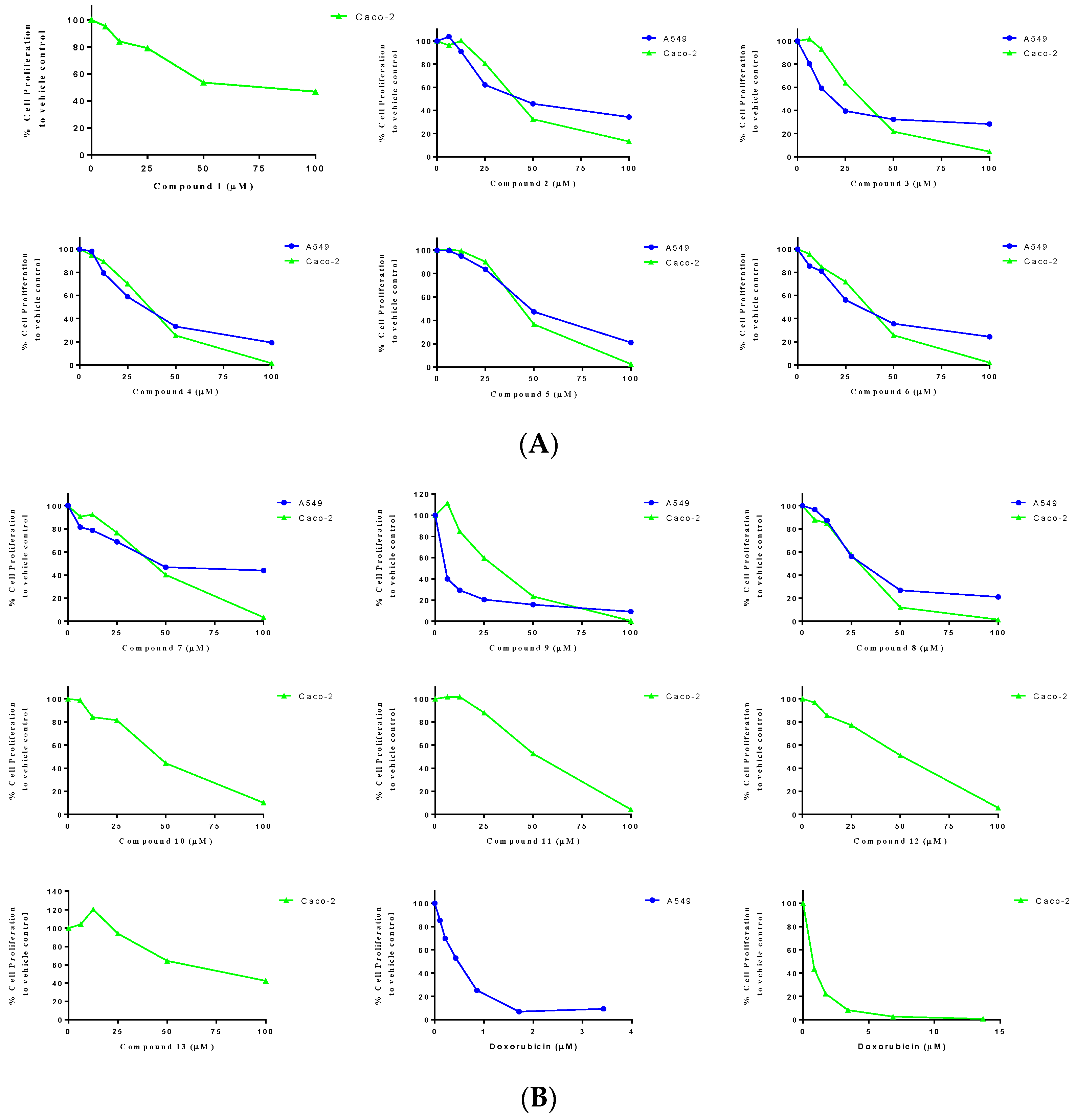
| Compound | IC50 on Caco-2 (µM) a | IC50 on A549 (µM) a |
|---|---|---|
| 1 | 75.8 (0.950) | >100 |
| 2 | 40.5 (0.989) | 48.5 (0.927) |
| 3 | 31.0 (0.999) | 21.5 (0.924) |
| 4 | 33.2 (0.993) | 32.8 (0.988) |
| 5 | 43.5 (0.999) | 50.1 (0.9960) |
| 6 | 33.3 (0.984) | 33.1 (0.983) |
| 7 | 40.3 (0.979) | 60.3 (0.937) |
| 8 | 26.1 (0.979) | 31.3 (0.971) |
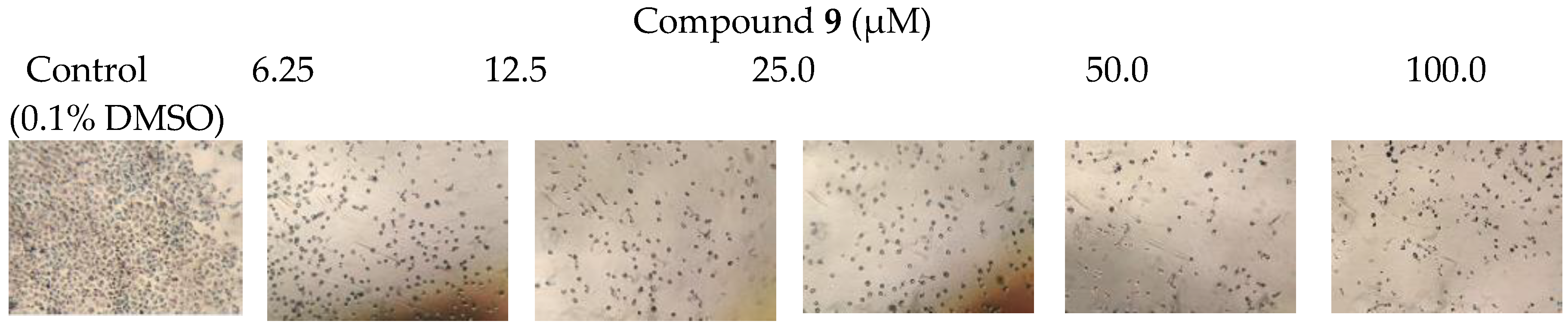
| 9 | 29.4 (0.972) | 3.3 (0.996) |
| 10 | 43.9 (0.975) | >100 |
| 11 | 50.2 (0.993) | >100 |
| 12 | 44.7 (0.961) | >100 |
| 13 | 79.4 (0.843) | >100 |
| Doxorubicin HCl | 0.7 (0.999) | 0.4 (0.987) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegazy, M.-E.F.; Hamed, A.R.; Ibrahim, M.A.A.; Talat, Z.; Reda, E.H.; Abdel-Azim, N.S.; Hammouda, F.M.; Nakamura, S.; Matsuda, H.; Haggag, E.G.; et al. Euphosantianane A–D: Antiproliferative Premyrsinane Diterpenoids from the Endemic Egyptian Plant Euphorbia Sanctae-Catharinae. Molecules 2018, 23, 2221. https://doi.org/10.3390/molecules23092221
Hegazy M-EF, Hamed AR, Ibrahim MAA, Talat Z, Reda EH, Abdel-Azim NS, Hammouda FM, Nakamura S, Matsuda H, Haggag EG, et al. Euphosantianane A–D: Antiproliferative Premyrsinane Diterpenoids from the Endemic Egyptian Plant Euphorbia Sanctae-Catharinae. Molecules. 2018; 23(9):2221. https://doi.org/10.3390/molecules23092221
Chicago/Turabian StyleHegazy, Mohamed-Elamir F., Ahmed R. Hamed, Mahmoud A. A. Ibrahim, Zienab Talat, Eman H. Reda, Nahla S. Abdel-Azim, Faiza M. Hammouda, Seikou Nakamura, Hisashi Matsuda, Eman G. Haggag, and et al. 2018. "Euphosantianane A–D: Antiproliferative Premyrsinane Diterpenoids from the Endemic Egyptian Plant Euphorbia Sanctae-Catharinae" Molecules 23, no. 9: 2221. https://doi.org/10.3390/molecules23092221
APA StyleHegazy, M.-E. F., Hamed, A. R., Ibrahim, M. A. A., Talat, Z., Reda, E. H., Abdel-Azim, N. S., Hammouda, F. M., Nakamura, S., Matsuda, H., Haggag, E. G., Paré, P. W., & Efferth, T. (2018). Euphosantianane A–D: Antiproliferative Premyrsinane Diterpenoids from the Endemic Egyptian Plant Euphorbia Sanctae-Catharinae. Molecules, 23(9), 2221. https://doi.org/10.3390/molecules23092221










