Effects of Modified Processing Methods on Structural Changes of Black Soybean Protein Isolate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Changes of Modified Productions of BSPI
2.1.1. Determination of Amino Acid Composition of BSPI and Its Modification Products
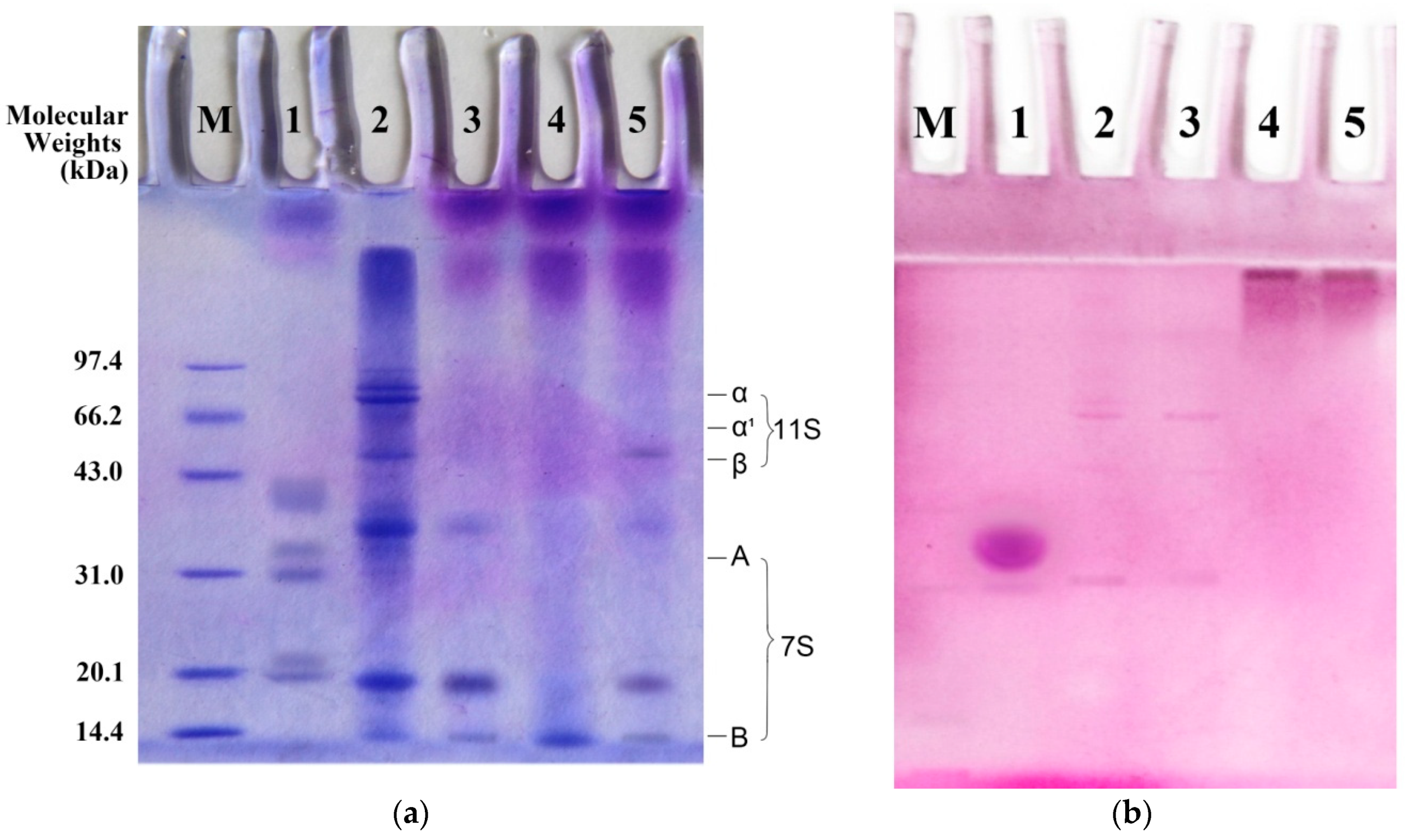
2.1.2. SDS-PAGE Analysis of BSPI and Its Modification Products
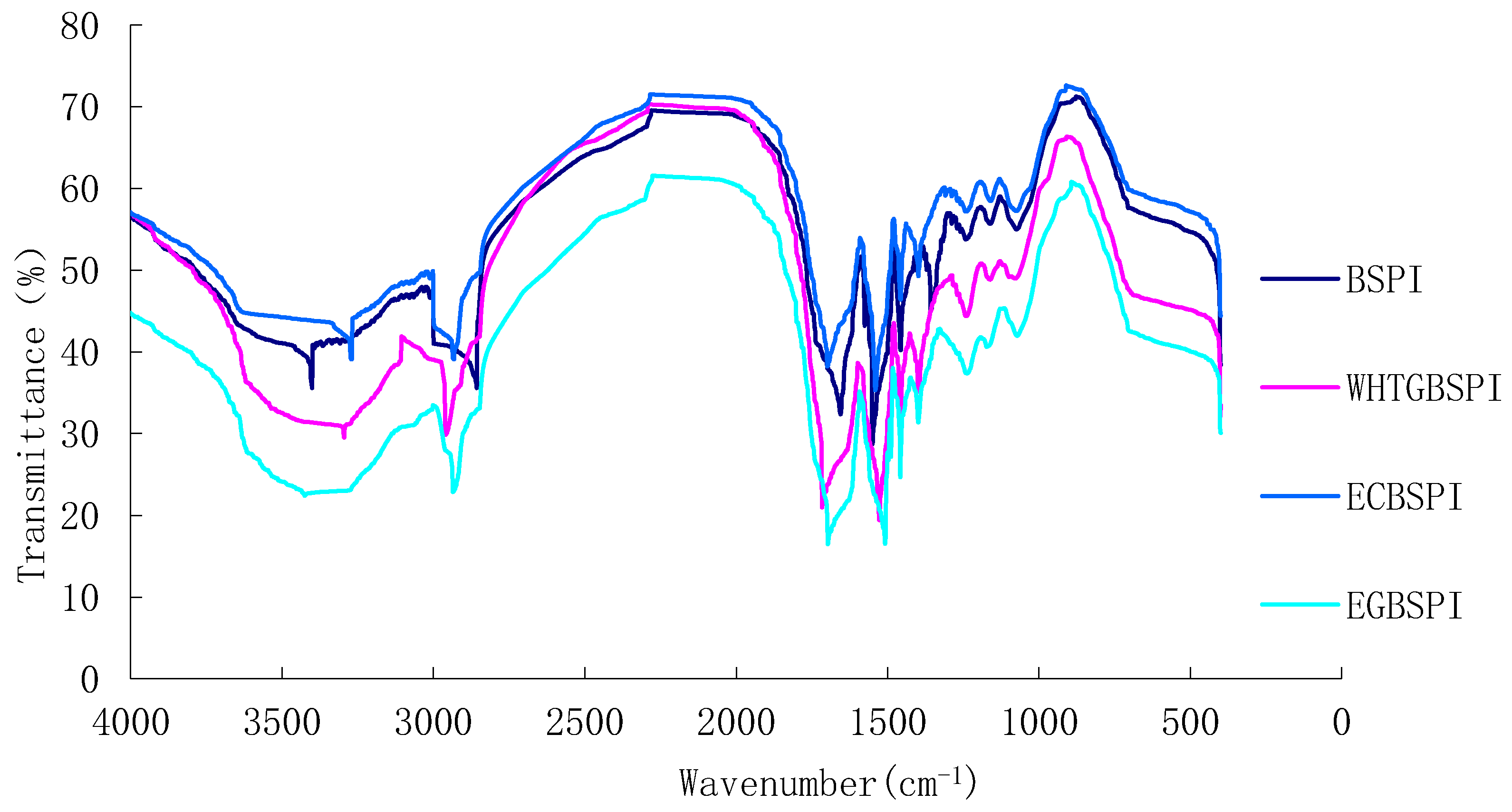
2.1.3. FT-IR Spectroscopy Analysis of BSPI and Its Modified Products
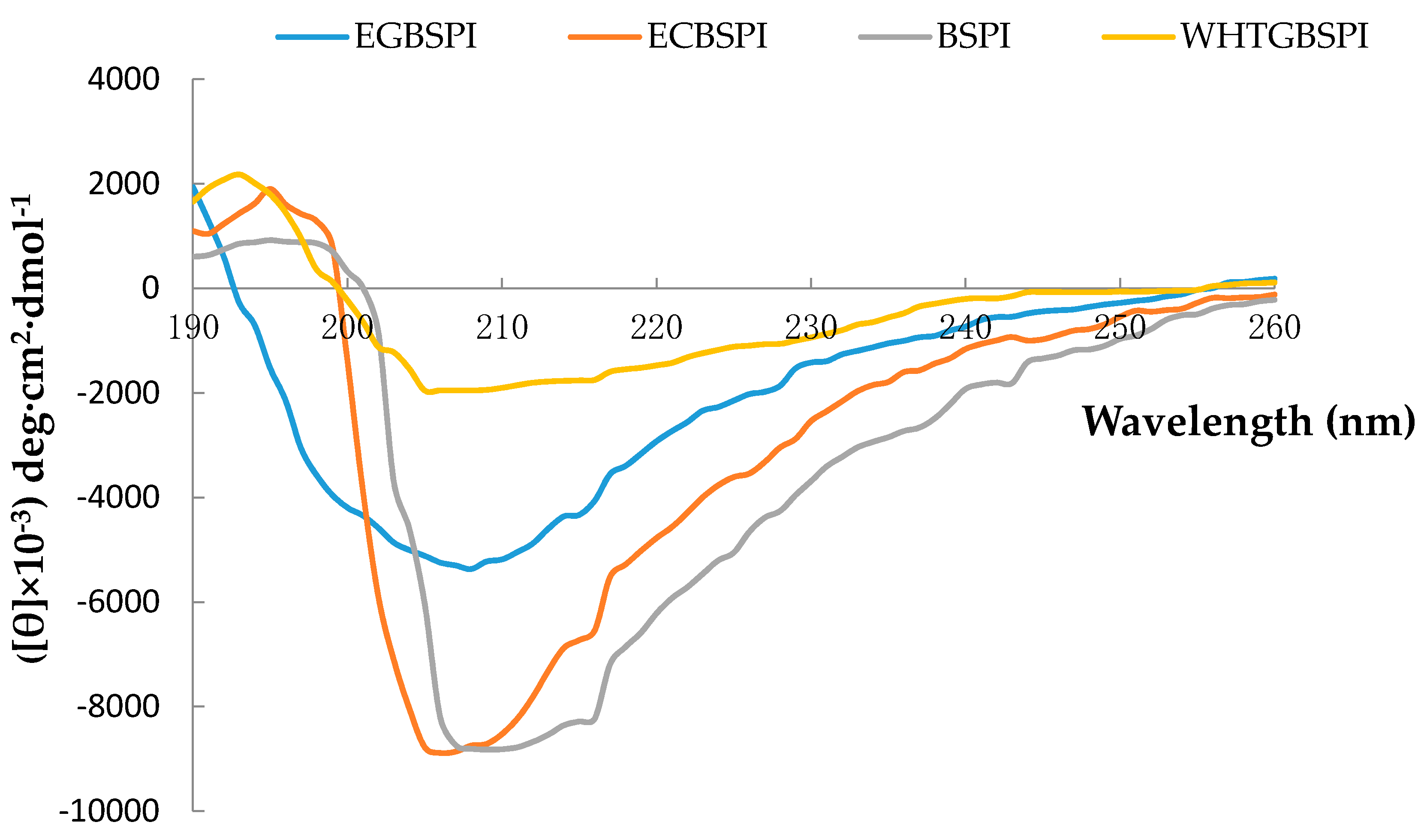
2.1.4. Circular Dichroism (CD) Spectroscopy Analysis of BSPI and Its Modified Products
2.2. Structural Changes Characterized by the Properties of BSPI Modified Products
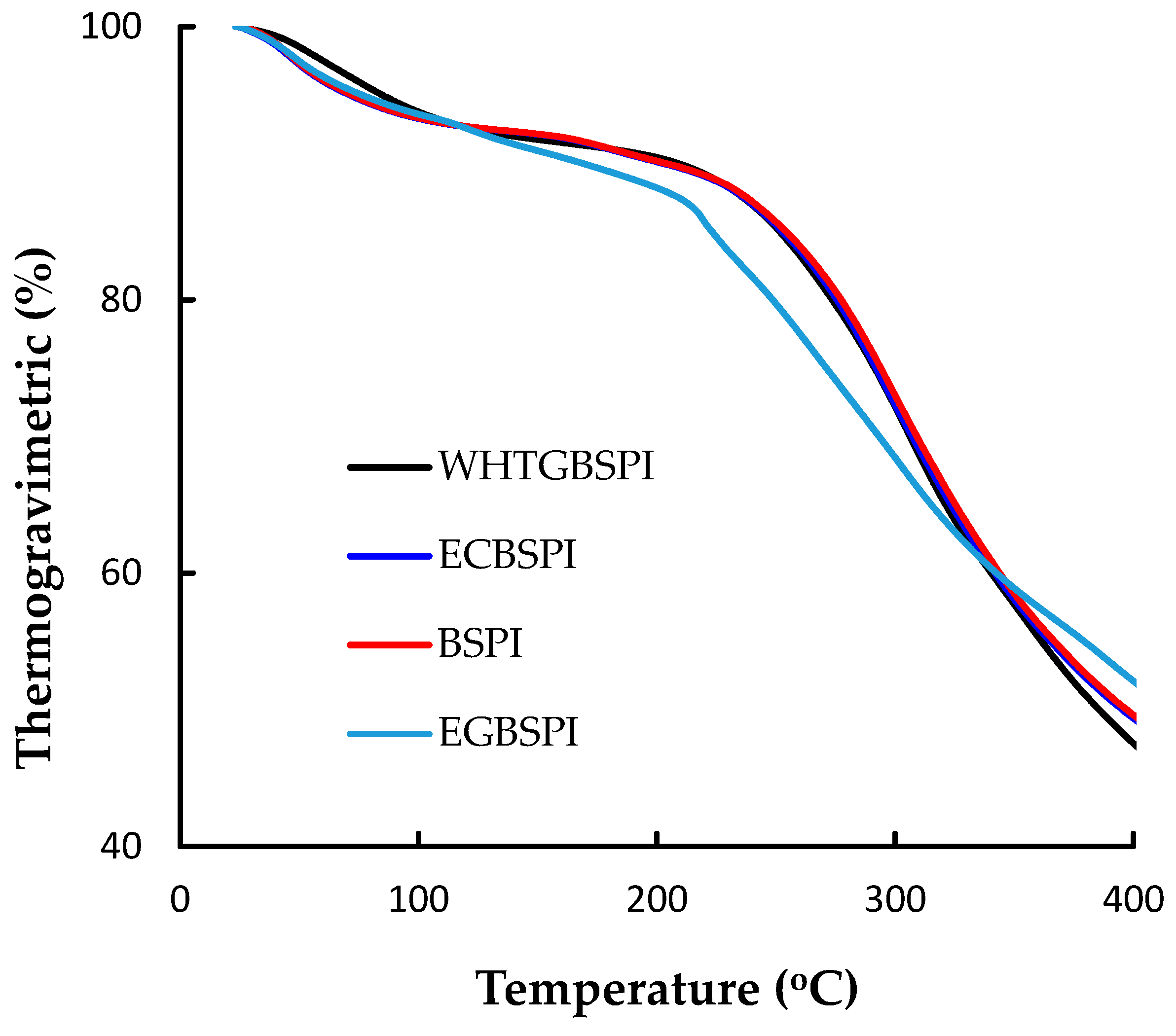
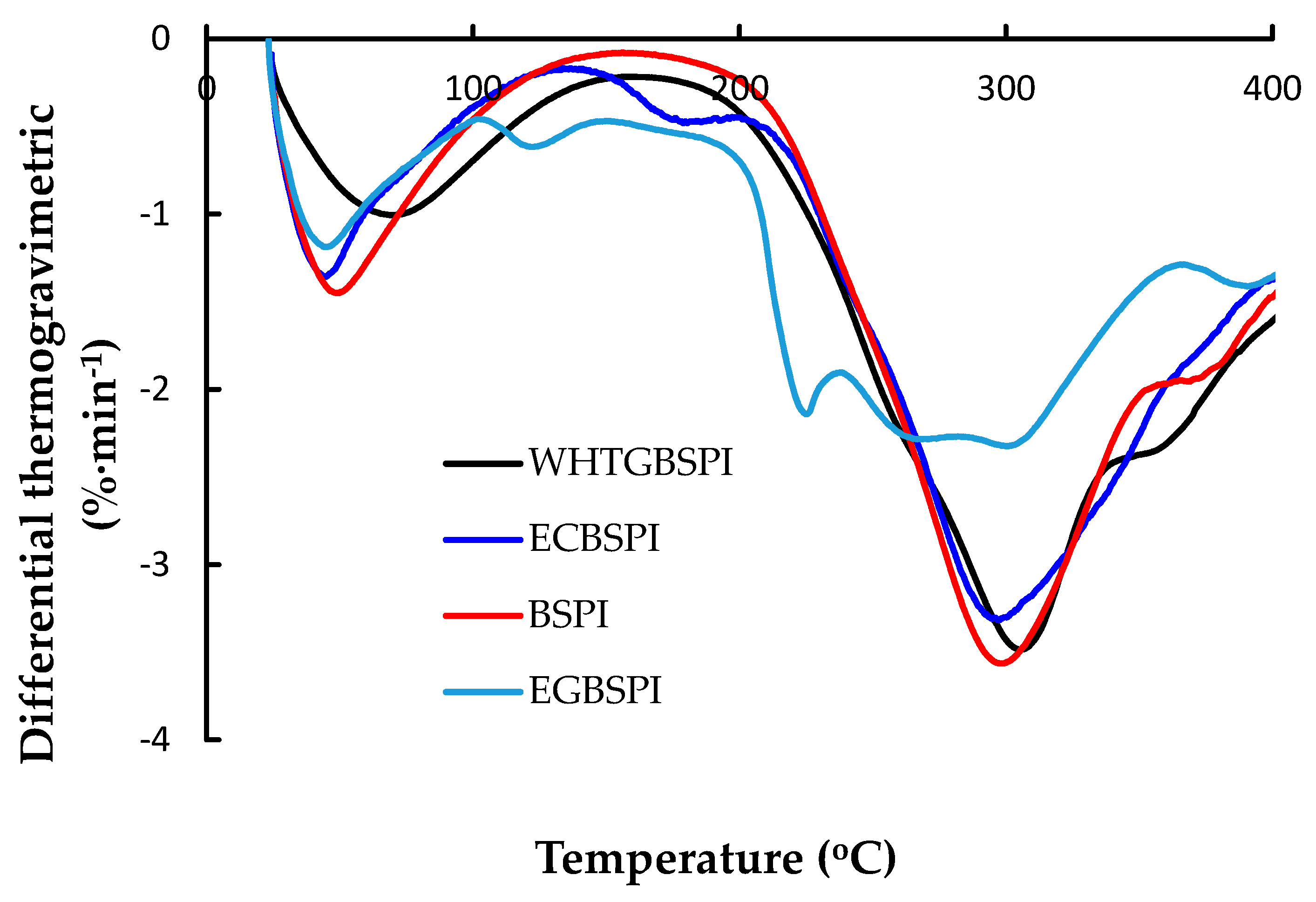
2.2.1. Thermogravimetric Analysis of BSPI and Its Modified Products
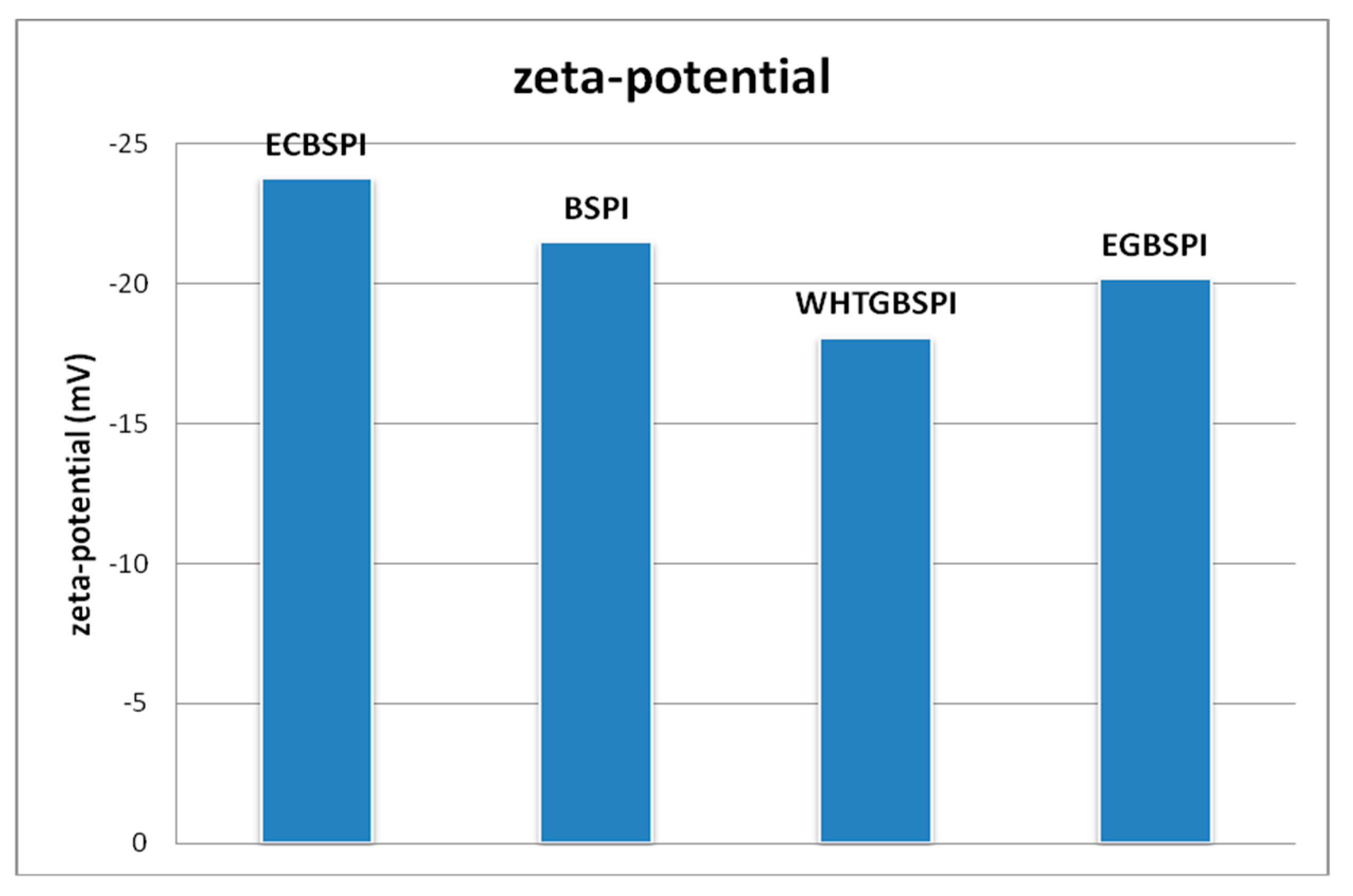
2.2.2. Zeta-Potential Analysis of BSPI and Its Modified Products
2.2.3. Surface Hydrophobicity Analysis of BSPI and Its Modified Products
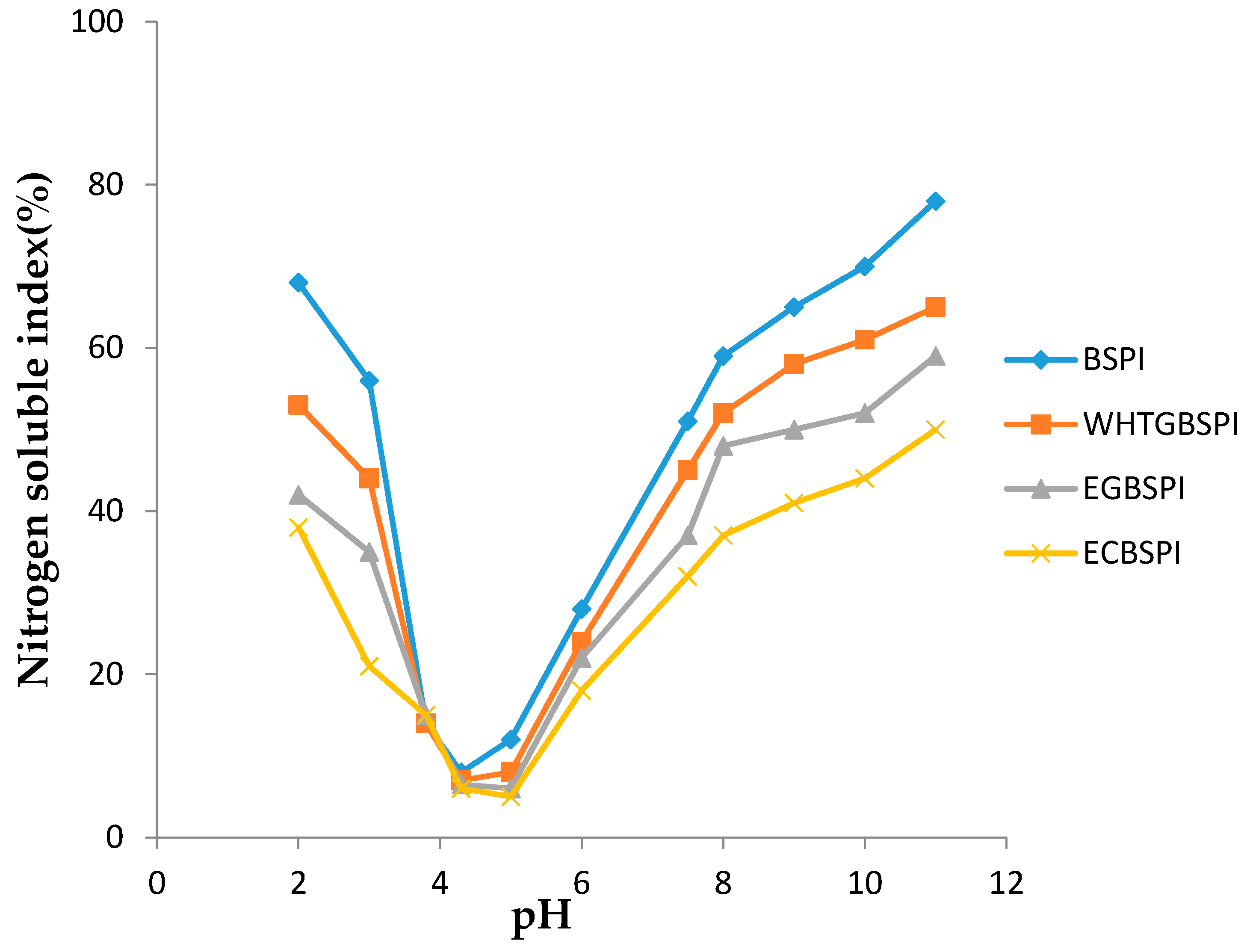
2.2.4. Solubility of BSPI and Its Modified Products
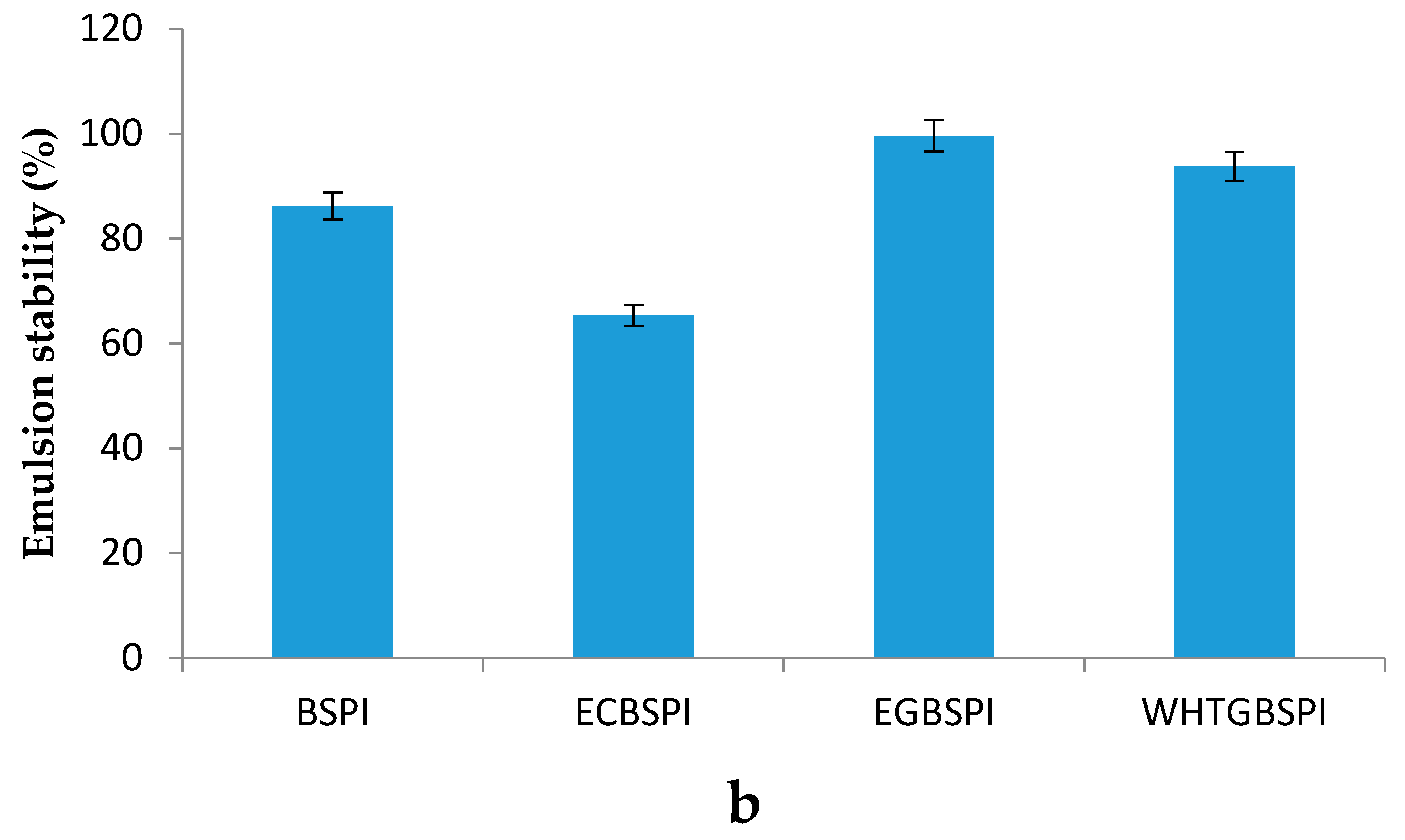
2.2.5. Emulsification and Emulsion Stability of BSPI and Its Modified Products
2.2.6. Gelation Properties of BSPI and Its Modified Products
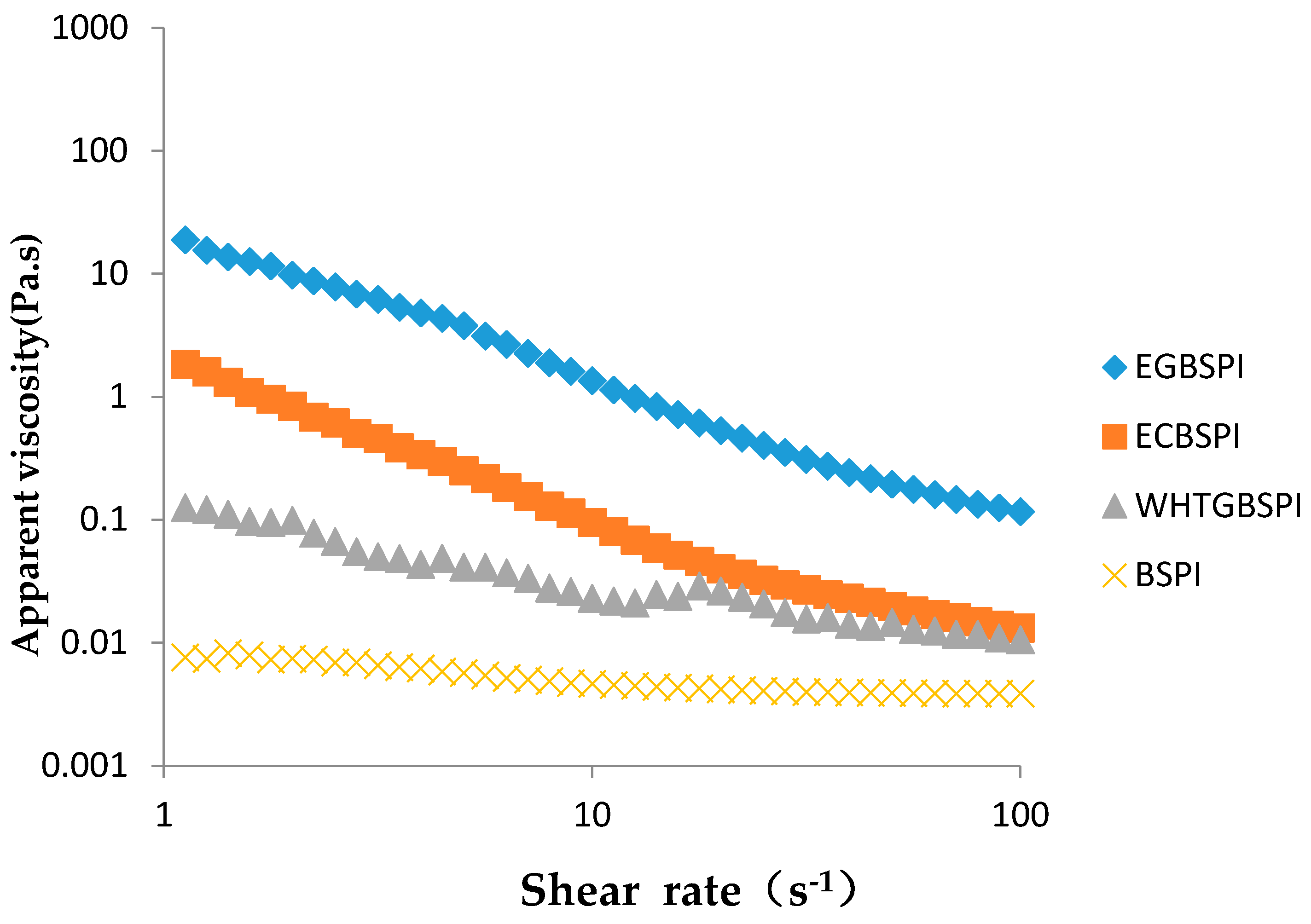
2.2.7. Rheological Property of BSPI and Its Modified Products
3. Materials and Methods
3.1. Materials
3.1.1. Materials
3.1.2. Reagents
3.1.3. Instruments
3.2. Preparation of BSPI and Its Modification Products
3.2.1. Preparation of Black Soybean Protein Isolated (BSPI) [18]
3.2.2. Preparation of Enzymatic Glycosylation Black Soybean Protein Isolated (EGBSPI)
3.2.3. Preparation of Enzymatic Crossing-Linked Black Soybean Protein Isolated (ECBSPI)
3.2.4. Preparation of Wet Heating Treatment Glycosylation Black Soybean Protein Isolated (WHTGBSPI)
3.3. Methods
3.3.1. Structure Changes of Modified Products of BSPI
- (1)
- SDS-PAGE analysis of BSPI and its modified products
- (2)
- Determination of amino acid content of BSPI and its modified products
- (3)
- Fourier transform infrared spectroscopy (FTIR) analysis of BSPI and its modified products
- (4)
- Circular dichroism analysis of BSPI and its modified products
3.3.2. Structure Changes Characterized by Properties of BSPI Modified Productions
- (1)
- Thermogravimetric analysis of BSPI and its modified products
- (2)
- The measurement of zeta-potential analysis of BSPI and its modified products
- (3)
- Surface hydrophobicity analysis of of BSPI and its modified products
- (4)
- Solubility of BSPI and its modified products
- (5)
- Emulsification and emulsion stability of BSPI and its modified products
- (6)
- Gelation of BSPI and its modified products
- (7)
- Rheological property of BSPI and its modified products
3.3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, B.; Chang, S.K. Characterization of phenolic substances and antioxidant properties of food soybeans grown in the north Dakota-Minnesota region. J. Agric. Food Chem. 2008, 56, 9102–9113. [Google Scholar] [CrossRef] [PubMed]
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes. Int. J. Mol. Sci. 2010, 11, 4973–4990. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.S.; Han, Y.S.; Lee, S.S. The effects of yellow soybean, black soybean, and sword bean on lipid levels and oxidative stress in ovariectomized rats. Int. J. Vitam. Nutr. Res. 2010, 80, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Mine, Y.; Ma, C.Y. Characterization of heat-induced aggregates of globulin from common buckwheat (Fagopyrum esculentum Moench). Int. J. Biol. Macromol. 2006, 39, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, F.; Ji, W.; Yokoyama, W.; Shoemaker, C.F.; Zhu, S.; Xia, W. Functional properties of maillard reaction products of rice protein hydrolysates with mono-, oligo- and polysaccharides. Food Hydrocoll. 2013, 30, 53–60. [Google Scholar] [CrossRef]
- Munch, G.; Apelt, J.; Rosemarie-Kientsch, E.; Stahl, P.; Luth, H.J.; Schliebs, R. Advanced glycation endproducts and pro-inflammatory cytokines in transgenic TG2576 mice with amyloid plaque pathology. J. Neurochem. 2003, 86, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.B.; Wu, N.N.; Yang, X.Q.; He, X.T.; Wang, L.J. Improvement of emulsifying properties of maillard reaction products from β-conglycinin and dextran using controlled enzymatic hydrolysis. Food Hydrocoll. 2012, 28, 301–312. [Google Scholar] [CrossRef]
- Lorand, L.; Conrad, S.M. Transglutaminases. Mol. Cell. Biochem. 1984, 58, 9–35. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.C.B.; Wold, F. Neoglycoproteins: In vitro introduction of glycosyl units at glutamines in beta-casein using transglutaminase. Biochemistry 1984, 23, 3759–3765. [Google Scholar] [CrossRef] [PubMed]
- Villalonga, R.; Fernández, M.; Fragoso, A.; Cao, R.; Mariniello, L.; Porta, R. Thermal stabilization of trypsin by enzymic modification with β-cyclodextrin derivatives. Biotechnol. Appl. Biochem. 2010, 38, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Wu, H.; Chen, Z.; Yang, X.Q. Formation and properties of glycinin-rich and β-conglycinin-rich soy protein isolate gels induced by microbial transglutaminase. Food Res. Int. 2006, 39, 87–97. [Google Scholar] [CrossRef]
- Boostani, S.; Aminlari, M.; Moosavi-nasab, M.; Niakosari, M.; Mesbahi, G. Fabrication and characterisation of soy protein isolate-grafted dextran biopolymer: A novel ingredient in spray-dried soy beverage formulation. Int. J. Biol. Macromol. 2017, 102, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Wooster, T.J.; Augustin, M.A. Rheology of whey protein–dextran conjugate films at the air/water interface. Food Hydrocoll. 2007, 21, 1072–1080. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, Q.; Hu, Z. Nanoscale understanding of thermal aggregation of whey protein pretreated by transglutaminase. J. Agric. Food Chem. 2013, 61, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Tang, C.H. Influence of glycation on microencapsulating properties of soy protein isolate-lactose blends. J. Sci. Food Agric. 2013, 93, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Hiller, B.; Lorenzen, P.C. Surface hydrophobicity of physicochemically and enzymatically treated milk proteins in relation to techno-functional properties. J. Agric. Food Chem. 2008, 56, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.Y.; Gu, R.Z.; Li, C.Y.; Ma, Y.; Dong, Z.; Liu, W.Y.; Jin, Z.T.; Lu, J.; Yi, W.X. Pilot-scale production of soybean oligopeptides and antioxidant and antihypertensive effects in vitro and in vivo. J. Food Sci. Technol. 2014, 51, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, S.; Anon, M.C. Relationship between the method of obtention and the structural and functional properties of soy protein isolates. 1. Structural and hydration properties. [Erratum to document cited in ca121:203669]. J. Agric. Food Chem. 1995, 43, 854. [Google Scholar] [CrossRef]
- Tang, S.; Hettiarachchy, N.S.; Shellhammer, T.H. Protein extraction from heat-stabilized defatted rice bran. 1. Physical processing and enzyme treatments. J. Agric. Food Chem. 2002, 50, 7444–7448. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.J.; Zhao, X.H. Cross-linking and glucosamine conjugation of casein by transglutaminase and the emulsifying property and digestibility in vitro of the modified product. Int. J. Food Prop. 2012, 15, 1286–1299. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.Y.; Zhao, X.H.; Yun, F.; Zhang, G.L. Enzymatic synthesis of chitosan–gelatin antimicrobial copolymer and its characterisation. J. Sci. Food Agric. 2010, 90, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Gómez, P.; Rosales-Rivera, A.; Rodríguez-García Mario, E. Effect of the thermo-alkaline treatment over the thermal degradation of corn starch. Starch-Stärke 2012, 64, 776–785. [Google Scholar] [CrossRef]
- Shigeru, H.; Shuryo, N. Relationships of hydrophobicity and net charge to the solubility of milk and soy proteins. J. Food Sci. 1985, 50, 486–491. [Google Scholar]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Campbell, L.J.; Gu, X.; Dewar, S.J.; Euston, S.R. Effects of heat treatment and glucono-δ-lactone-induced acidification on characteristics of soy protein isolate. Food Hydrocoll. 2009, 23, 344–351. [Google Scholar] [CrossRef]
- Song, C.L.; Zhao, X.H. Rheological, gelling and emulsifying properties of a glycosylated and cross-linked caseinate generated by transglutaminase. Int. J. Food Sci. Technol. 2013, 48, 2595–2602. [Google Scholar] [CrossRef]
Samples of the compounds are available from the authors. |

| Amino Acid | BSPI (%) | EGBSPI (%) | ECBSPI (%) | WHTGBSPI (%) |
|---|---|---|---|---|
| Asp | 8.4 ± 0.3 | 8.1 ± 0.2 | 8.7 ± 0.3 | 7.4 ± 0.2 |
| Thr | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.0 ± 0.1 |
| Ser | 3.5 ± 0.1 | 3.4 ± 0.1 | 3.7 ± 0.1 | 3.0 ± 0.1 |
| Glu | 16 ± 0.3 | 15 ± 0.4 | 16 ± 0.4 | 13 ± 0.5 |
| Gly | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.9 ± 0.1 | 2.4 ± 0.1 |
| Ala | 2.7 ± 0.1 | 2.6 ± 0.1 | 2.9 ± 0.1 | 2.3 ± 0.1 |
| Cys | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 1.1 ± 0.0 |
| Val | 2.5 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.1 | 2.2 ± 0.1 |
| Met | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.0 | 0.6 ± 0.0 |
| Ile | 2.5 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.1 | 2.1 ± 0.1 |
| Leu | 4.9 ± 0.2 | 4.8 ± 0.1 | 5.1 ± 0.2 | 4.2 ± 0.1 |
| Tyr | 2.2 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.1 | 1.8 ± 0.1 |
| Phe | 3.5 ± 0.1 | 3.4 ± 0.1 | 3.4 ± 0.1 | 2.9 ± 0.1 |
| His | 8.2 ± 0.2 | 8.8 ± 0.2 | 2.7 ± 0.1 | 6.8 ± 0.2 |
| Lys | 5.1 ± 0.1 | 4.5 ± 0.2 | 5.3 ± 0.2 | 4.3 ± 0.1 |
| Arg | 5.4 ± 0.1 | 4.9 ± 0.2 | 5.5 ± 0.2 | 4.5 ± 0.1 |
| Pro | 3.2 ± 0.1 | 2.8 ± 0.1 | 3.1 ± 0.1 | 2.4 ± 0.1 |
| Protein Type | BSPI | ECBSPI | EGBSPI | WHTGBSPI |
|---|---|---|---|---|
| Surface hydrophobicity | 16.8 ± 0.03 | 19.5 ± 0.06 | 5.07 ± 0.07 | 7.02 ± 0.05 |
| Textural Properties | BSPI | EGBSPI | ECBSPI | WHTGBSPI |
|---|---|---|---|---|
| Hardness (g) | 167 ± 4.4 b | 148 ± 5.6 b | 380 ± 16.9 c | 71.4 ± 2.95 a |
| Adhesiveness (g·s) | 233 ± 11.4 a | 235 ± 10.7 a | 351 ± 13.0 b | 505 ± 21.8 c |
| Springness | 0.784 ± 0.039 a | 0.764 ± 0.034 a | 0.864 ± 0.039 b | 0.943 ± 0.046 c |
| Cohesiveness | 0.413 ± 0.016 c | 0.455 ± 0.018 c | 0.285 ± 0.013 b | 0.158 ± 0.006 a |
| Guminess | 69.1 ± 3.54 b | 67.2 ± 4.63 b | 108.3 ± 3.15 c | 11.3 ± 0.43 a |
| Chewness | 54.2 ± 1.37 b | 59.0 ± 2.95 b | 93.6 ± 3.56 c | 10.6 ± 1.63 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yin, Y.; Lu, S.; Yao, X.; Zheng, X.; Zhao, R.; Li, Z.; Shen, H.; Zhang, S. Effects of Modified Processing Methods on Structural Changes of Black Soybean Protein Isolate. Molecules 2018, 23, 2127. https://doi.org/10.3390/molecules23092127
Zhang Y, Yin Y, Lu S, Yao X, Zheng X, Zhao R, Li Z, Shen H, Zhang S. Effects of Modified Processing Methods on Structural Changes of Black Soybean Protein Isolate. Molecules. 2018; 23(9):2127. https://doi.org/10.3390/molecules23092127
Chicago/Turabian StyleZhang, Yinglei, Yanyang Yin, Shuwen Lu, Xinmiao Yao, Xianzhe Zheng, Rui Zhao, Zhebin Li, Huifang Shen, and Shouwen Zhang. 2018. "Effects of Modified Processing Methods on Structural Changes of Black Soybean Protein Isolate" Molecules 23, no. 9: 2127. https://doi.org/10.3390/molecules23092127
APA StyleZhang, Y., Yin, Y., Lu, S., Yao, X., Zheng, X., Zhao, R., Li, Z., Shen, H., & Zhang, S. (2018). Effects of Modified Processing Methods on Structural Changes of Black Soybean Protein Isolate. Molecules, 23(9), 2127. https://doi.org/10.3390/molecules23092127




