Evaluation of Amino-Functional Polyester Dendrimers Based on Bis-MPA as Nonviral Vectors for siRNA Delivery
Abstract
1. Introduction
2. Results
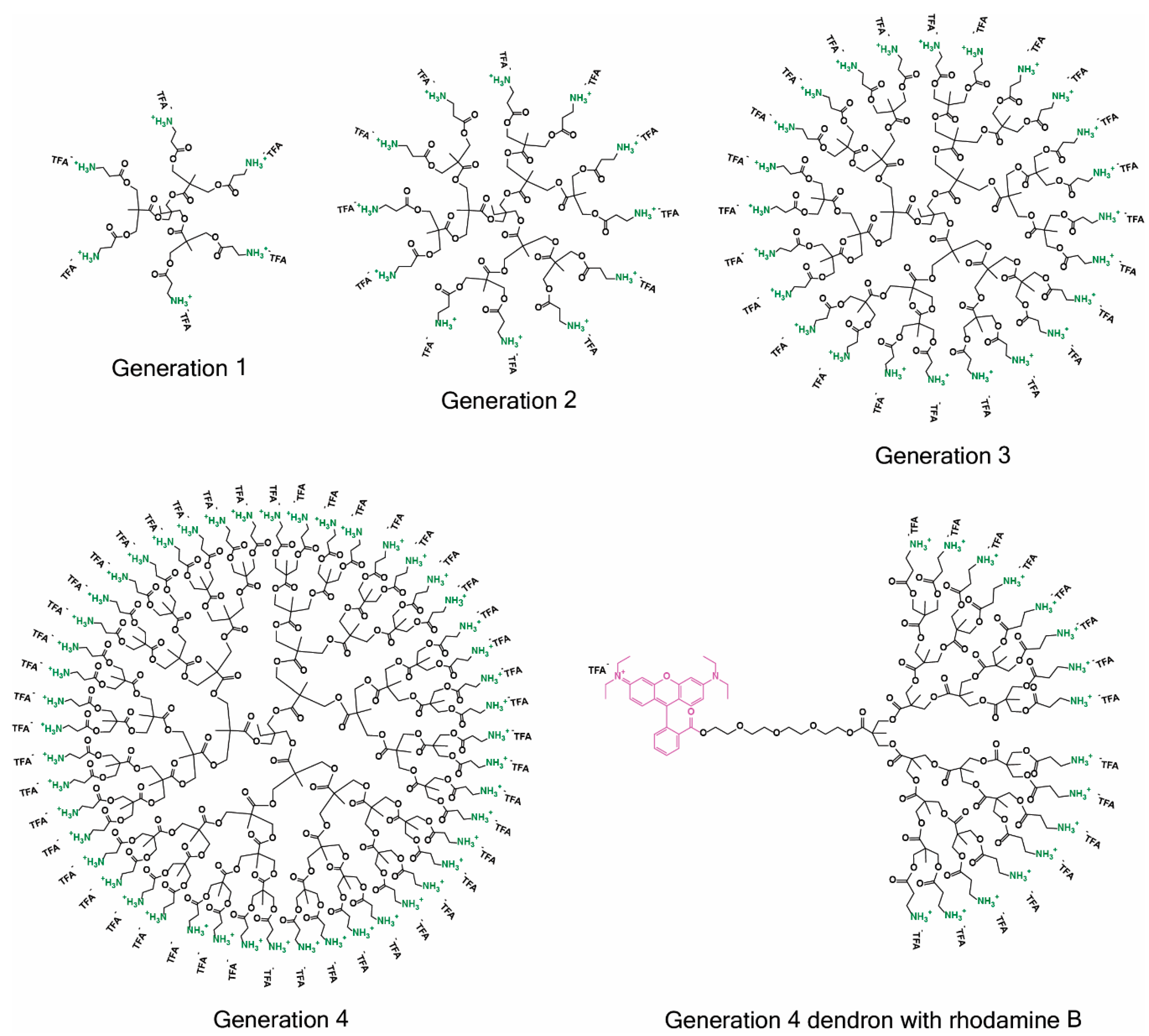
2.1. Synthesis
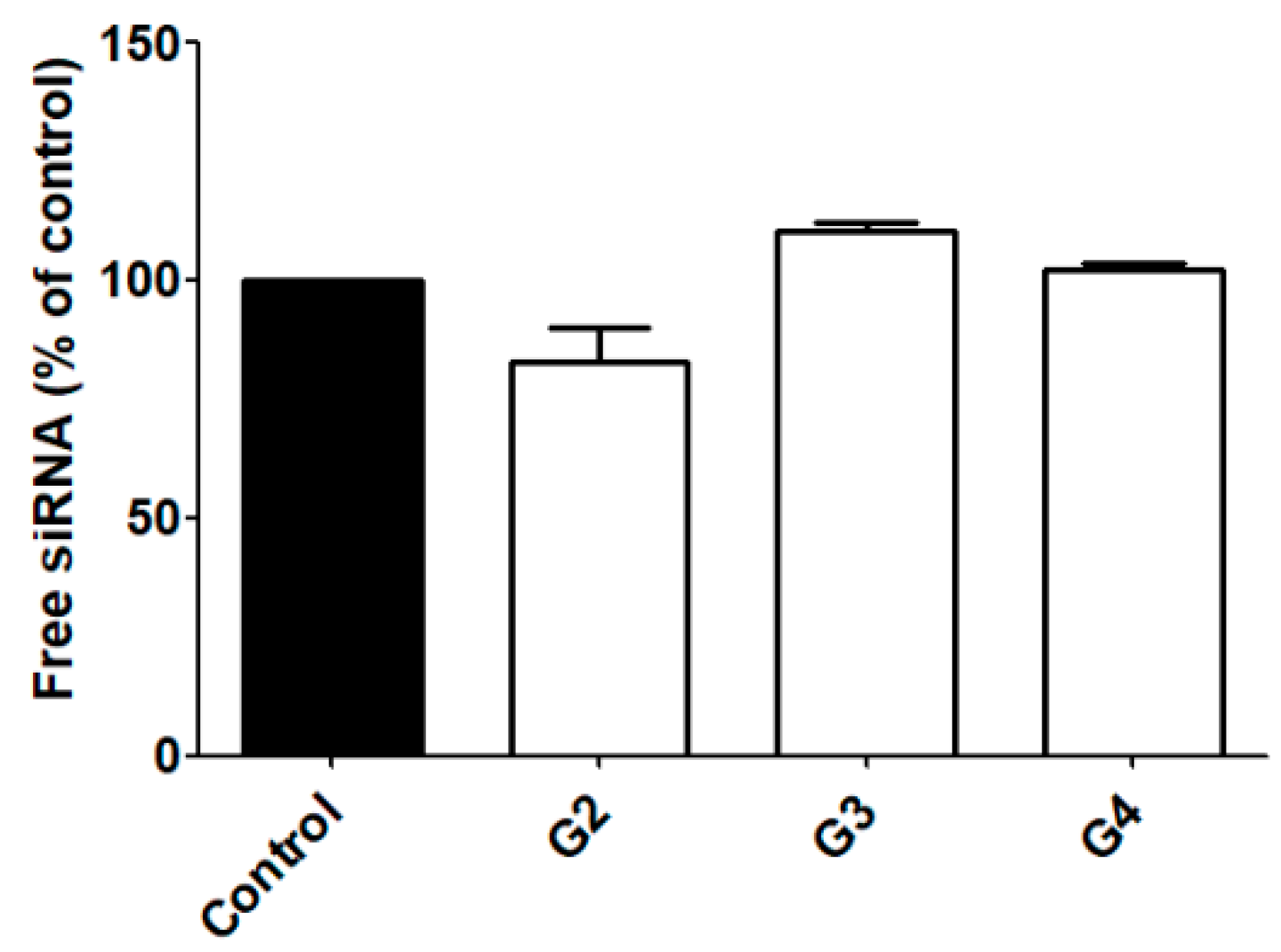
2.2. siRNA Complexation and RNase Protection
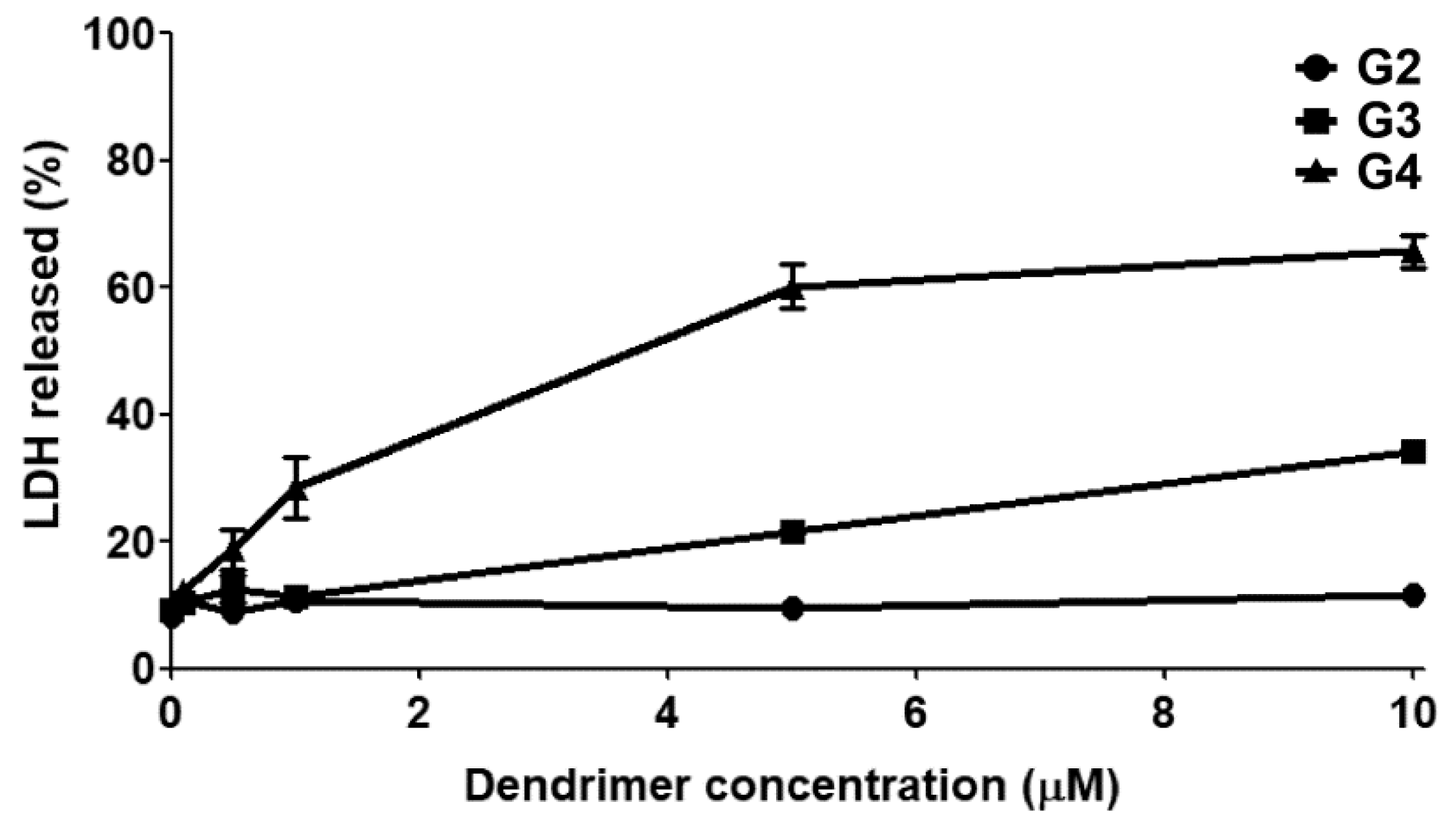
2.3. Cytotoxicity
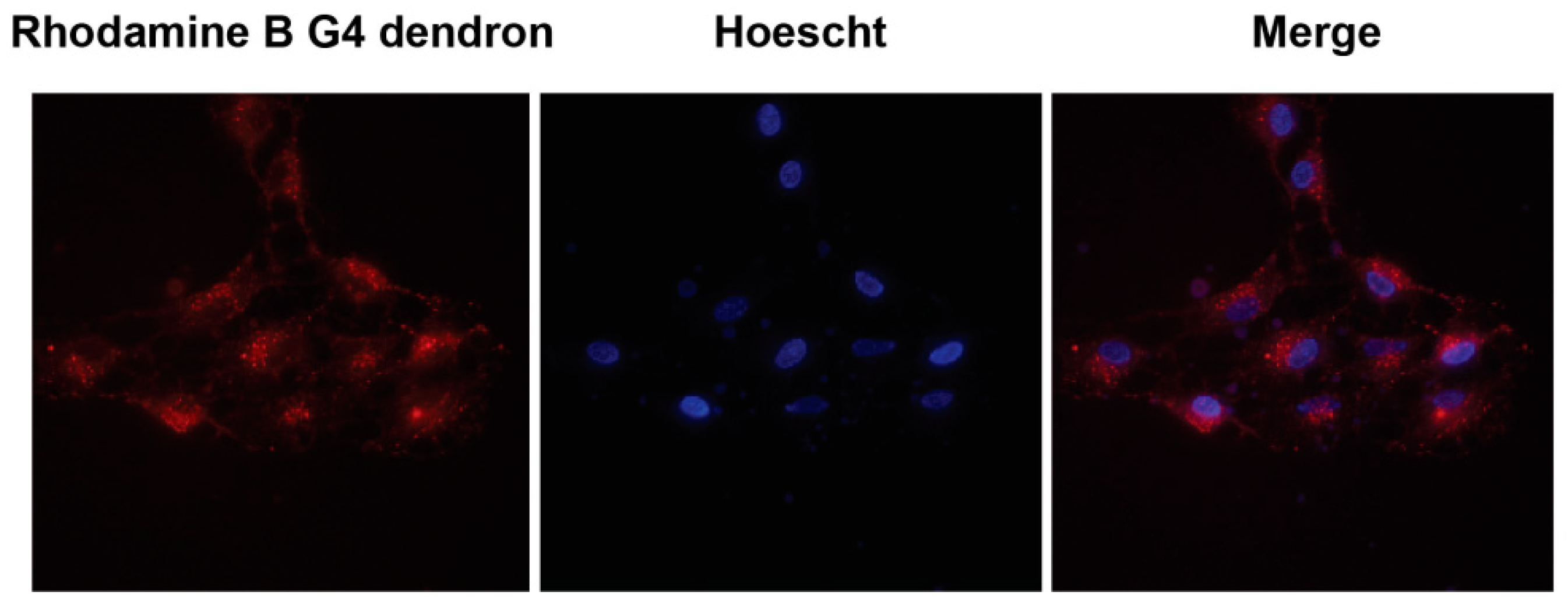
2.4. Subcellular Localization
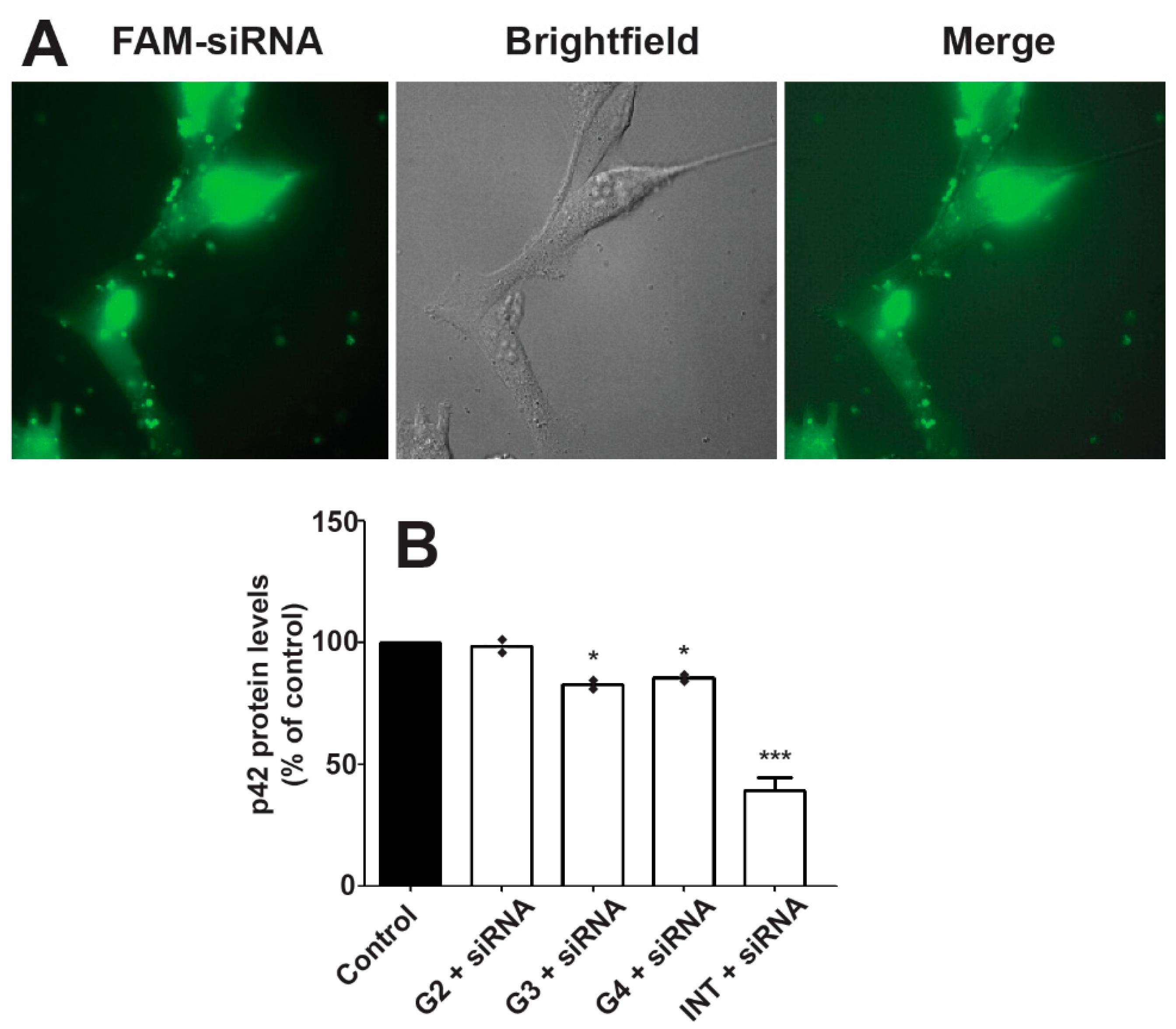
2.5. Transfection
3. Discussion
3.1. siRNA Complexation and RNase Protection
3.2. Cytotoxicity
3.3. Subcellular Localization
3.4. Transfection
4. Materials and Methods
4.1. Synthesis Protocols
4.2. MALDI-ToF-MS
4.3. NMR Spectroscopy
4.4. Cell Cultures
4.5. Fluorescence Spectroscopy
4.6. Fluorescence Microscopy
4.7. Agarose Gel Retardation
4.8. siRNA Protection against RNases
4.9. siRNA Transfection & Western Blot Analysis
4.10. Cytotoxicity Studies
4.11. Degradation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fire, A.; Xu, S.Q.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. The first targeted delivery of sirna in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol. Pharm. 2009, 6, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.E.; Gritli, I.; Tolcher, A.; Heidel, J.D.; Lim, D.; Morgan, R.; Chmielowski, B.; Ribas, A.; Davis, M.E.; Yen, Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA 2014, 111, 11449–11454. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.; Adams, D.; Silva, A.; Lozeron, P.; Hawkins, P.N.; Mant, T.; Perez, J.; Chiesa, J.; Warrington, S.; Tranter, E.; et al. Safety and efficacy of rnai therapy for transthyretin amyloidosis. N. Engl. J. Med. 2013, 369, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Suhr, O.B.; Coelho, T.; Buades, J.; Pouget, J.; Conceicao, I.; Berk, J.; Schmidt, H.; Waddington-Cruz, M.; Campistol, J.M.; Bettencourt, B.R.; et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: A phase II multi-dose study. Orphanet J. Rare Dis. 2015, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Hickerson, R.P.; Vlassov, A.V.; Wang, Q.; Leake, D.; Ilves, H.; Gonzalez-Gonzalez, E.; Contag, C.H.; Johnston, B.H.; Kaspar, R.L. Stability study of unmodified siRNA and relevance to clinical use. Oligonucleotides 2008, 18, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Judge, A.D.; Sood, V.; Shaw, J.R.; Fang, D.; McClintock, K.; MacLachlan, I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005, 23, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M.; Bernstein, E.; Beach, D.; Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000, 404, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Sen, G.L.; Blau, H.M. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 2005, 7, 633–636. [Google Scholar] [CrossRef] [PubMed]
- De Fougerolles, A.; Vornlocher, H.P.; Maraganore, J.; Lieberman, J. Interfering with disease: A progress report on siRNA-based therapeutics. Nat. Rev. Drug Discov. 2007, 6, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Wittrup, A.; Lieberman, J. Knocking down disease: A progress report on siRNA therapeutics. Nat. Rev. Genet. 2015, 16, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.A. State-of-the-art gene-based therapies: The road ahead. Nat. Rev. Genet. 2011, 12, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Sharei, A.; Ding, X.Y.; Sahay, G.; Langer, R.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Dalby, B.; Cates, S.; Harris, A.; Ohki, E.C.; Tilkins, M.L.; Price, P.J.; Ciccarone, V.C. Advanced transfection with Lipofectamine 2000 reagent: Primary neurons, siRNA, and high-throughput applications. Methods 2004, 33, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Urban-Klein, B.; Werth, S.; Abuharbeid, S.; Czubayko, F.; Aigner, A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005, 12, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Green, J.J.; Love, K.T.; Sunshine, J.; Langer, R.; Anderson, D.G. Gold, poly(beta-amino ester) nanoparticles for small interfering rna delivery. Nano Lett. 2009, 9, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Karra, D.; Dahm, R. Transfection techniques for neuronal cells. J. Neurosci. 2010, 30, 6171–6177. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martinez, F.C.; Guerra, J.; Posadas, I.; Ceña, V. Barriers to nonviral vector-mediated gene delivery in the nervous system. Pharm. Res. 2011, 28, 1843–1858. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Choi, J.S.; Macala, L.J.; Tyrrell, L.; Black, J.A.; Cummins, T.R.; Waxman, S.G. Transfection of rat or mouse neurons by biolistics or electroporation. Nat. Protoc. 2009, 4, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Royo, N.C.; Vandenberghe, L.H.; Ma, J.Y.; Hauspurg, A.; Yu, L.; Maronski, M.; Johnston, J.; Dichter, M.A.; Wilson, J.M.; Watson, D.J. Specific AAV serotypes stably transduce primary hippocampal and cortical cultures with high efficiency and low toxicity. Brain Res. 2008, 1190, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Luo, J.G.; Jia, M.R.; Li, H.; Li, K.Z.; Fu, Z.J. Small interfering RNA-mediated knockdown of NF-kappa Bp65 attenuates neuropathic pain following peripheral nerve injury in rats. Eur. J. Pharmacol. 2012, 682, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Jativa, P.; Ceña, V. Use of nanoparticles for glioblastoma treatment: A new approach. Nanomedicine (Lond.) 2017, 12, 2533–2554. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Garbuzenko, O.B.; Kirkpatrick, P.; Pandya, I.; Savla, R.; Pozharov, V.P.; He, H.X.; Minko, T. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J. Control. Release 2009, 140, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; DeLong, R.; Fisher, M.H.; Juliano, R.L. Tat-conjugated PAMAM dendrimers as delivery agents for antisense and siRNA oligonucleotides. Pharm. Res. 2005, 22, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; Wu, J.Y.; Hafdi, N.; Behr, J.P.; Erbacher, P.; Peng, L. PAMAM dendrimers for efficient siRNA delivery and potent gene silencing. Chem. Commun. 2006, 2362–2364. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Nam, K.; Hahn, H.J.; Kim, B.H.; Lim, H.J.; Kim, H.J.; Choi, J.S.; Park, J.S. Biodegradable PAMAM ester for enhanced transfection efficiency with low cytotoxicity. Biomaterials 2009, 30, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, A.C.; Rivilla, I.; Perez-Martinez, F.C.; Monteagudo, S.; Ocana, V.; Guerra, J.; Garcia-Martinez, J.C.; Merino, S.; Sanchez-Verdu, P.; Ceña, V.; et al. Efficient, nontoxic hybrid ppv-pamam dendrimer as a gene carrier for neuronal cells. Biomacromolecules 2011, 12, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Kaneshiro, T.L.; Lu, Z.R. Targeted intracellular codelivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nanoglobular carrier. Biomaterials 2009, 30, 5660–5666. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Ortega, P.; Clemente, M.I.; Shcharbin, D.; Bryszewska, M.; de la Mata, F.J.; Gomez, R.; Munoz-Fernandez, M.A. Characterization of carbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes. J. Control. Release 2008, 132, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; Lopez-Hernandez, B.; Clemente, M.I.; Jimenez, J.L.; Ortega, P.; de la Mata, J.; Gomez, R.; Munoz-Fernandez, M.A.; Ceña, V. Highly efficient transfection of rat cortical neurons using carbosilane dendrimers unveils a neuroprotective role for HIF-1 α in early chemical hypoxia-mediated neurotoxicity. Pharm. Res. 2009, 26, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Gillies, E.R.; Dy, E.; Frechet, J.M.J.; Szoka, F.C. Biological evaluation of polyester dendrimer: Poly(ethylene oxide) “Bow-Tie” hybrids with tunable molecular weight and architecture. Mol. Pharm. 2005, 2, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Feliu, N.; Walter, M.V.; Montanez, M.I.; Kunzmann, A.; Hult, A.; Nystrom, A.; Malkoch, M.; Fadeel, B. Stability and biocompatibility of a library of polyester dendrimers in comparison to polyamidoamine dendrimers. Biomaterials 2012, 33, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Stenström, P.; Hjorth, E.; Zhang, Y.N.; Andrén, O.C.J.; Guette-Marquet, S.; Schultzberg, M.; Malkoch, M. Synthesis and in vitro evaluation of monodisperse amino-functional polyester dendrimers with rapid degradability and antibacterial properties. Biomacromolecules 2017, 18, 4323–4330. [Google Scholar] [CrossRef] [PubMed]
- Stenström, P.; Andrén, O.C.J.; Malkoch, M. Fluoride-promoted esterification (fpe) chemistry: A robust route to bis-mpa dendrons and their postfunctionalization. Molecules 2016, 21, 366. [Google Scholar] [CrossRef] [PubMed]
- García-Gallego, S.; Hult, D.; Olsson, J.V.; Malkoch, M. Fluoride-promoted esterification with imidazolide-activated compounds: A modular and sustainable approach to dendrimers. Angew. Chem. Int. Ed. Engl. 2015, 54, 2416–2419. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, I.; Peretz, P.; Muszkat, K.A. Thermochromic and hyperchromic effects in rhodamine-b solutions. J. Phys. Chem. 1979, 83, 350–353. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine (Lond.) 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Shi, J.; Chiu, E.; Leshchiner, E.S.; Langer, R.; Anderson, D.G. Biodegradable polymeric vectors for gene delivery to human endothelial cells. Bioconjug. Chem. 2006, 17, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, K.A.; Cu, Y.; Booth, C.J.; Saucier-Sawyer, J.K.; Wood, M.J.; Saltzman, W.M. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat. Mater. 2009, 8, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, A.C.; Herruzo, E.T.; Rausell, E.; Cena, V.; Garcia, R. Unbinding forces and energies between a siRNA molecule and a dendrimer measured by force spectroscopy. Nanoscale 2015, 7, 20267–20276. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Li, Y.X.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Ohsaki, M.; Okuda, T.; Wada, A.; Hirayama, T.; Niidome, T.; Aoyagi, H. In vitro gene Transfection using dendritic poly(L-lysine). Bioconjug. Chem. 2002, 13, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Kaneshiro, T.L.; Wang, X.; Lu, Z.R. Synthesis, characterization, and gene delivery of Poly-l-lySine octa(3-aminopropyl)silsesquioxane dendrimers: Nanoglobular drug carriers with precisely defined molecular Architectures. Mol. Pharm. 2007, 4, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Duran-Lara, E.F.; Marple, J.L.; Giesen, J.A.; Fang, Y.L.; Jordan, J.H.; Godbey, W.T.; Marican, A.; Santos, L.S.; Grayson, S.M. Investigation of lysine-functionalized dendrimers as dichlorvos detoxification agents. Biomacromolecules 2015, 16, 3434–3444. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bexiga, M.G.; Varela, J.A.; Wang, F.J.; Fenaroli, F.; Salvati, A.; Lynch, I.; Simpson, J.C.; Dawson, K.A. Cationic nanoparticles induce caspase 3-, 7- and 9-mediated cytotoxicity in a human astrocytoma cell line. Nanotoxicology 2011, 5, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, H.R.; Lan, A.P.; Hu, Y.; Li, H.P.; Chen, J. Mitochondria-targeted nanocarriers using hyperbranched polycations wrapped carbon nanotubes for augment photodynamic therapeutic effects. J. Control. Release 2017, 259, E170–E171. [Google Scholar] [CrossRef]
- Sliwka, L.; Wiktorska, K.; Suchocki, P.; Milczarek, M.; Mielczarek, S.; Lubelska, K.; Cierpial, T.; Lyzwa, P.; Kielbasinski, P.; Jaromin, A.; et al. The comparison of mtt and cvs assays for the assessment of anticancer agent interactions. PLoS ONE 2016, 11, e0155772. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.V.; Walsh, M.L.; Chen, L.B. Localization of mitochondria in living cells with rhodamine-123. Proc. Natl. Acad. Sci. USA 1980, 77, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Moyle, J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur. J. Biochem. 1969, 7, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.V.; Walsh, M.L.; Bockus, B.J.; Chen, L.B. Monitoring of relative mitochondrial-membrane potential in living cells by fluorescence microscopy. J. Cell Biol. 1981, 88, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Marrache, S.; Dhar, S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc. Natl. Acad. Sci. USA 2012, 109, 16288–16293. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Peng, H.S.; Yang, L.; You, F.T.; Teng, F.; Tang, A.W.; Zhang, F.J.; Li, X.H. Poly-l-lysine assisted synthesis of core-shell nanoparticles and conjugation with triphenylphosphonium to target mitochondria. J. Mater. Chem. B 2013, 1, 5143–5152. [Google Scholar] [CrossRef]
- Orosz, A.; Bosze, S.; Mezo, G.; Szabo, I.; Herenyi, L.; Csik, G. Oligo- and polypeptide conjugates of cationic porphyrins: Binding, cellular uptake, and cellular localization. Amino Acids 2017, 49, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Liu, G.H.; Zhang, Y.; Yan, M.Q.; Bi, H. TPP-modified protein-polymer bioconjugate as a mitochondria-targeting nanocarrier. J. Control. Release 2017, 259, E169–E170. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J. Before and after endosomal escape: Roles of stimuli-converting siRNA/polymer interactions in determining gene silencing efficiency. Acc. Chem. Res. 2012, 45, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, D.; Araya-Duran, I.; Gallego-Yerga, L.; Jativa, P.; Marquez-Miranda, V.; Canan, J.; Blanco, J.L.J.; Mellet, C.O.; Gonzalez-Nilo, F.D.; Fernandez, J.M.G.; et al. Molecular determinants for cyclooligosaccharide-based nanoparticle-mediated effective siRNA transfection. Nanomedicine (Lond.) 2017, 12, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Salmon-Chemin, L.; Buisine, E.; Yardley, V.; Kohler, S.; Debreu, M.A.; Landry, V.; Sergheraert, C.; Croft, S.L.; Krauth-Siegel, R.L.; Davioud-Charvet, E. 2- and 3-substituted 1,4-naphthoquinone derivatives as subversive substrates of trypanothione reductase and lipoamide dehydrogenase from Trypanosoma cruzi: Synthesis and correlation between redox cycling activities and in vitro cytotoxicity. J. Med. Chem. 2001, 44, 548–565. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; Perez-Martinez, F.C.; Guerra, J.; Sanchez-Verdu, P.; Ceña, V. Cofilin activation mediates Bax translocation to mitochondria during excitotoxic neuronal death. J. Neurochem. 2012, 120, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Kipp, M.; Karakaya, S.; Pawlak, J.; Araujo-Wright, G.; Arnold, S.; Beyer, C. Estrogen and the development and protection of nigrostriatal dopaminergic neurons: Concerted action of a multitude of signals, protective molecules, and growth factors. Front. Neuroendocr. 2006, 27, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, S.; Perez-Martinez, F.C.; Perez-Carrion, M.D.; Guerra, J.; Merino, S.; Sanchez-Verdu, M.P.; Ceña, V. Inhibition of p42 MAPK using a nonviral vector-delivered siRNA potentiates the anti-tumor effect of metformin in prostate cancer cells. Nanomedicine (Lond.) 2012, 7, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, J.; Posadas, I.; Jativa, P.; Bugaj-Zarebska, M.; Urbanczyk-Lipkowska, Z.; Ceña, V. Second generation amphiphilic poly-lysine dendrons inhibit glioblastoma cell proliferation without toxicity for neurons or astrocytes. PLoS ONE 2016, 11, e0165704. [Google Scholar] [CrossRef] [PubMed]
- Perez-Carrion, M.D.; Ceña, V. Knocking down hmgb1 using dendrimer-delivered sirna unveils its key role in nmda-induced autophagy in rat cortical neurons. Pharm. Res. 2013, 30, 2584–2595. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; Vellecco, V.; Santos, P.; Prieto-Lloret, J.; Ceña, V. Acetaminophen potentiates staurosporine-induced death in a human neuroblastoma cell line. Br. J. Pharm. 2007, 150, 577–585. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenström, P.; Manzanares, D.; Zhang, Y.; Ceña, V.; Malkoch, M. Evaluation of Amino-Functional Polyester Dendrimers Based on Bis-MPA as Nonviral Vectors for siRNA Delivery. Molecules 2018, 23, 2028. https://doi.org/10.3390/molecules23082028
Stenström P, Manzanares D, Zhang Y, Ceña V, Malkoch M. Evaluation of Amino-Functional Polyester Dendrimers Based on Bis-MPA as Nonviral Vectors for siRNA Delivery. Molecules. 2018; 23(8):2028. https://doi.org/10.3390/molecules23082028
Chicago/Turabian StyleStenström, Patrik, Dario Manzanares, Yuning Zhang, Valentin Ceña, and Michael Malkoch. 2018. "Evaluation of Amino-Functional Polyester Dendrimers Based on Bis-MPA as Nonviral Vectors for siRNA Delivery" Molecules 23, no. 8: 2028. https://doi.org/10.3390/molecules23082028
APA StyleStenström, P., Manzanares, D., Zhang, Y., Ceña, V., & Malkoch, M. (2018). Evaluation of Amino-Functional Polyester Dendrimers Based on Bis-MPA as Nonviral Vectors for siRNA Delivery. Molecules, 23(8), 2028. https://doi.org/10.3390/molecules23082028