Experimental Characterization of the Thermal Conductivity and Microstructure of Opacifier-Fiber-Aerogel Composite
Abstract
1. Introduction
2. Experimental Investigation
2.1. Test Materials
2.2. Sample Characterization
2.3. Thermal Conductivity Measurement
3. Results and Discussion
3.1. Thermal Conductivity
3.2. Microscopic Morphology
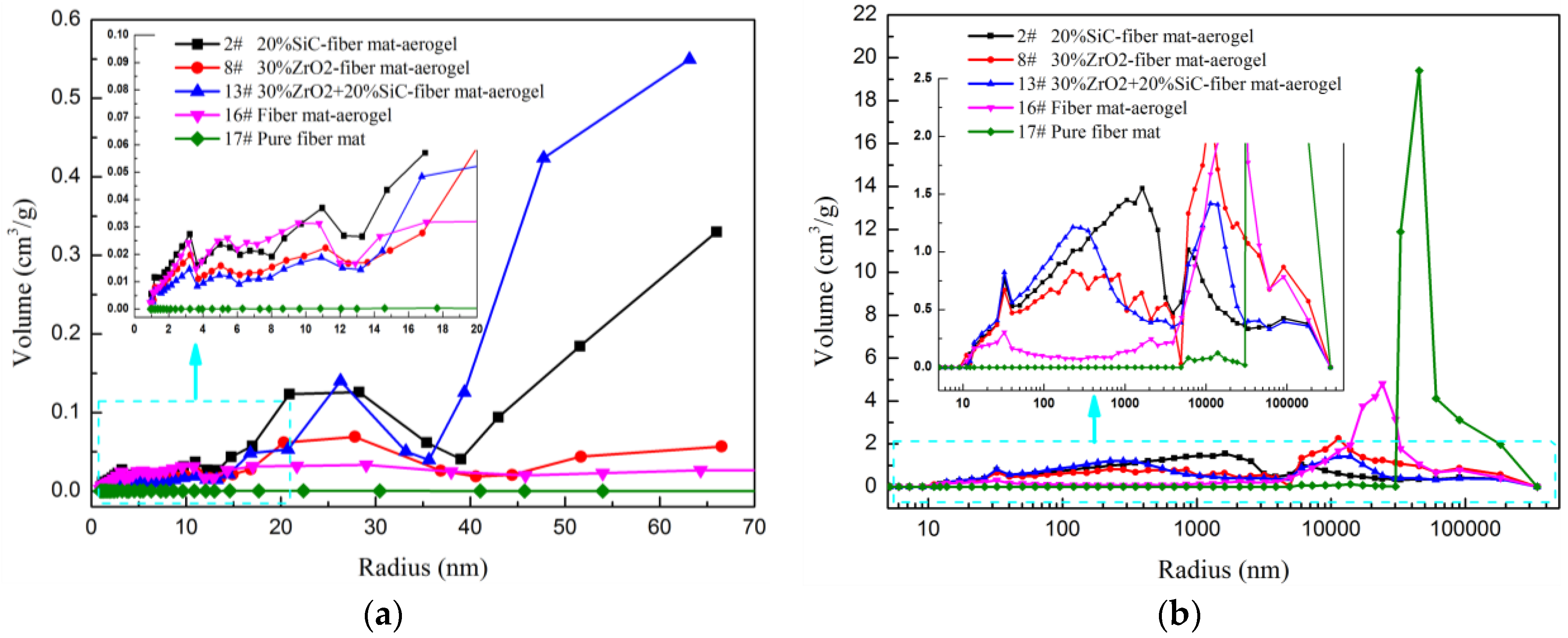
3.3. Specific Surface Area and Pore Size Distribution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hüsing, N.; Schubert, U. Aerogels—Airy materials: Chemistry, structure, and properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.K.; Park, H.H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 23. [Google Scholar] [CrossRef]
- Lei, C.; Li, J.; Sun, C.; Yang, H.; Xia, T.; Hu, Z.; Zhang, Y. A Co-precursor approach coupled with a supercritical modification method for constructing highly transparent and superhydrophobic polymethylsilsesquioxane aerogels. Molecules 2018, 23, 797. [Google Scholar] [CrossRef] [PubMed]
- Marquezescalante, J.; Carvajalmillan, E.; Mikiyoshida, M.; Alvarezcontreras, L.; Lizardimendoza, J. Water extractable arabinoxylan aerogels prepared by supercritical CO2 drying. Molecules 2013, 18, 5531–5542. [Google Scholar] [CrossRef] [PubMed]
- Koebel, M.; Rigacci, A.; Achard, P. Aerogel-based thermal superinsulation: An overview. J. Sol-Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef]
- Zeng, S.Q.; Hunt, A.; Greif, R. Geometric structure and thermal conductivity of porous medium silica aerogel. ASME J. Heat Transf. 1995, 117, 1055–1058. [Google Scholar] [CrossRef]
- Lu, X.; Arduini-Schuster, M.; Kuhn, J.; Nilsson, O.; Fricke, J.; Pekala, R. Thermal conductivity of monolithic organic aerogels. Science 1992, 255, 971. [Google Scholar] [CrossRef] [PubMed]
- Carriço, C.S.; Fraga, T.; Carvalho, V.E.; Vmd, P. Polyurethane foams for thermal insulation uses produced from castor oil and crude glycerol biopolyols. Molecules 2017, 22, 1091. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer: New York, NY, USA, 2011. [Google Scholar]
- Lee, O.J.; Lee, K.H.; Yim, T.J.; Kim, S.Y.; Yoo, K.P. Determination of mesopore size of aerogels from thermal conductivity measurements. J. Non-Cryst. Solids 2002, 298, 287–292. [Google Scholar] [CrossRef]
- Zeng, S.; Hunt, A.; Greif, R. Mean free path and apparent thermal conductivity of a gas in a porous medium. ASME J. Heat Transf. 1995, 117, 758–761. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, W.; Li, Z.; Tao, W. The influence of gaseous heat conduction to the effective thermal conductivity of nano-porous materials. Int. Commun. Heat Mass Transf. 2015, 68, 158–161. [Google Scholar] [CrossRef]
- Bi, C.; Tang, G. Effective thermal conductivity of the solid backbone of aerogel. Int. J. Heat Mass Transf. 2013, 64, 452–456. [Google Scholar] [CrossRef]
- Tang, G.H.; Bi, C.; Zhao, Y.; Tao, W.Q. Thermal transport in nano-porous insulation of aerogel: Factors, models and outlook. Energy 2015, 90, 701–721. [Google Scholar] [CrossRef]
- Xie, T.; He, Y.L.; Hu, Z.J. Theoretical study on thermal conductivities of silica aerogel composite insulating material. Int. J. Heat Mass Transf. 2013, 58, 540–552. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, J.; Feng, J. Synthesis and characterization of ambient-dried microglass fibers/silica aerogel nanocomposites with low thermal conductivity. J. Sol-Gel Sci. Technol. 2017, 83, 64–71. [Google Scholar] [CrossRef]
- Dan, D.; Zhang, H.; Tao, W.-Q. Effective structure of aerogels and decomposed contributions of its thermal conductivity. Appl. Therm. Eng. 2014, 72, 2–9. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, W.Z.; Wang, X.; Li, Y.M.; Tao, W.Q. Thermal conductivity of fiber and opacifier loaded silica aerogel composite. Int. J. Heat Mass Transf. 2017, 115, 21–31. [Google Scholar] [CrossRef]
- Fang, W.Z.; Zhang, H.; Chen, L.; Tao, W.Q. Numerical predictions of thermal conductivities for the silica aerogel and its composites. Appl. Therm. Eng. 2016, 115, 1277–1286. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, W.; Li, M.J.; Fang, W.Z.; Li, Z.Y.; Tao, W.Q. Influence of environmental factors on the adsorption capacity and thermal conductivity of silica nano-porous materials. J. Nanosci. Nanotechnol. 2015, 15, 3048–3054. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Hunt, A.; Greif, R. Theoretical modeling of carbon content to minimize heat transfer in silica aerogel. J. Non-Cryst. Solids 1995, 186, 271–277. [Google Scholar] [CrossRef]
- Wei, G.; Liu, Y.; Zhang, X.; Yu, F.; Du, X. Thermal conductivities study on silica aerogel and its composite insulation materials. Int. J. Heat Mass Transf. 2011, 54, 2355–2366. [Google Scholar] [CrossRef]
- Gao, Q.; Feng, J.; Zhang, C.; Wu, W.; Jiang, Y. Mechanical properties of ceramic fiber-reinforced silica aerogel insulation composites. J. Chin. Ceram. Soc. 2009, 37, 1–5. [Google Scholar]
- Yao, X.Z.; Hu, Z.J.; Fang, J.C.; Sun, C.C.; Zhang, H.B. Orthogonal optimization of reactive mixture ratio and thermal conductivity of silica aerogels. Aerosp. Mater. Technol. 2009, 39, 32–34. [Google Scholar]
- Gao, F.Q.; Zeng, L.K.; Wang, H.; Cheng, X.S.; Liu, P.A. Characterization of SiO2 aerogels/fiber composites prepared by the sol-gel methods. J. Ceram. 2010, 31, 368–371. [Google Scholar]
- Haq, E.U.; Zaidi, S.F.A.; Zubair, M.; Karim, M.R.A.; Padmanabhan, S.K.; Licciulli, A. Hydrophobic silica aerogel glass-fibre composite with higher strength and thermal insulation based on methyltrimethoxysilane (MTMS) Precursor. Energy Build. 2017, 151, 494–500. [Google Scholar] [CrossRef]
- He, Y. Rapid thermal conductivity measurement with a hot disk sensor: Part 1. Theoretical considerations. Thermochim. Acta 2005, 436, 122–129. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Y.; Gu, W.; Li, Z.Y.; Tao, W.Q. A numerical study on the influence of insulating layer of the hot disk sensor on the thermal conductivity measuring accuracy. Prog. Comput. Fluid Dyn. 2013, 13, 191–201. [Google Scholar] [CrossRef]
- Zeng, S.; Hunt, A.; Cao, W.; Greif, R. Pore size distribution and apparent gas thermal conductivity of silica aerogel. ASME J. Heat Transf. 1994, 116, 756–759. [Google Scholar] [CrossRef]
- Reichenauer, G.; Heinemann, U.; Ebert, H.P. Relationship between pore size and the gas pressure dependence of the gaseous thermal conductivity. Colloids Surf. A Physicochem. Eng. Asp. 2007, 300, 204–210. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.; Yoo, T.; Park, D.; Han, H. Preparation and characterization of spherical polyimide aerogel microparticles. Macromol. Mater. Eng. 2014, 299, 1081–1088. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, J.; Kim, S.; Kim, M.; Lee, D.; Lee, S.; Han, H. One-step synthesis of nano-porous monolithic polyimide aerogel. Microporous Mesoporous Mater. 2016, 234, 35–42. [Google Scholar] [CrossRef]
- Seraji, M.M.; Ghafoorian, N.S.; Bahramian, A.R. Investigation of microstructure and mechanical properties of novolac/silica and C/SiO2/SiC aerogels using mercury porosimetry method. J. Non-Cryst. Solids 2016, 435, 1–7. [Google Scholar] [CrossRef]
- Pang, X.; Zhu, J.; Shao, T.; Luo, X.; Zhang, L. Facile fabrication of gradient density organic aerogel foams via density gradient centrifugation and UV curing in one-step. J. Sol.-Gel Sci. Technol. 2018, 85, 243–250. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
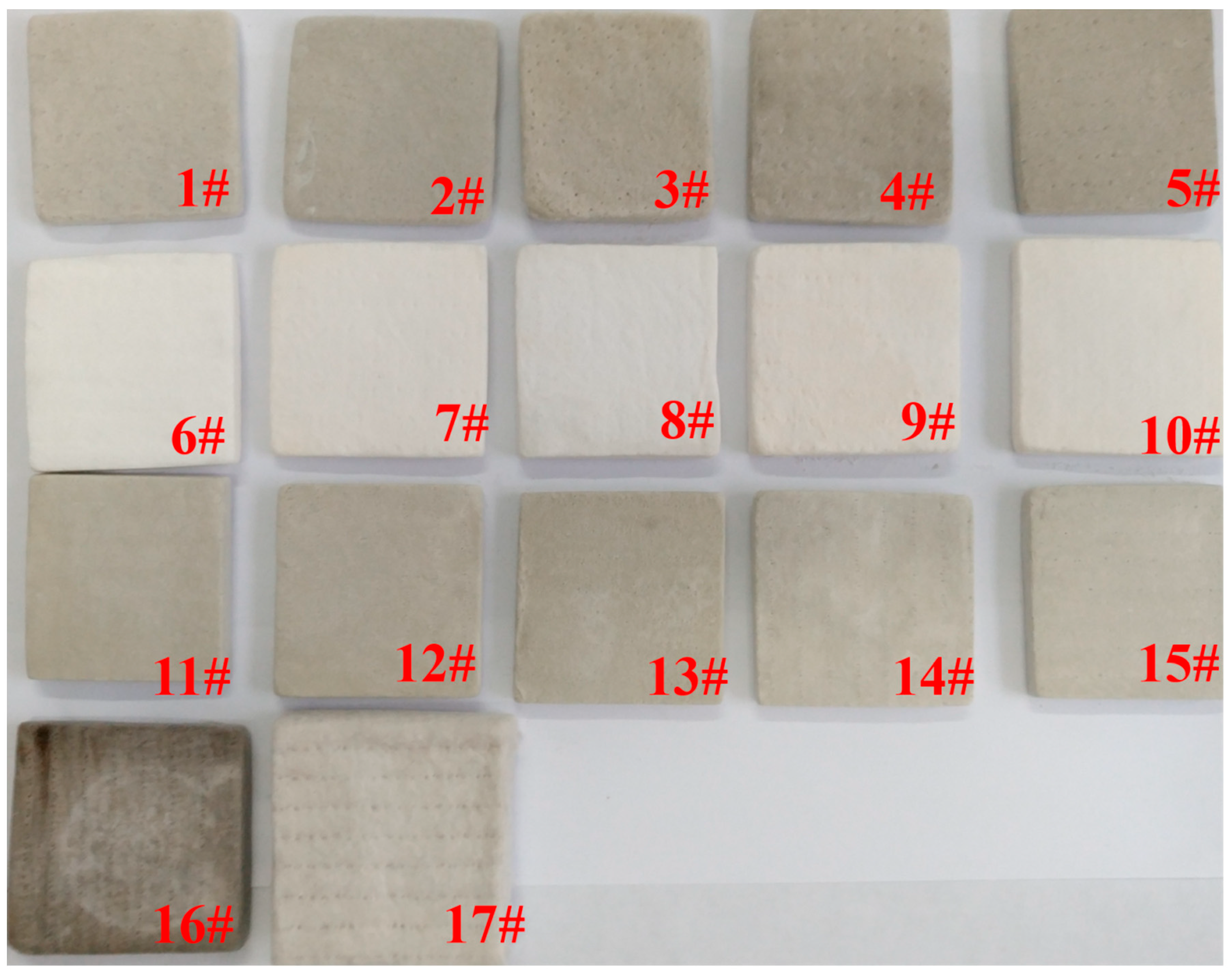
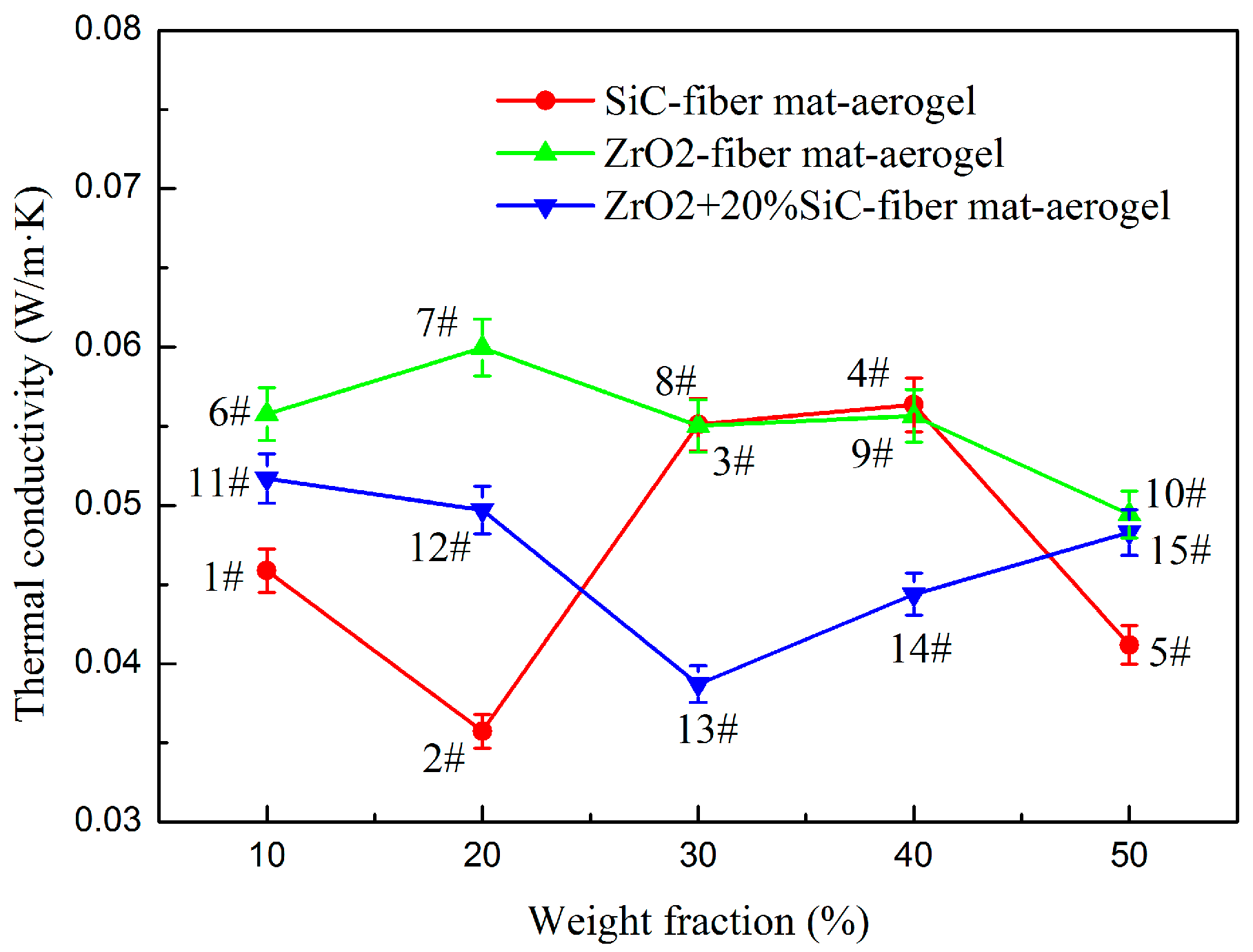

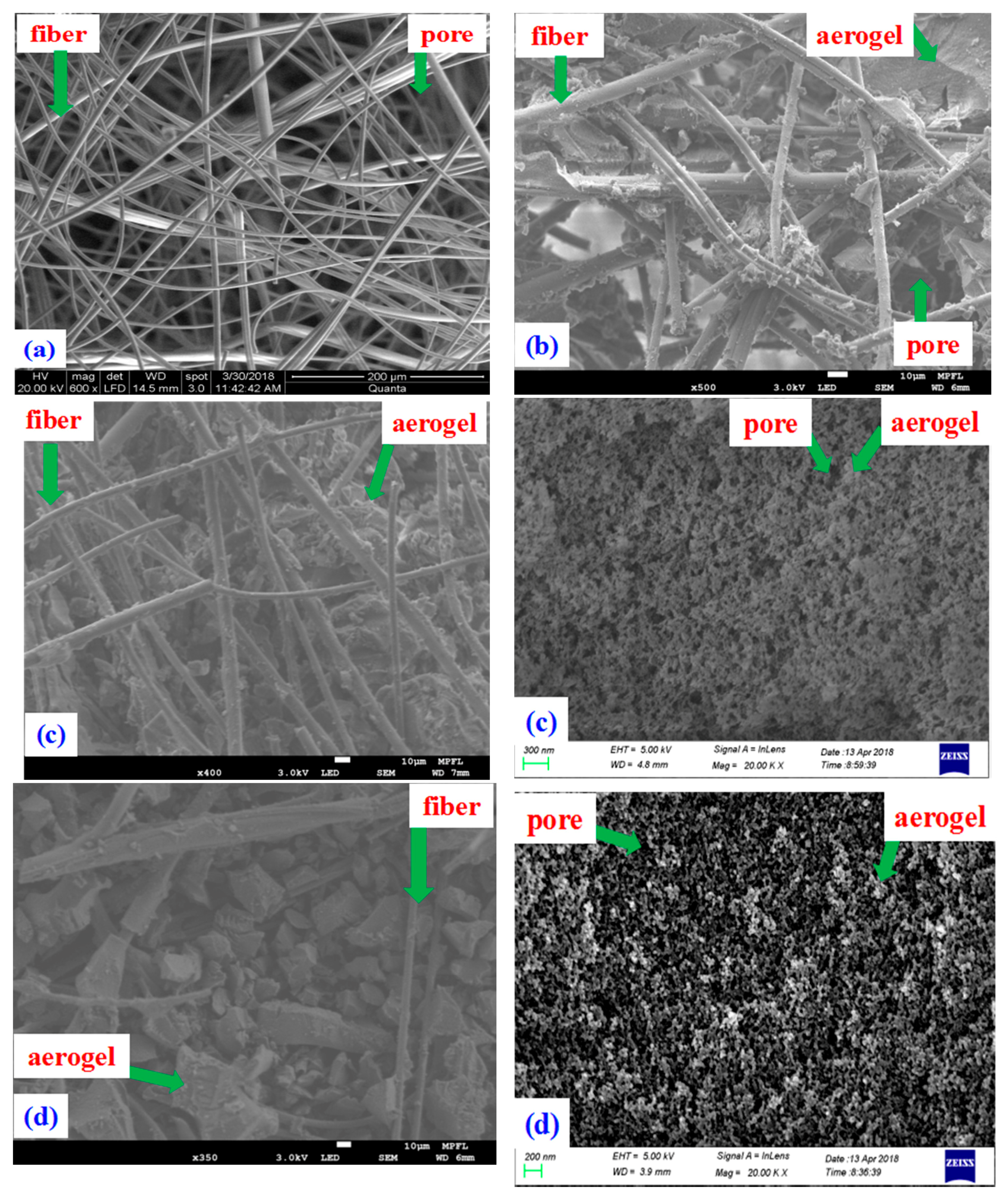
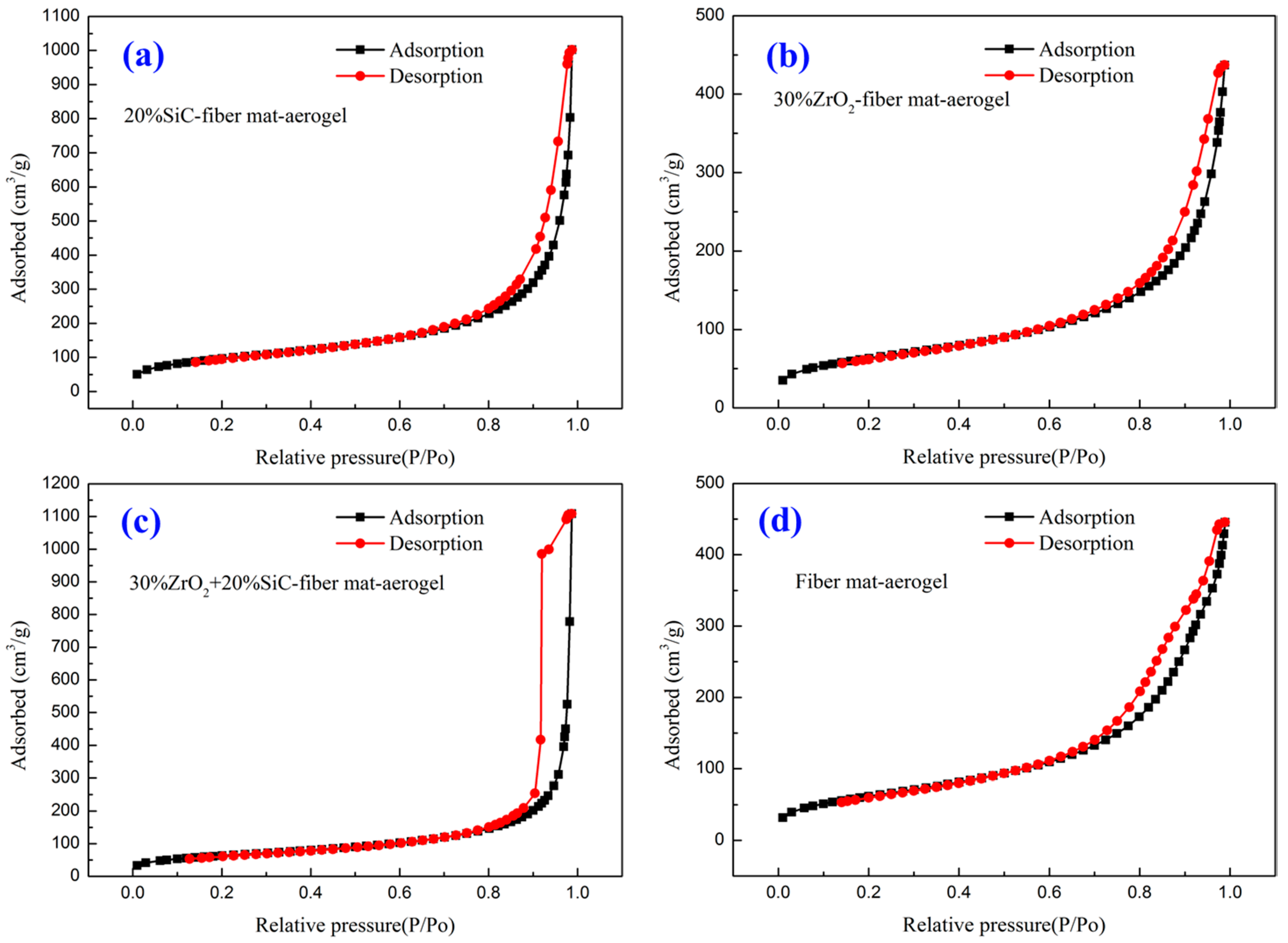
| Sample | Material | Density (kg/m3) | Porosity |
|---|---|---|---|
| 1 | 10% SiC-fiber mat-aerogel | 244.7 | 89.7 |
| 2 | 20% SiC-fiber mat-aerogel | 202.0 | 92.6 |
| 3 | 30% SiC-fiber mat-aerogel | 267.6 | 91.8 |
| 4 | 40% SiC-fiber mat-aerogel | 290.8 | 92.7 |
| 5 | 50% SiC-fiber mat-aerogel | 265.8 | 94.8 |
| 6 | 10% ZrO2-fiber mat-aerogel | 242.5 | 89.2 |
| 7 | 20% ZrO2-fiber mat-aerogel | 255.4 | 89.4 |
| 8 | 30% ZrO2-fiber mat-aerogel | 276.4 | 89.4 |
| 9 | 40% ZrO2-fiber mat-aerogel | 271.7 | 90.4 |
| 10 | 50% ZrO2-fiber mat-aerogel | 280.6 | 90.9 |
| 11 | 10% ZrO2 +20% SiC-fiber mat-aerogel | 277.6 | 88.6 |
| 12 | 20% ZrO2 + 20% SiC-fiber mat-aerogel | 255.4 | 89.6 |
| 13 | 30% ZrO2 + 20% SiC-fiber mat-aerogel | 263.8 | 90.4 |
| 14 | 40% ZrO2 + 20% SiC-fiber mat-aerogel | 256.0 | 91.4 |
| 15 | 50% ZrO2 + 20% SiC-fiber mat-aerogel | 284.7 | 91.9 |
| 16 | Fiber mat-aerogel | 262.9 | 87.5 |
| 17 | Pure fiber mat | 160.4 | 92.4 |
| Sample | N2 Adsorption-BET (m2/g) | N2 Adsorption-Langmuir (m2/g) | Hg Injection (m2/g) |
|---|---|---|---|
| 2 | 343.5 | 508.6 | 71.4 |
| 8 | 222.7 | 328.7 | 51.7 |
| 13 | 224.8 | 333.1 | 64.0 |
| 16 | 222.0 | 329.1 | 71.4 |
| 17 | 1.5 | 2.3 | 0.44 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, C.; Ji, W.; Wang, X.; Li, Y.; Tao, W. Experimental Characterization of the Thermal Conductivity and Microstructure of Opacifier-Fiber-Aerogel Composite. Molecules 2018, 23, 2198. https://doi.org/10.3390/molecules23092198
Zhang H, Zhang C, Ji W, Wang X, Li Y, Tao W. Experimental Characterization of the Thermal Conductivity and Microstructure of Opacifier-Fiber-Aerogel Composite. Molecules. 2018; 23(9):2198. https://doi.org/10.3390/molecules23092198
Chicago/Turabian StyleZhang, Hu, Chao Zhang, Wentao Ji, Xian Wang, Yueming Li, and Wenquan Tao. 2018. "Experimental Characterization of the Thermal Conductivity and Microstructure of Opacifier-Fiber-Aerogel Composite" Molecules 23, no. 9: 2198. https://doi.org/10.3390/molecules23092198
APA StyleZhang, H., Zhang, C., Ji, W., Wang, X., Li, Y., & Tao, W. (2018). Experimental Characterization of the Thermal Conductivity and Microstructure of Opacifier-Fiber-Aerogel Composite. Molecules, 23(9), 2198. https://doi.org/10.3390/molecules23092198






