Synthesis of New 5-Aryl-benzo[f][1,7]naphthyridines via a Cascade Process (Ugi-3CR/Intramolecular Aza-Diels-Alder Cycloaddition)/Aromatization
Abstract
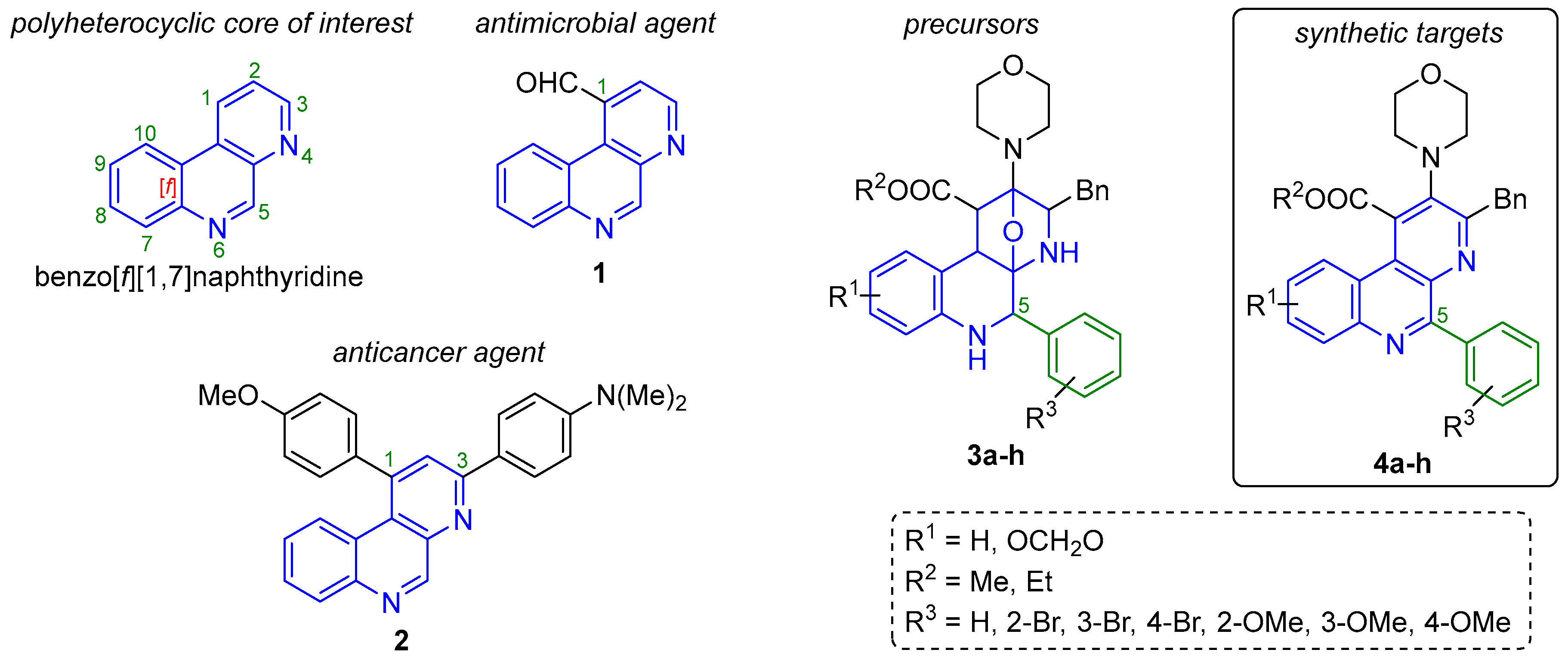
1. Introduction
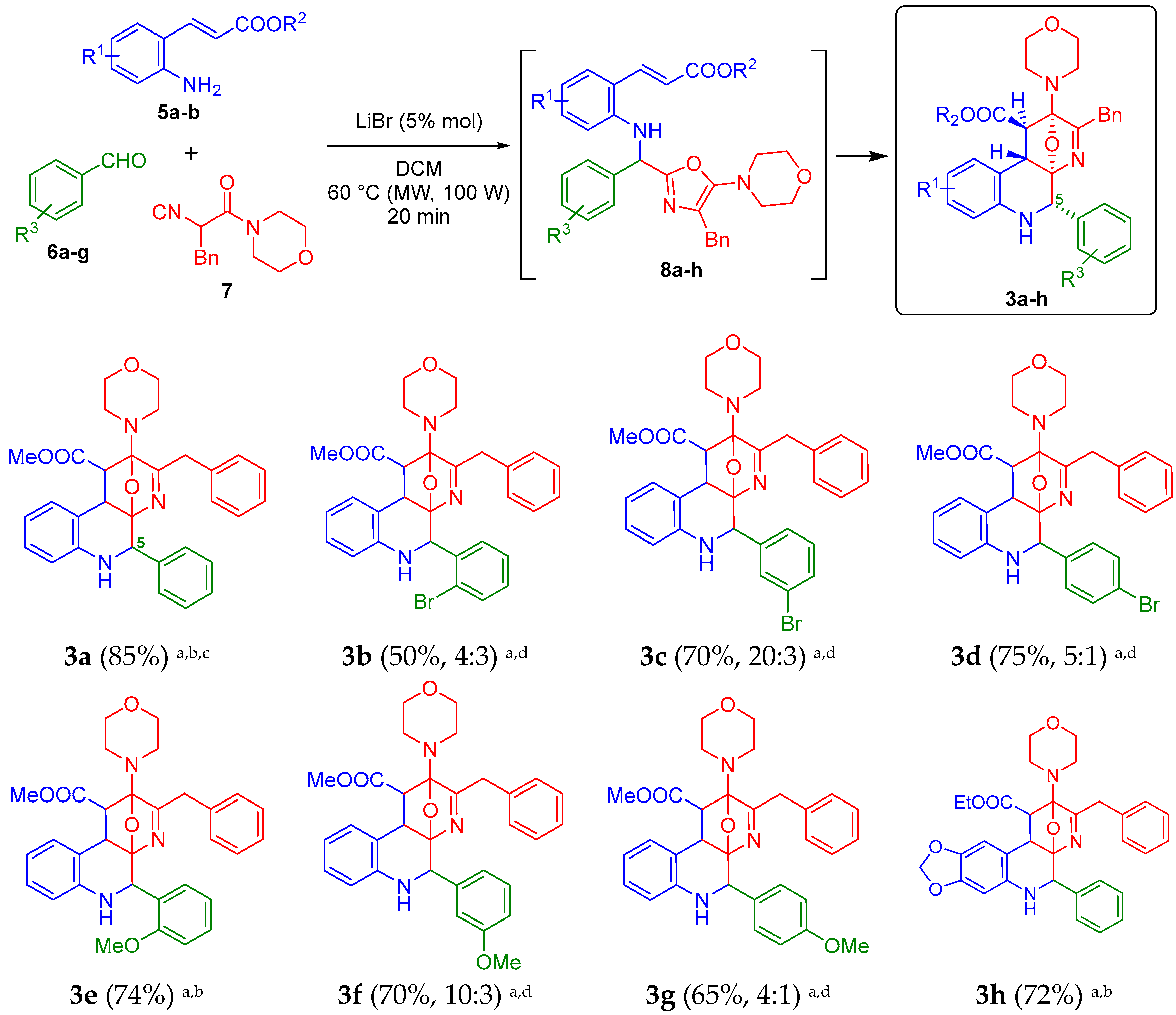
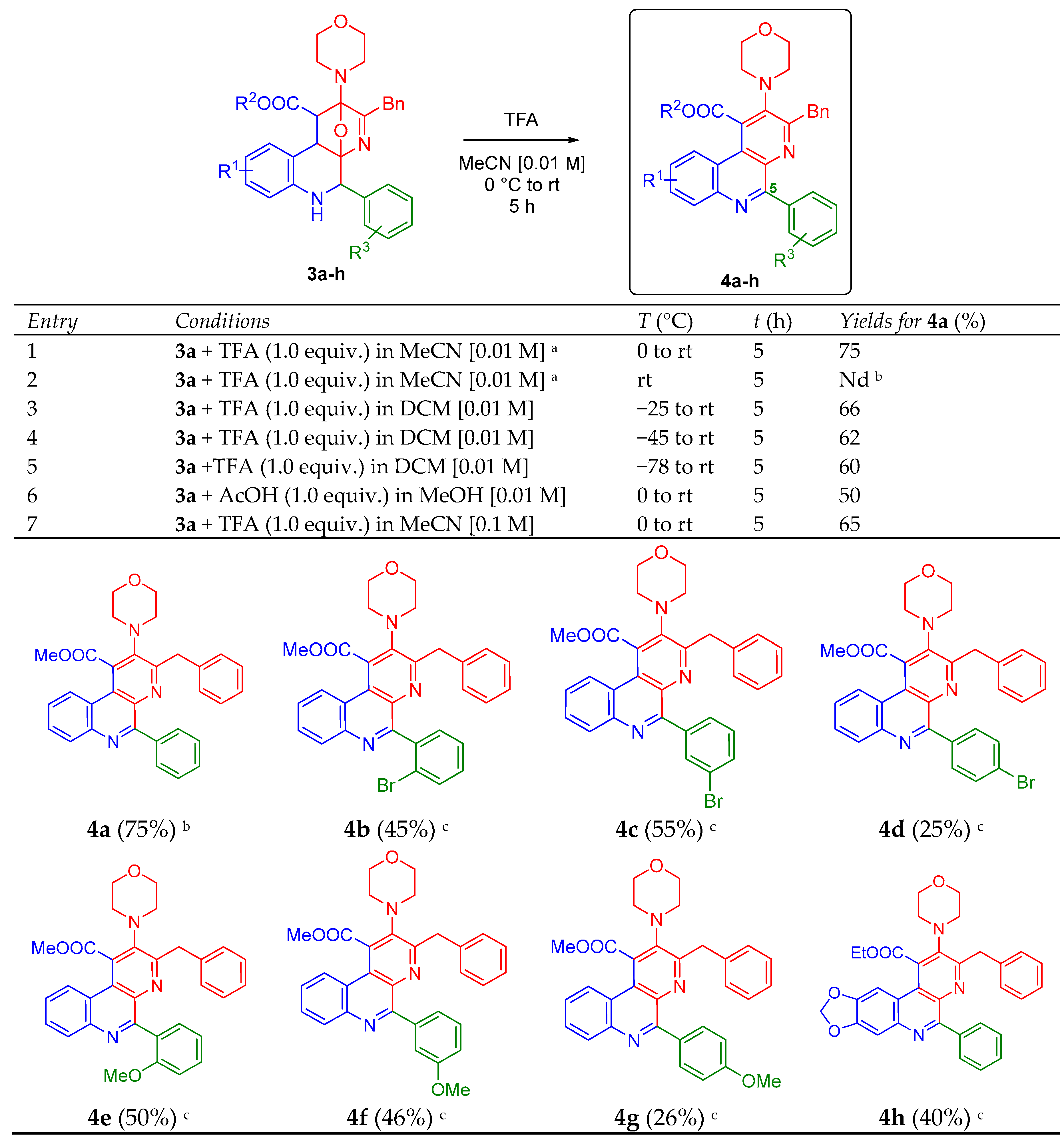
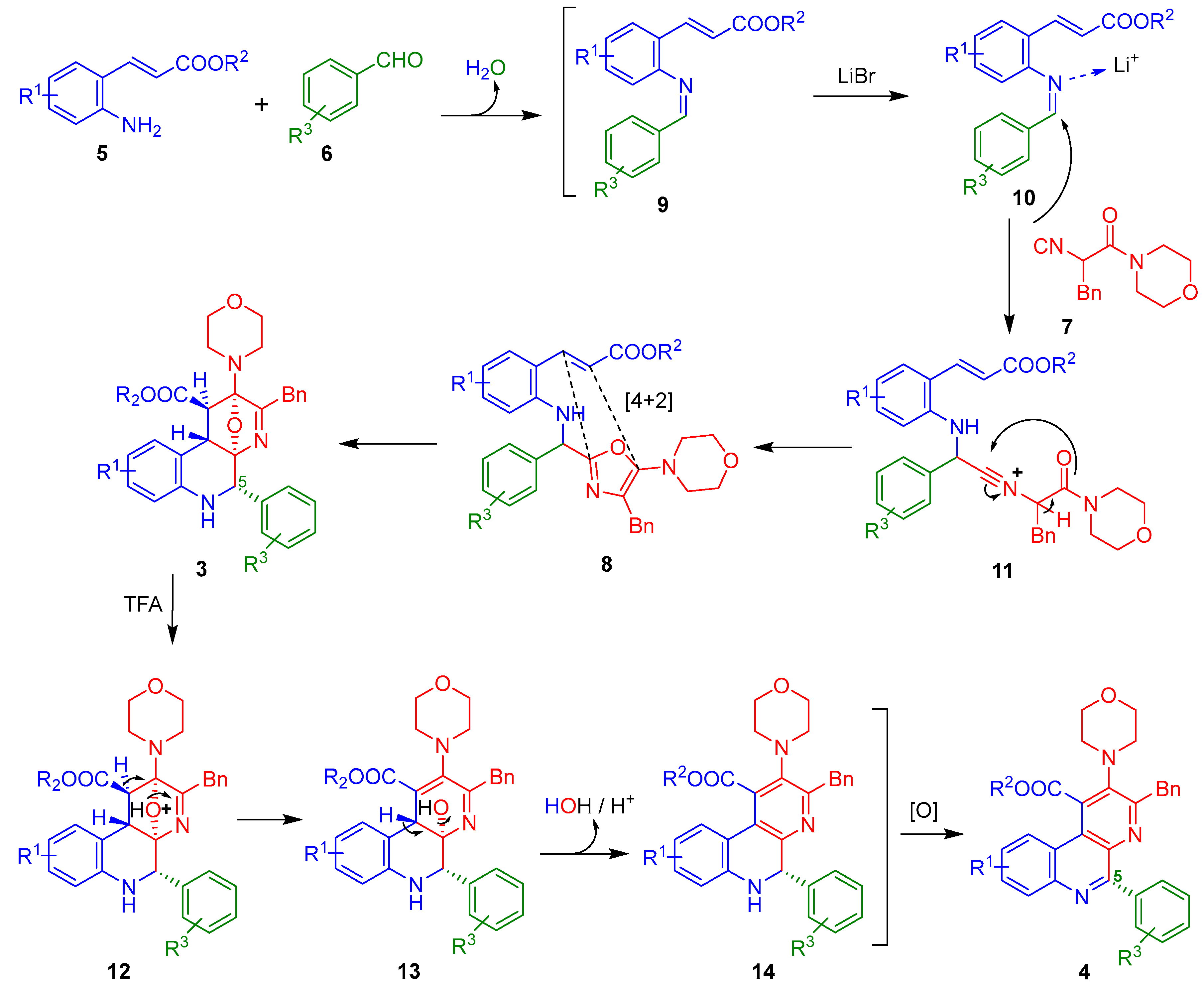
2. Results and Discussion
3. Experimental Section
3.1. General Information, Instrumentation, Software, Solvents and Chemicals
3.2. Synthesis and Characterization of the Oxa-Bridged 5-arylbenzo[f][1,7]naphthyridines 3a–h
3.2.1. Methyl 3-Benzyl-2-morpholino-5-phenyl-1,5,6,10b-tetrahydro-2H-2,4a-epoxybenzo[f][1,7]-naphthyridine-1-carboxylate (3a)
3.2.2. Methyl 3-Benzyl-5-(2-bromophenyl)-2-morpholino-1,5,6,10b-tetrahydro-2H-2,4a-epoxybenzo[f][1,7]-naphthyridine-1-carboxylate (3b)
3.2.3. Methyl 3-Benzyl-5-(3-bromophenyl)-2-morpholino-1,5,6,10b-tetrahydro-2H-2,4a-epoxybenzo[f][1,7]-naphthyridine-1-carboxylate (3c)
3.2.4. Methyl 3-Benzyl-5-(4-bromophenyl)-2-morpholino-1,5,6,10b-tetrahydro-2H-2,4a-epoxybenzo[f][1,7]-naphthyridine-1-carboxylate (3d)
3.2.5. Methyl 3-Benzyl-5-(2-methoxyphenyl)-2-morpholino-1,5,6,10b-tetrahydro-2H-2,4a-epoxybenzo[f][1,7]-naphthyridine-1-carboxylate (3e)
3.2.6. Methyl 3-Benzyl-5-(3-methoxyphenyl)-2-morpholino-1,5,6,10b-tetrahydro-2H-2,4a-epoxybenzo[f][1,7]-naphthyridine-1-carboxylate (3f)
3.2.7. Methyl 3-Benzyl-5-(4-methoxyphenyl)-2-morpholino-1,5,6,10b-tetrahydro-2H-2,4a-epoxybenzo[f][1,7]-naphthyridine-1-carboxylate (3g)
3.2.8. Ethyl 3-Benzyl-2-morpholino-5-phenyl-1,5,6,11b-tetrahydro-2H-2,4a-epoxy[1,3]dioxolo[4′,5′:4,5]-benzo[1–2-f][1,7]naphthyridine-1-carboxylate (3h)
3.3. Synthesis and Characterization of the Polysubstituted 5-Arylbenzo[f][1,7]naphthyridines 4a–h
3.3.1. Methyl 3-Benzyl-2-morpholino-5-phenylbenzo[f][1,7]naphthyridine-1-carboxylate (4a)
3.3.2. Methyl 3-Benzyl-5-(2-bromophenyl)-2-morpholinobenzo[f][1,7]naphthyridine-1-carboxylate (4b)
3.3.3. Methyl 3-Benzyl-5-(3-bromophenyl)-2-morpholinobenzo[f][1,7]naphthyridine-1-carboxylate (4c)
3.3.4. Methyl 3-Benzyl-5-(4-bromophenyl)-2-morpholinobenzo[f][1,7]naphthyridine-1-carboxylate (4d)
3.3.5. 3-Benzyl-5-(2-methoxyphenyl)-2-morpholinobenzo[f][1,7]naphthyridine-1-carboxylate (4e)
3.3.6. 3-Benzyl-5-(3-Methoxyphenyl)-2-morpholinobenzo[f][1,7]naphthyridine-1-carboxylate (4f)
3.3.7. 3-Benzyl-5-(4-methoxyphenyl)-2-morpholinobenzo[f][1,7]naphthyridine-1-carboxylate (4g)
3.3.8. Ethyl 3-Benzyl-2-morpholino-5-phenyl-[1,3]dioxolo[4′,5′:4,5]benzo[1,2-f][1,7]naphthyridine-1-carboxylate (4h)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bachowska, B.; Zujewska, T. Chemistry and applications of benzonaphthyridines. ARKIVOC 2001, 6, 77–84. [Google Scholar] [CrossRef]
- Li, X.-L.; Xu, M.-J.; Zhao, Y.-L.; Xu, J. A Novel Benzo[f][1,7]Naphthyridine Produced by Streptomyces albogriseolus from Mangrove Sediments. Molecules 2010, 15, 9298–9307. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Jiao, X.; Li, X.; Li, R.; Dong, L.; Liu, X.; Zhang, Z.; Xu, J.; Xu, M.; Xie, P. First total synthesis and determination of the absolute configuration of 1-N-methyl-3-methylamino-[N-butanoicacid-3-(9-methyl-8-propen-7-one)-amide]-benzo[f][1,7]naphthyridine-2-one, a novel benzonaphthyridine alkaloid. Tetrahedron Lett. 2012, 53, 4892–4895. [Google Scholar] [CrossRef]
- Litvinov, V.P. Advances in the Chemistry of Naphthyridines. Adv. Heterocycl. Chem. 2006, 91, 65–300. [Google Scholar] [CrossRef]
- Dondela, B.; Chrzastek, L. Theoretical study of UV spectra of diazaphenanthrenesulfonamides calculated by AM1 and DFT B3-LYP methods. J. Phys. Conf. Ser. 2007, 79, 012042. [Google Scholar] [CrossRef]
- Chrzastek, L.; Mianowska, B.; Sliwa, W. Synthesis and Properties of Methyl-, Formyl- and Amino-Diazaphenanthrene. Aust. J. Chem. 1994, 47, 2129–2133. [Google Scholar] [CrossRef]
- Arepalli, S.K.; Park, B.; Lee, K.; Jo, H.; Jun, K.-Y.; Kwon, Y.; Kang, J.-S.; Jung, J.-K.; Lee, H. Design, synthesis and biological evaluation of 1,3-diphenylbenzo[f][1,7]naphthyrdines. Bioorg. Med. Chem. 2017, 25, 5586–5597. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Takeuchi, I.; Hirota, M. Synthesis of Nitrogen-containing Heterocyclic Compounds. XX. Improvement of One-step Synthesis of Phenanthrolines and Some Reactions of 4,6-Phenanthroline. Chem. Pharm. Bull. 1974, 22, 485–492. [Google Scholar] [CrossRef][Green Version]
- Paudler, W.W.; Sheets, R.M. Recent Developments in Naphthyridine Chemistry. Adv. Heterocycl. Chem. 1983, 33, 147–184. [Google Scholar] [CrossRef]
- Chen, W.-L.; Chen, C.-Y.; Chen, Y.-F.; Hsieh, J.C. Hydride-Induced Anionic Cyclization: An Efficient Method for the Synthesis of 6-H-Phenanthridines via a Transition-Metal-Free Process. Org. Lett. 2015, 17, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Strekowski, L.; Kiselyov, A.S.; Hojjat, M. The o-Amino-Trifluoromethyl Functionality as a Novel Synthon for 4-Fluoroquinoline. J. Org. Chem. 1994, 59, 5856–5890. [Google Scholar] [CrossRef]
- Bachowska, B.; Zujewska, T. Vicarious Nucleophilic Substitution of Hydrogen and Formation of Aziridine Rings in Reactions of Benzonaphthyridines and their N-Oxides with Chloromethyl Phenyl Sulfone. Motasch. Chem. 2001, 132, 849–854. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Ramón, D.J.; Yus, M. Asymmetric Multicomponent Reactions (AMCRs): The New Frontier. Angew. Chem. Int. Ed. 2005, 44, 1602–1634. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.A.; Islas-Jácome, A.; González-Zamora, E. Synthesis of polyheterocycles via multicomponent reactions. Org. Biomol. Chem. 2018, 16, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Biggs-Houck, J.E.; Younai, A.; Shaw, J.T. Recent advances in multicomponent reactions for diversity-oriented synthesis. Curr. Op. Chem. Biol. 2010, 14, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Ulaczyk-Lesanko, A.; Hall, D.G. Wanted: New multicomponent reactions for generating libraries of polycyclic natural products. Curr. Opin. Chem. Biol. 2005, 9, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, H.; Dömling, A. Efficient Multicomponent Reaction Synthesis of the Schistosomiasis Drug Praziquantel. Chem. Eur. J. 2010, 16, 12296–12298. [Google Scholar] [CrossRef] [PubMed]
- González-Zamora, E.; Fayol, A.; Bois-Choussy, M.; Chiaroni, A.; Zhu, J. Three component synthesis of oxa-bridged tetracyclic tetrahydroquinolines. Chem. Commun. 2001, 1684–1685. [Google Scholar] [CrossRef]
- Fayol, A.; González-Zamora, E.; Bois-Choussy, M.; Zhu, J. Lithium Bromide-Promoted Three-Component Synthesis of Oxa-Bridged Tetracyclic Tetrahydroisoquinolines. Heterocycles 2007, 73, 729–742. [Google Scholar] [CrossRef]
- Marcaccini, S.; Torroba, T. The use of the Ugi four-component condensation. Nat. Protoc. 2007, 2, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.F. Recent applications of imines as key intermediates in the synthesis of alkaloids and novel nitrogen heterocycles. Pure Appl. Chem. 2009, 81, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Li, P.; Shimizu, K.D. Synergy between experimental and computational studies of aromatic stacking interactions. Org. Biomol. Chem. 2017, 15, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Janvier, P.; Sun, X.; Bienaymé, H.; Zhu, J. Ammonium Chloride-Promoted Four-Component Synthesis of Pyrrolo[3,4-b]pyridin-5-one. J. Am. Chem. Soc. 2002, 124, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Moghimi, S. Catalytic Multicomponent Reactions Based on Isocyanides. J. Iran. Chem. Soc. 2011, 8, 306–373. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.-X.; Wang, D.-X.; Zhu, J. Asymmetric Lewis Acid Catalyzed Addition of Isocyanides to Aldehydes—Synthesis of 5-Amino-2-(1-hydroxyalkyl)oxazoles. Eur. J. Org. Chem. 2007, 4076–4080. [Google Scholar] [CrossRef]
- Okandeji, B.O.; Gordon, J.R.; Sello, J.K. Catalysis of UGI Four Component Coupling Reactions by Rare Earth Metal Triflates. J. Org. Chem. 2008, 73, 5595–5597. [Google Scholar] [CrossRef] [PubMed]
- Islas-Jácome, A.; González-Zamora, E.; Gámez-Montaño, R. A short microwave-assisted synthesis of tetrahydroisoquinolinpyrrolopyridinones by a triple process: Ugi-3CR–aza Diels–Alder/S-oxidation/Pummerer. Tetrahedron Lett. 2011, 52, 5245–5248. [Google Scholar] [CrossRef]
- Perreux, L.; Loupy, A. A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron 2001, 57, 9199–9223. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3a–h and 4a–h are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Vera, Ó.; Segura-Olvera, D.; Rincón-Guevara, M.A.; Gutiérrez-Carrillo, A.; García-Sánchez, M.A.; Ibarra, I.A.; Lomas-Romero, L.; Islas-Jácome, A.; González-Zamora, E. Synthesis of New 5-Aryl-benzo[f][1,7]naphthyridines via a Cascade Process (Ugi-3CR/Intramolecular Aza-Diels-Alder Cycloaddition)/Aromatization. Molecules 2018, 23, 2029. https://doi.org/10.3390/molecules23082029
Vázquez-Vera Ó, Segura-Olvera D, Rincón-Guevara MA, Gutiérrez-Carrillo A, García-Sánchez MA, Ibarra IA, Lomas-Romero L, Islas-Jácome A, González-Zamora E. Synthesis of New 5-Aryl-benzo[f][1,7]naphthyridines via a Cascade Process (Ugi-3CR/Intramolecular Aza-Diels-Alder Cycloaddition)/Aromatization. Molecules. 2018; 23(8):2029. https://doi.org/10.3390/molecules23082029
Chicago/Turabian StyleVázquez-Vera, Óscar, Daniel Segura-Olvera, Mónica A. Rincón-Guevara, Atilano Gutiérrez-Carrillo, Miguel A. García-Sánchez, Ilich A. Ibarra, Leticia Lomas-Romero, Alejandro Islas-Jácome, and Eduardo González-Zamora. 2018. "Synthesis of New 5-Aryl-benzo[f][1,7]naphthyridines via a Cascade Process (Ugi-3CR/Intramolecular Aza-Diels-Alder Cycloaddition)/Aromatization" Molecules 23, no. 8: 2029. https://doi.org/10.3390/molecules23082029
APA StyleVázquez-Vera, Ó., Segura-Olvera, D., Rincón-Guevara, M. A., Gutiérrez-Carrillo, A., García-Sánchez, M. A., Ibarra, I. A., Lomas-Romero, L., Islas-Jácome, A., & González-Zamora, E. (2018). Synthesis of New 5-Aryl-benzo[f][1,7]naphthyridines via a Cascade Process (Ugi-3CR/Intramolecular Aza-Diels-Alder Cycloaddition)/Aromatization. Molecules, 23(8), 2029. https://doi.org/10.3390/molecules23082029





