UPLC-QTOF/MS-Based Metabolomics Applied for the Quality Evaluation of Four Processed Panax ginseng Products
Abstract
1. Introduction
2. Results
2.1. Metabolite Profiling of Processed Ginseng Products by UPLC-QTOF/MS
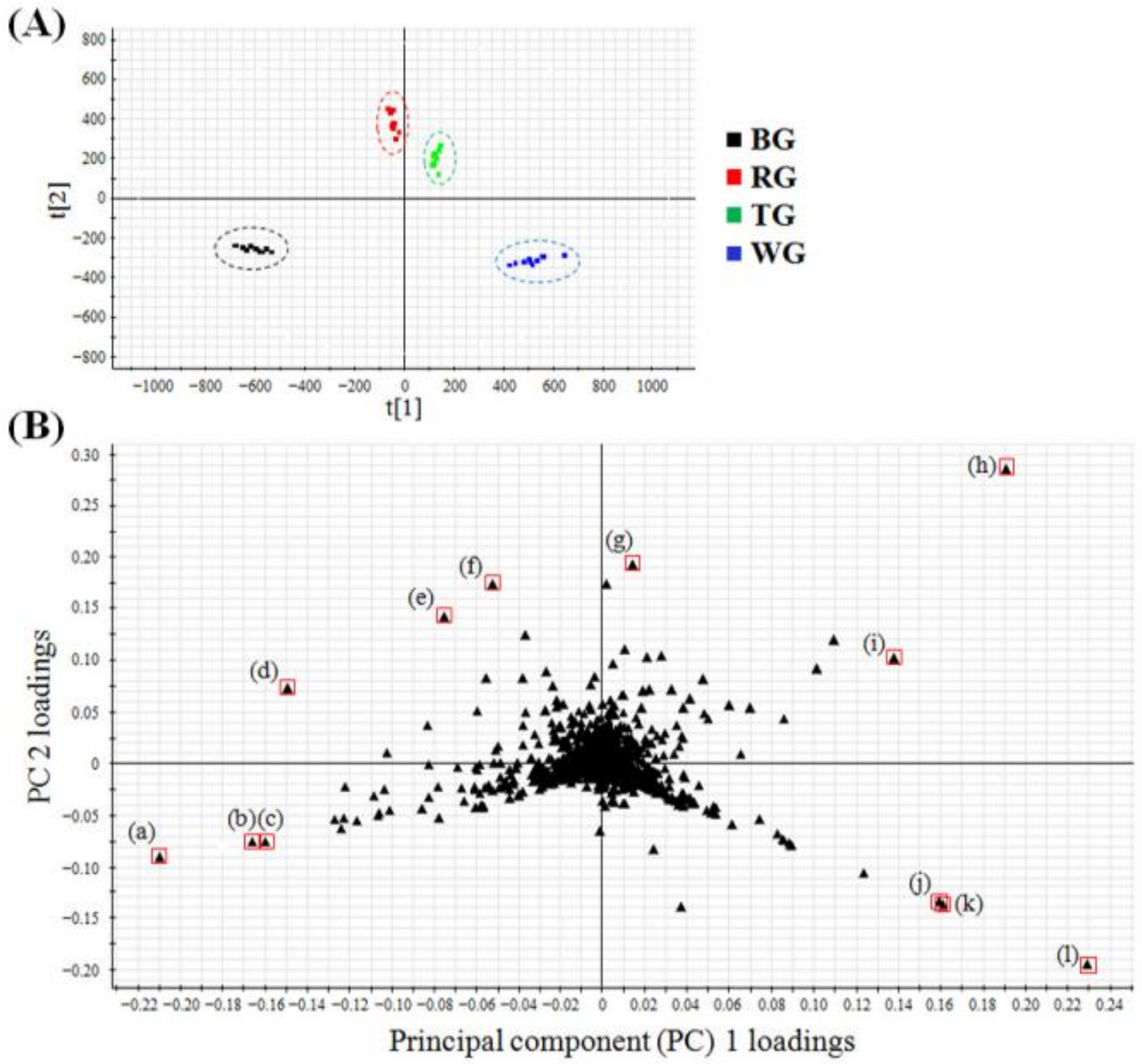
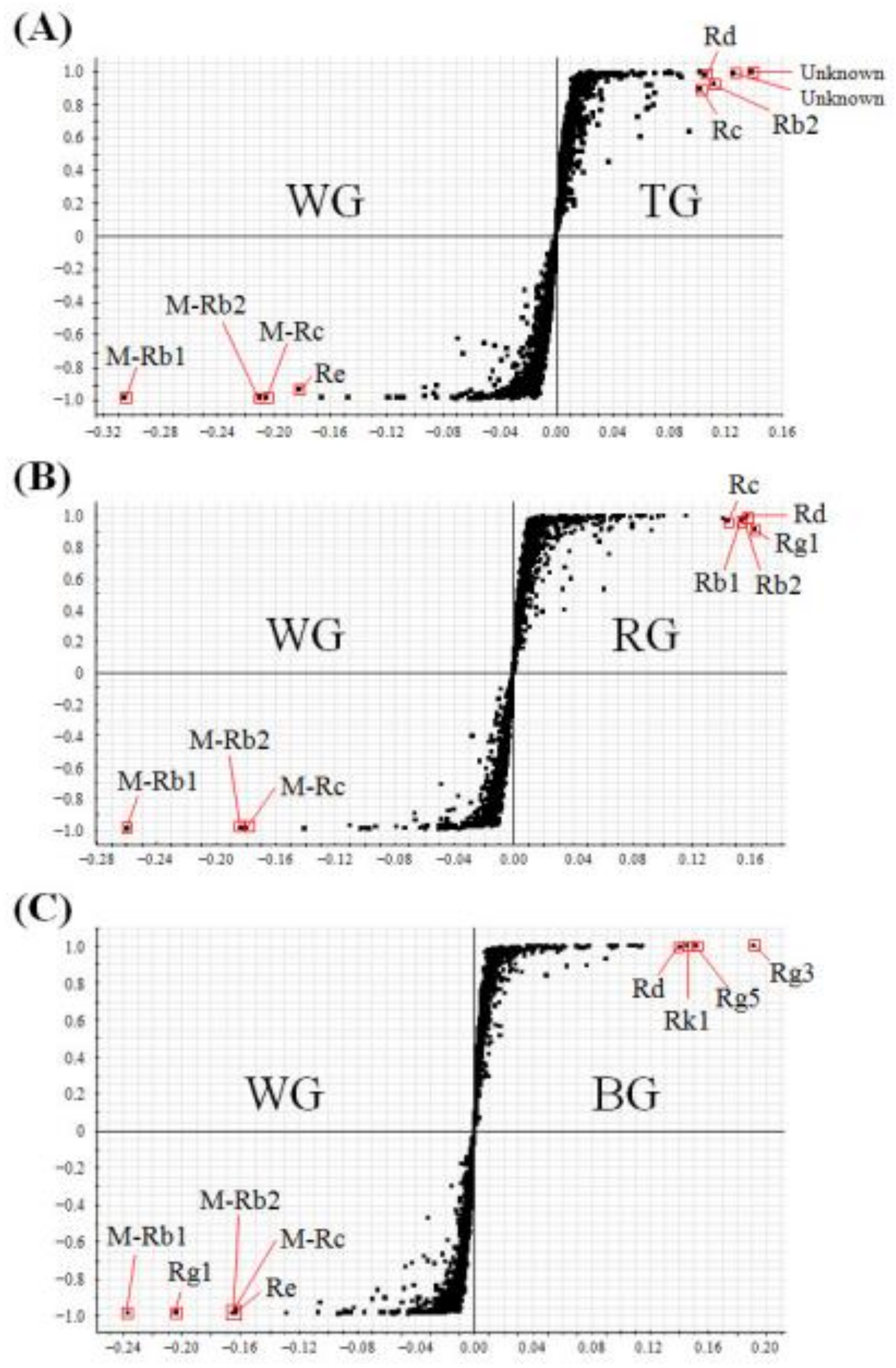
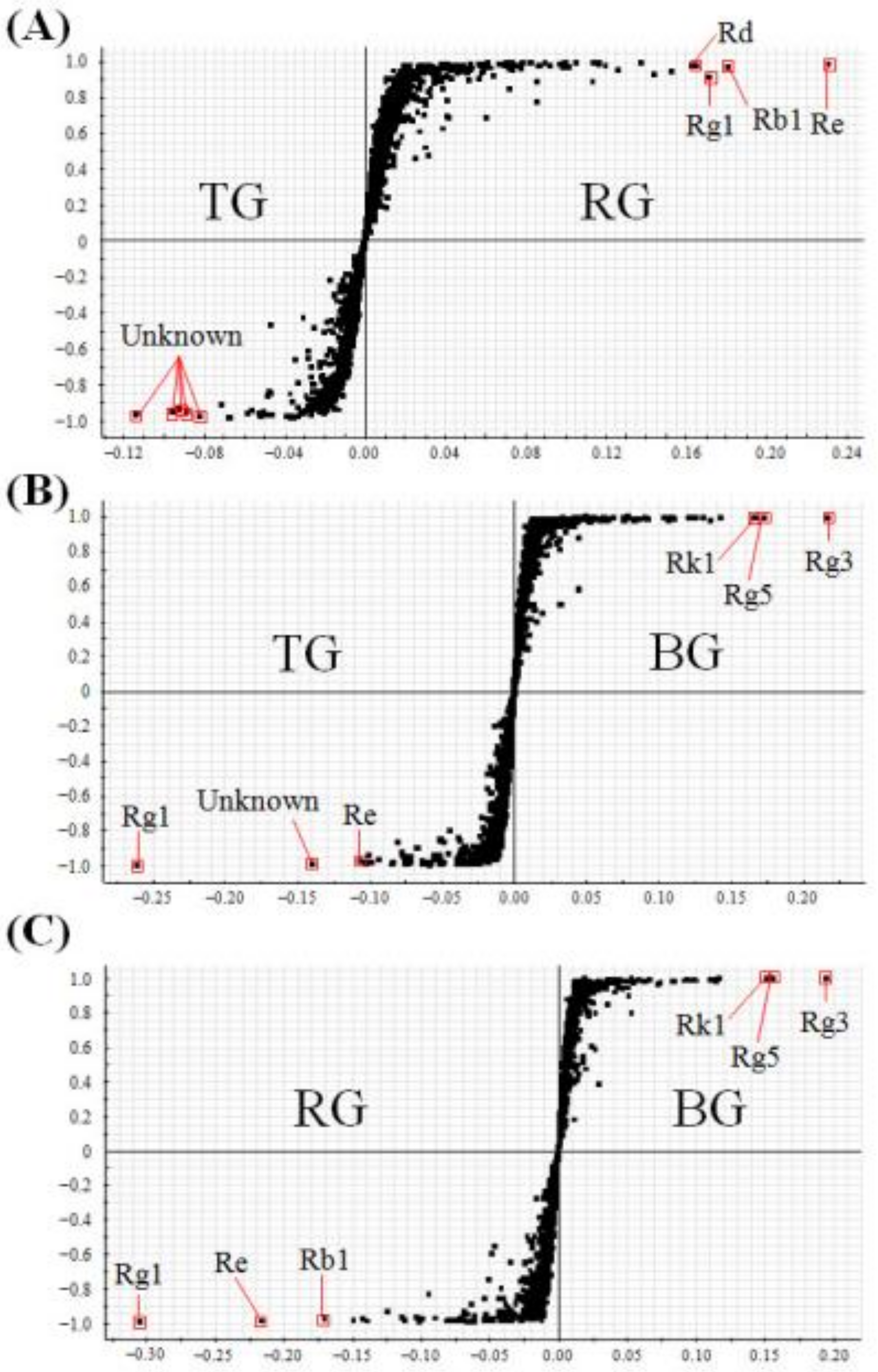
2.2. Multivariate Analysis to Differentiate Four Processed Ginseng Products
2.3. Quantification of Various Ginsenosides from Four Processed ginseng Products
3. Discussion
4. Materials and Methods
4.1. Standard Constituents and Reagents
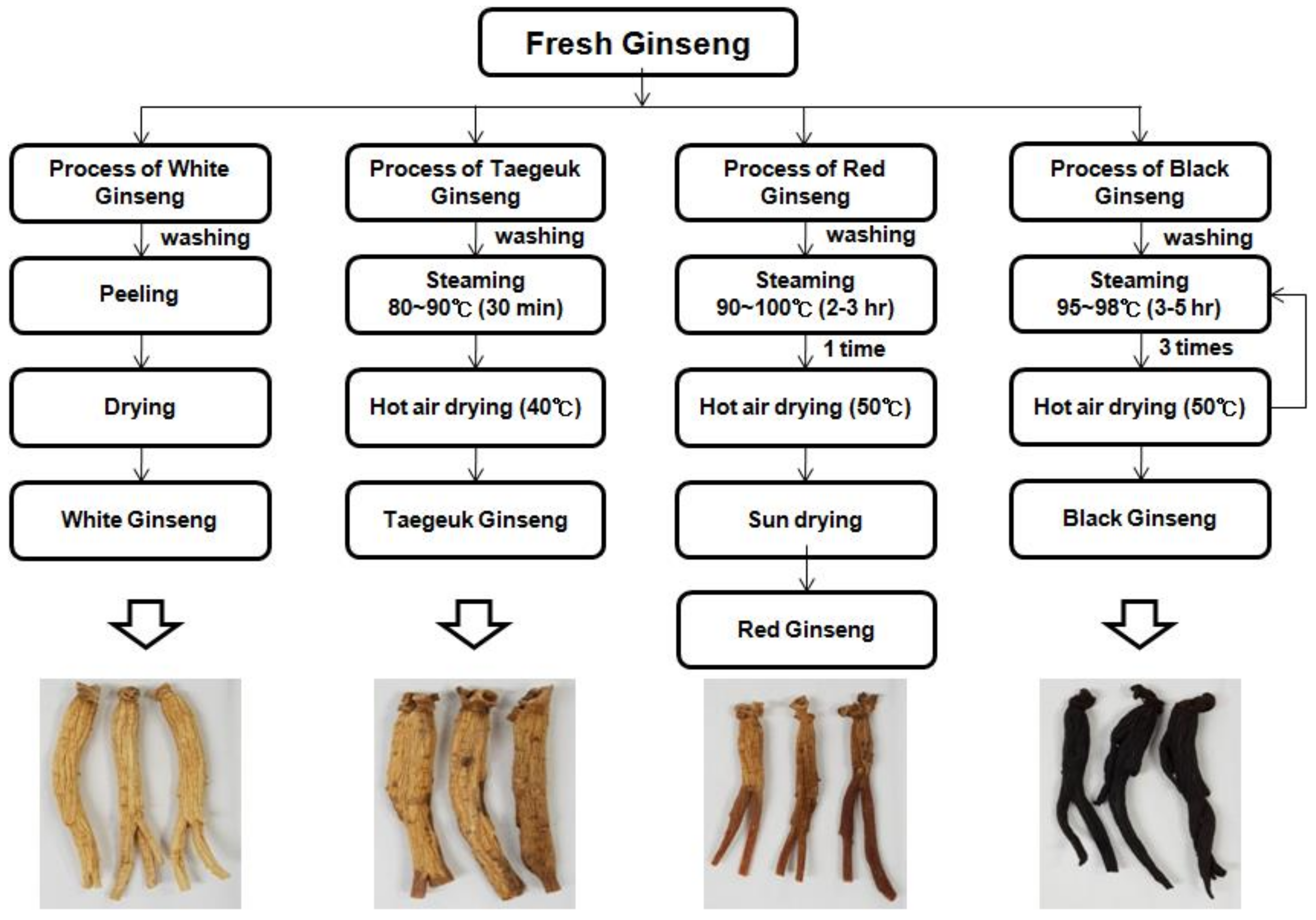
4.2. Processed Panax ginseng Samples
4.3. Sample Preparation
4.4. UPLC-QTOF/MS Conditions
4.5. Data Processing and Multivariate Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| UPLC | Ultra-performance liquid chromatography |
| QTOF | Quadrupole time of flight |
| ESI | Electrospray ionization |
| MS | Mass spectrometry |
| LOD | Limits of detection |
| RT | Retention time |
References
- Jang, H.-J.; Han, I.-H.; Kim, Y.-J.; Yamabe, N.; Lee, D.; Hwang, G.S.; Oh, M.; Choi, K.-C.; Kim, S.-N.; Ham, J. Anticarcinogenic effects of products of heat-processed ginsenoside Re, a major constituent of ginseng berry, on human gastric cancer cells. J. Agric. Food Chem. 2014, 62, 2830–2836. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, Y.Z.; Jin, X.; Kim, Y.K.; Uddin, M.R.; Kim, Y.B.; Bae, H.; Kim, Y.C.; Lee, S.W.; Park, S.U. Ginsenoside content in the leaves and roots of Panax ginseng at different ages. Life Sci. J. 2012, 9, 670–683. [Google Scholar]
- Lee, S.-R.; Kim, M.-R.; Yon, J.-M.; Baek, I.-J.; Park, C.G.; Lee, B.J.; Yun, Y.W.; Nam, S.-Y. Black ginseng inhibits ethanol-induced teratogenesis in cultured mouse embryos through its effects on antioxidant activity. Toxicol. In Vitro 2009, 23, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Han, C. Suitable drying model for far infrared drying of taegeuk ginseng. Food Sci. Biotechnol. 2012, 21, 1087–1094. [Google Scholar] [CrossRef]
- Ning, X.; Han, C. Drying characteristics and quality of taegeuk ginseng (Panax ginseng CA Meyer) using far-infrared rays. Int. J. Food Sci. Technol. 2013, 48, 477–483. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Li, S.-L.; Zhang, H.; Wang, Y.; Zhao, Z.-L.; Chen, S.-L.; Xu, H.-X. Holistic quality evaluation of commercial white and red ginseng using a UPLC-QTOF-MS/MS-based metabolomics approach. J. Pharm. Biomed. Anal. 2012, 62, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.-S.; Gu, L.-J.; Fang, Z.-M.; Wang, C.-Y.; Wang, Z.; Lee, M.-R.; Li, Z.; Li, J.-J.; Sung, C.-K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC–ELSD. J. Pharm. Biomed. Anal. 2009, 50, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.-S.; Gu, L.-J.; Fang, Z.-M.; Wang, C.Y.; Wang, Z.; Sung, C.-K. Determination of 11 ginsenosides in black ginseng developed from Panax ginseng by high performance liquid chromatography. Food Sci. Biotechnol. 2009, 18, 561–564. [Google Scholar]
- Lee, H.; Park, D.; Yoon, M. Korean red ginseng (Panax ginseng) prevents obesity by inhibiting angiogenesis in high fat diet-induced obese C57BL/6J mice. Food Chem. Toxicol. 2013, 53, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Luo, D.; Cheng, Y.; Ma, J.-F.; Wang, Y.; Liang, Q.; Luo, G. Steaming-induced chemical transformations and holistic quality assessment of red ginseng derived from Panax ginseng by means of HPLC-ESI-MS/MS n-based multicomponent quantification fingerprint. J. Agric. Food Chem. 2012, 60, 8213–8224. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Jo, H.K.; Im, B.O.; Kim, S.; Whang, W.K.; Ko, S.K. Changes in the contents of prosapogenin in the red ginseng (Panax ginseng) depending on steaming batches. J. Ginseng Res. 2012, 36, 102. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Kim, J.-W.; Seguin, P.; Jun, Y.-M.; Kim, S.-H. Ginsenosides and phenolics in fresh and processed Korean ginseng (Panax ginseng CA Meyer): Effects of cultivation location, year, and storage period. Food Chem. 2012, 130, 73–83. [Google Scholar] [CrossRef]
- Kim, S.N.; Ha, Y.W.; Shin, H.; Son, S.H.; Wu, S.J.; Kim, Y.S. Simultaneous quantification of 14 ginsenosides in Panax ginseng CA Meyer (Korean red ginseng) by HPLC-ELSD and its application to quality control. J. Pharm. Biomed. Anal. 2007, 45, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-Y.; Park, E.-Y.; Kim, H.-J.; Park, S.-U.; Nam, K.-Y.; Choi, J.-E.; Jun, H.-S. Effect of white, taegeuk, and red ginseng root extracts on insulin-stimulated glucose uptake in muscle cells and proliferation of β-cells. J. Ginseng Res. 2010, 34, 192–197. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, A.K. Anti-breast cancer activity of Fine Black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. J. Ginseng Res. 2015, 39, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-T.; Mehendale, S.R.; Li, X.; Quigg, R.; Wang, X.; Wang, C.-Z.; Wu, J.A.; Aung, H.H.; Rue, P.A.; Bell, G.I. Anti-diabetic effect of ginsenoside Re in ob/ob mice. Biochim Biophys. Acta Mol. Basis Dis. 2005, 1740, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.Y.K.; Mak, N.K.; Cheng, Y.K.; Leung, K.W.; Ng, T.B.; Fan, D.T.P.; Yeung, H.W.; Wong, R.N.S. Pharmacogenomics and the Yin/Yang actions of ginseng: Anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin. Med. 2007, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.-H.; Xie, J.-T.; Hoek, T.L.V.; Mehendale, S.; Aung, H.; Li, C.-Q.; Qin, Y.; Schumacker, P.T.; Becker, L.B.; Yuan, C.-S. Antioxidant effects of American ginseng berry extract in cardiomyocytes exposed to acute oxidant stress. Biochim. Biophys. Acta General Subj. 2004, 1670, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.-K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-Z.; Hu, Y.; Wu, W.-Y.; Ye, M.; Guo, D.-A. Saponins in the genus Panax L.(Araliaceae): A systematic review of their chemical diversity. Phytochemistry 2014, 106, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, O.; Uto, T.; Yuan, C.-S.; Tanaka, H.; Shoyama, Y. Evaluation of a new eastern blotting technique for the analysis of ginsenoside Re in American ginseng berry pulp extracts. Fitoterapia 2010, 81, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Wu, J.A.; McEntee, E.; Yuan, C.-S. Saponins composition in American ginseng leaf and berry assayed by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Van Le, T.H.; Lee, S.Y.; Lee, G.J.; Nguyen, N.K.; Park, J.H.; Nguyen, M.D. Effects of steaming on saponin compositions and antiproliferative activity of Vietnamese ginseng. J. Ginseng Res. 2015, 39, 274–278. [Google Scholar]
- Kim, S.; Shin, B.-K.; Lim, D.K.; Yang, T.-J.; Lim, J.; Park, J.H.; Kwon, S.W. Expeditious discrimination of four species of the Panax genus using direct infusion-MS/MS combined with multivariate statistical analysis. J. Chromatogr. B 2015, 1002, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, J.Y.; Xiao, X.Y.; Lin, R.C.; Cheng, Y.Y. Simultaneous determination of ginsenosides in Panax ginseng with different growth ages using high-performance liquid chromatography–mass spectrometry. Phytochem. Anal. 2006, 17, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Bai, M.; Xu, J.-D.; Kong, M.; Zhu, L.-Y.; Zhu, H.; Wang, Q.; Li, S.-L. Discrimination of leaves of Panax ginseng and P. quinquefolius by ultra high performance liquid chromatography quadrupole/time-of-flight mass spectrometry based metabolomics approach. J. Pharm. Biomed. Anal. 2014, 97, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-H.; Moon, J.Y.; Ryu, H.W.; Noh, B.-S.; Kim, J.-H.; Lee, H.-K.; Oh, S.-R. Discrimination of white ginseng origins using multivariate statistical analysis of data sets. J. Ginseng Res. 2014, 38, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.X.; Ni, Y.; Su, M.M.; Zhang, Y.Y.; Zhao, A.H.; Gao, X.F.; Liu, Z.; Xiao, P.G.; Jia, W. Application of ultra-performance LC-TOF MS metabolite profiling techniques to the analysis of medicinal Panax herbs. Metabolomics 2008, 4, 248–260. [Google Scholar] [CrossRef]
- Wan, J.-Y.; Liu, P.; Wang, H.-Y.; Qi, L.-W.; Wang, C.-Z.; Li, P.; Yuan, C.-S. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2013, 1286, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-W.; In, G.; Kim, J.-H.; Cho, B.-G.; Han, G.-H.; Chang, I.-M. Metabolomic approach for discrimination of processed ginseng genus (Panax ginseng and Panax quinquefolius) using UPLC-QTOF MS. J. Ginseng Res. 2014, 38, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, B.-R.; Kim, Y.-C.; Choi, D.J.; Lee, Y.-S.; Kim, G.-S.; Baek, N.-I.; Kim, S.-Y.; Lee, D.Y. Comprehensive Profiling and Quantification of Ginsenosides in the Root, Stem, Leaf, and Berry of Panax ginseng by UPLC-QTOF/MS. Molecules 2017, 22, 2147. [Google Scholar] [CrossRef] [PubMed]
- Kenarova, B.; Neychev, H.; Hadjiivanova, C.; Petkov, V.D. Immunomodulating activity of ginsenoside Rg1 from Panax ginseng. Jpn. J. Pharmacol. 1990, 54, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Zhou, Y.-P.; Xie, J.-T.; Wu, J.A.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.A.; Polonsky, K.S.; Yuan, C.-S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-K.; Cho, D.H.; Lee, K.-S.; Lee, D.-K.; Park, C.-W.; Kim, W.G.; Lee, S.J.; Ha, K.-S.; Goo Taeg, O.; Kwon, Y.-G. Ginseng berry extract prevents atherogenesis via anti-inflammatory action by upregulating phase II gene expression. Evid.-Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.-M.; Luo, J.-G.; Huang, F.; Kong, L.-Y. Chemical characteristics combined with bioactivity for comprehensive evaluation of Panax ginseng CA Meyer in different ages and seasons based on HPLC-DAD and chemometric methods. J. Pharm. Biomed. Anal. 2014, 89, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yuan, Z.; Sun, Y.; Bu, Y.; Li, W.; Fei, Z. Ginsenoside Rg3 enhances the anti-proliferative activity of erlotinib in pancreatic cancer cell lines by downregulation of EGFR/PI3K/Akt signaling pathway. Biomed. Pharmacother. 2017, 96, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Junmin, S.; Hongxiang, L.; Zhen, L.; Chao, Y.; Chaojie, W. Ginsenoside Rg3 inhibits colon cancer cell migration by suppressing nuclear factor kappa B activity. J. Tradit. Chin. Med. 2015, 35, 440–444. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, Y.Y.; Kim, S.Y.; Pyo, J.S.; Yun-Choi, H.S.; Park, J.H. Platelet antiaggregating activity of ginsenosides isolated from processed ginseng. Die Pharm. Int. J. Pharmacol. Sci. 2009, 64, 602–604. [Google Scholar]
- Kim, J.H.; Yi, Y.-S.; Kim, M.-Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, M.W.; Yuan, H.D.; Lee, H.J.; Kim, S.H.; Chung, S.H. Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. J. Agric. Food Chem. 2009, 57, 10573–10578. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Shin, J.A.; Jung, J.-S.; Hyun, J.-W.; Van Le, T.K.; Kim, D.-H.; Park, E.-M.; Kim, H.-S. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J. Pharmacol. Exp. Ther. 2012, 341, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Bae, E.A.; Han, M.J.; Kim, N.J.; Kim, D.H. Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int. 2005, 25, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
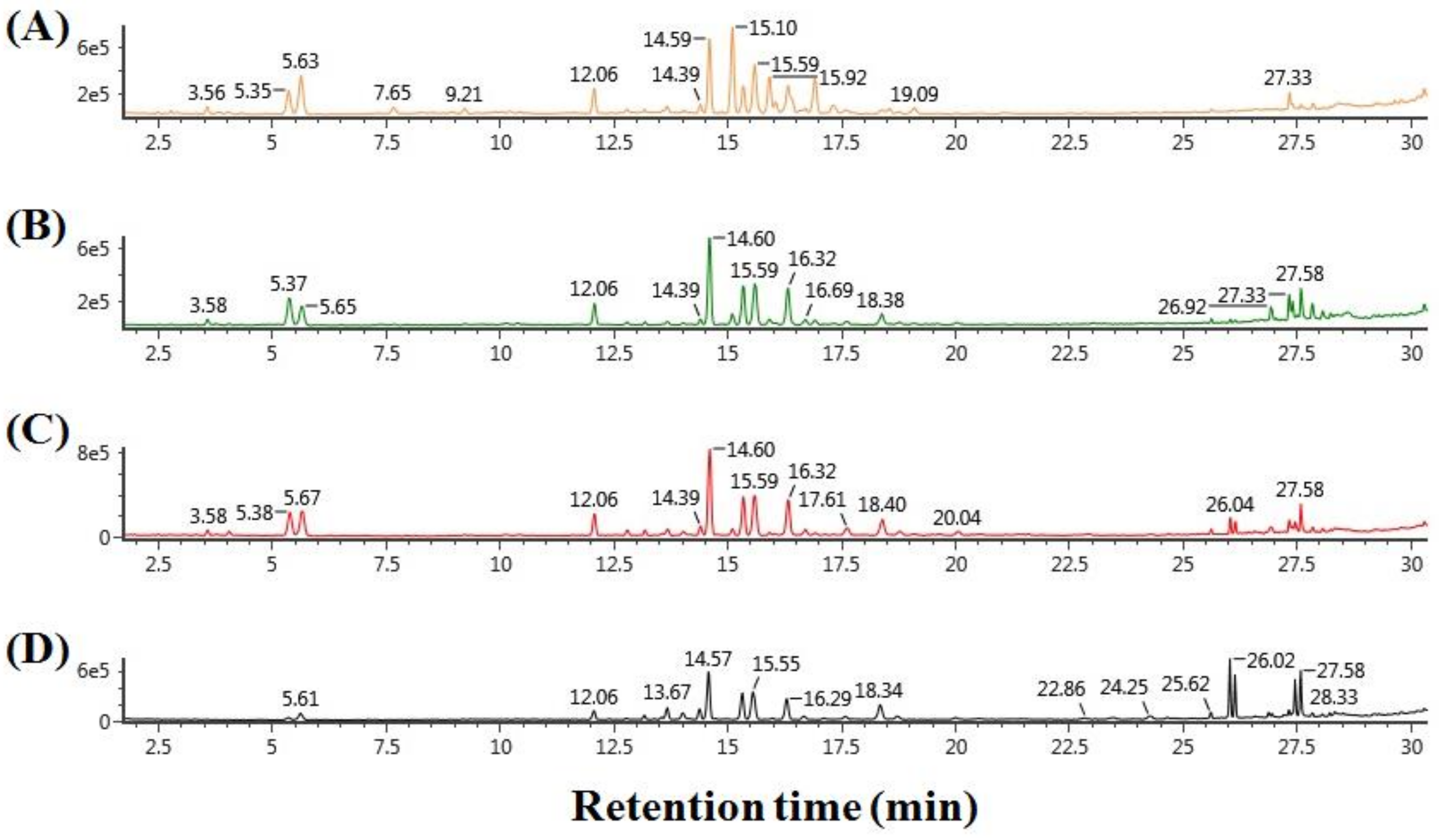
| No. | RT (min) | Ginsenosides | White Ginseng | Tae-Geuk Ginseng | Red Ginseng | Black Ginseng |
|---|---|---|---|---|---|---|
| 1 | 3.57 | 20-O-Glucoginsenoside Rf (gRf) | 2.2 ± 0.3 | 1.7 ± 0.2 | 2.6 ± 0.2 | 0.17 ± 0.04 |
| 2 | 5.37 | Ginsenoside Rg1 (Rg1) | 3.9 ± 0.4 | 4.5 ± 0.3 | 5.7 ± 0.3 | 0.63 ± 0.05 |
| 3 | 5.63 | Ginsenoside Re (Re) | 3.7 ± 0.5 | 1.7 ± 0.2 | 4.2 ± 0.4 | 0.88 ± 0.09 |
| 4 | 11.21 | vinaginsenoside R4 (vR4) | 0.008 ± 0.004 | 0.01 ± 0.004 | 0.024 ± 0.004 | 0.012 ± 0.004 |
| 5 | 12.05 | Ginsenoside Rf (Rf) | 1.1 ± 0.2 | 0.91 ± 0.07 | 1.4 ± 0.1 | 0.78 ± 0.04 |
| 6 | 12.78 | Notoginsenoside R2 (nR2) | 1.3 ± 0.2 | 1.1 ± 0.1 | 3.1 ± 0.3 | 0.9 ± 0.1 |
| 7 | 13.17 | Notoginsenoside R4 (nR4) | 0.17 ± 0.04 | 0.18 ± 0.03 | 0.57 ± 0.06 | 0.45 ± 0.06 |
| 8 | 13.55 | Ginsenoside Rh1 (Rh1) | 0.02 ± 0.01 | 0.09 ± 0.01 | 0.38 ± 0.03 | 0.8 ± 0.1 |
| 9 | 13.56 | 20(R)-Notoginsenoside R2 (nR2-R) | Not detected | Not detected | 0.01 ± 0.002 | 0.004 ± 0.002 |
| 10 | 13.67 | Ginsenoside Rg2 (Rg2) | 0.16 ± 0.02 | 0.082 ± 0.006 | 0.30 ± 0.02 | 0.66 ± 0.06 |
| 11 | 14.03 | 20(R)-Ginsenoside Rg2 (Rg2-R) | 0.004 ± 0.001 | 0.01 ± 0.002 | 0.09 ± 0.01 | 0.33 ± 0.03 |
| 12 | 14.04 | 20(R)-Ginsenoside Rh1 (Rh1-R) | Not detected | 0.018 ± 0.002 | 0.11 ± 0.02 | 0.35 ± 0.04 |
| 13 | 14.39 | Ginsenoside Ra2 (Ra2) | 3.4 ± 0.2 | 2.9 ± 0.1 | 4.9 ± 0.4 | 5.3 ± 0.4 |
| 14 | 14.56 | Ginsenoside Ra3 (Ra3) | 1.1 ± 0.2 | 1.2 ± 0.1 | 3.1 ± 0.4 | 4.5 ± 0.5 |
| 15 | 14.59 | Ginsenoside Rb1 (Rb1) | 4.7 ± 0.6 | 5.4 ± 0.4 | 8.9 ± 0.6 | 4.2 ± 0.4 |
| 16 | 15.1 | Malonyl ginsenoside Rb1 (M-Rb1) | 6.0 ± 0.7 | 0.3 ± 0.1 | 0.32 ± 0.07 | Not detected |
| 17 | 15.35 | Ginsenoside Rc (Rc) | 1.2 ± 0.2 | 1.8 ± 0.2 | 2.9 ± 0.2 | 2.2 ± 0.3 |
| 18 | 15.55 | Ginsenoside Ra1 (Ra1) | 1.1 ± 0.3 | 1.2 ± 0.4 | 5.5 ± 0.5 | 7.1 ± 1.5 |
| 19 | 15.6 | Ginsenoside Ro (Ro) | 0.17 ± 0.02 | 0.14 ± 0.02 | 0.19 ± 0.01 | 0.08 ± 0.01 |
| 20 | 15.91 | Malonyl ginsenoside Rc (M-Rc) | 1.1 ± 0.2 | 0.10 ± 0.03 | 0.04 ± 0.02 | Not detected |
| 21 | 16.31 | Ginsenoside Rb2 (Rb2) | 0.8 ± 0.1 | 1.2 ± 0.1 | 2.0 ± 0.2 | 1.2 ± 0.1 |
| 22 | 16.7 | Ginsenoside Rb3 (Rb3) | 0.19 ± 0.05 | Not detected | 0.45 ± 0.03 | 0.28 ± 0.09 |
| 23 | 16.92 | Malonyl ginsenoside Rb2 (M-Rb2) | 1.2 ± 0.2 | 0.03 ± 0.01 | 0.014 ± 0.002 | Not detected |
| 24 | 18.39 | Ginsenoside Rd (Rd) | 0.041 ± 0.005 | 0.01 ± 0.004 | 0.5 ± 0.1 | 0.6 ± 0.1 |
| 25 | 23.43 | Ginsenoside Rg4 (Rg4) | Not detected | Not detected | 0.11 ± 0.03 | 0.46 ± 0.05 |
| 26 | 23.54 | Compound O (CO) | 0.07 ± 0.03 | Not detected | 0.06 ± 0.03 | 0.14 ± 0.05 |
| 27 | 23.92 | Ginsenoside Rk3 (Rk3) | Not detected | 0.04 ± 0.01 | 0.23 ± 0.04 | 0.69 ± 0.07 |
| 28 | 24.3 | Ginsenoside F4 (F4) | Not detected | 0.40 ± 0.03 | 2.5 ± 0.3 | 9.0 ± 1.1 |
| 29 | 24.69 | Ginsenoside Rh4 (Rh4) | Not detected | 0.48 ± 0.07 | 3.4 ± 0.4 | 8.9 ± 0.7 |
| 30 | 25.26 | Ginsenoside F2 (F2) | 0.0020 ± 0.0001 | Not detected | 0.004 ± 0.002 | 0.016 ± 0.004 |
| 31 | 26.03 | Ginsenoside Rg3 (Rg3) | 0.002 ± 0.001 | 0.044 ± 0.004 | 0.30 ± 0.02 | 1.10 ± 0.07 |
| 32 | 26.4 | Ginsenoisde Mc (Mc) | Not detected | Not detected | 0.01 ± 0.002 | 0.08 ± 0.02 |
| 33 | 26.56 | Compound Y (CY) | Not detected | Not detected | Not detected | 0.09 ± 0.02 |
| 34 | 27.38 | Compound K (CK) | Not detected | Not detected | Not detected | 0.004 ± 0.002 |
| 35 | 27.45 | Ginsenoside Rk1 (Rk1) | Not detected | 1.60 ± 0.04 | 4.4 ± 0.3 | 14.6 ± 1.1 |
| 36 | 27.59 | Ginsenoside Rg5 (Rg5) | 0.06 ± 0.02 | 0.41 ± 0.05 | 2.2 ± 0.2 | 8.4 ± 0.6 |
| 37 | 27.73 | Ginsenoside Rh2 (Rh2) | Not detected | Not detected | Not detected | 0.004 ± 0.002 |
| 38 | 29.13 | Ginsenoside Rk2 (Rk2) | Not detected | Not detected | Not detected | 0.012 ± 0.002 |
| 39 | 29.14 | Ginsenoside Rh3 (Rh3) | Not detected | Not detected | Not detected | 0.036 ± 0.006 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.W.; Ji, S.-H.; Choi, B.-R.; Choi, D.J.; Lee, Y.-G.; Kim, H.-G.; Kim, G.-S.; Kim, K.; Lee, Y.-H.; Baek, N.-I.; et al. UPLC-QTOF/MS-Based Metabolomics Applied for the Quality Evaluation of Four Processed Panax ginseng Products. Molecules 2018, 23, 2062. https://doi.org/10.3390/molecules23082062
Lee JW, Ji S-H, Choi B-R, Choi DJ, Lee Y-G, Kim H-G, Kim G-S, Kim K, Lee Y-H, Baek N-I, et al. UPLC-QTOF/MS-Based Metabolomics Applied for the Quality Evaluation of Four Processed Panax ginseng Products. Molecules. 2018; 23(8):2062. https://doi.org/10.3390/molecules23082062
Chicago/Turabian StyleLee, Jae Won, Seung-Heon Ji, Bo-Ram Choi, Doo Jin Choi, Yeong-Geun Lee, Hyoung-Geun Kim, Geum-Soog Kim, Kyuil Kim, Youn-Hyung Lee, Nam-In Baek, and et al. 2018. "UPLC-QTOF/MS-Based Metabolomics Applied for the Quality Evaluation of Four Processed Panax ginseng Products" Molecules 23, no. 8: 2062. https://doi.org/10.3390/molecules23082062
APA StyleLee, J. W., Ji, S.-H., Choi, B.-R., Choi, D. J., Lee, Y.-G., Kim, H.-G., Kim, G.-S., Kim, K., Lee, Y.-H., Baek, N.-I., & Lee, D. Y. (2018). UPLC-QTOF/MS-Based Metabolomics Applied for the Quality Evaluation of Four Processed Panax ginseng Products. Molecules, 23(8), 2062. https://doi.org/10.3390/molecules23082062






