Design, Synthesis, and Antiproliferative Evaluation of Novel Coumarin/2-Cyanoacryloyl Hybrids as Apoptosis Inducing Agents by Activation of Caspase-Dependent Pathway
Abstract
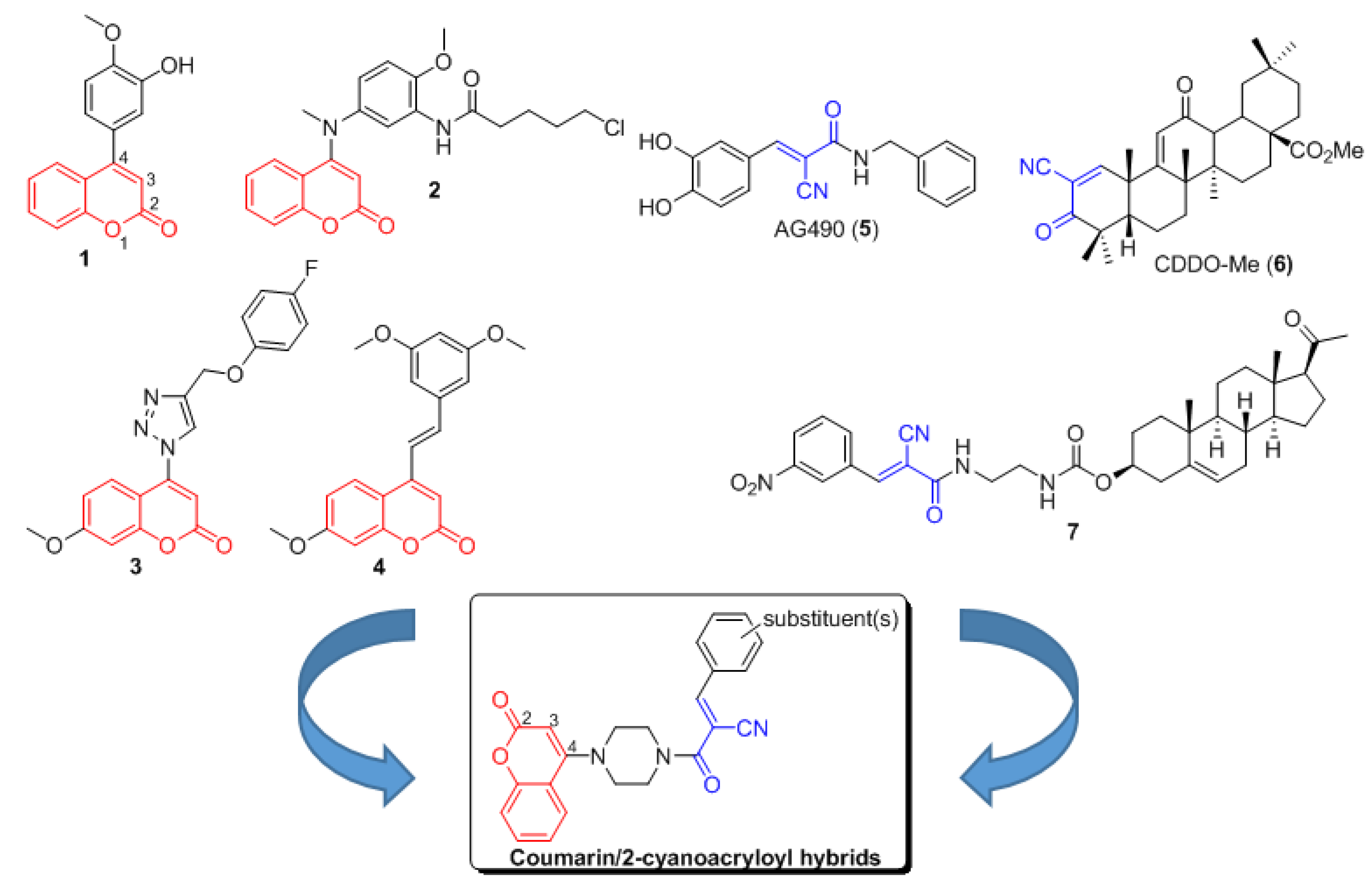
:1. Introduction
2. Results and Discussion
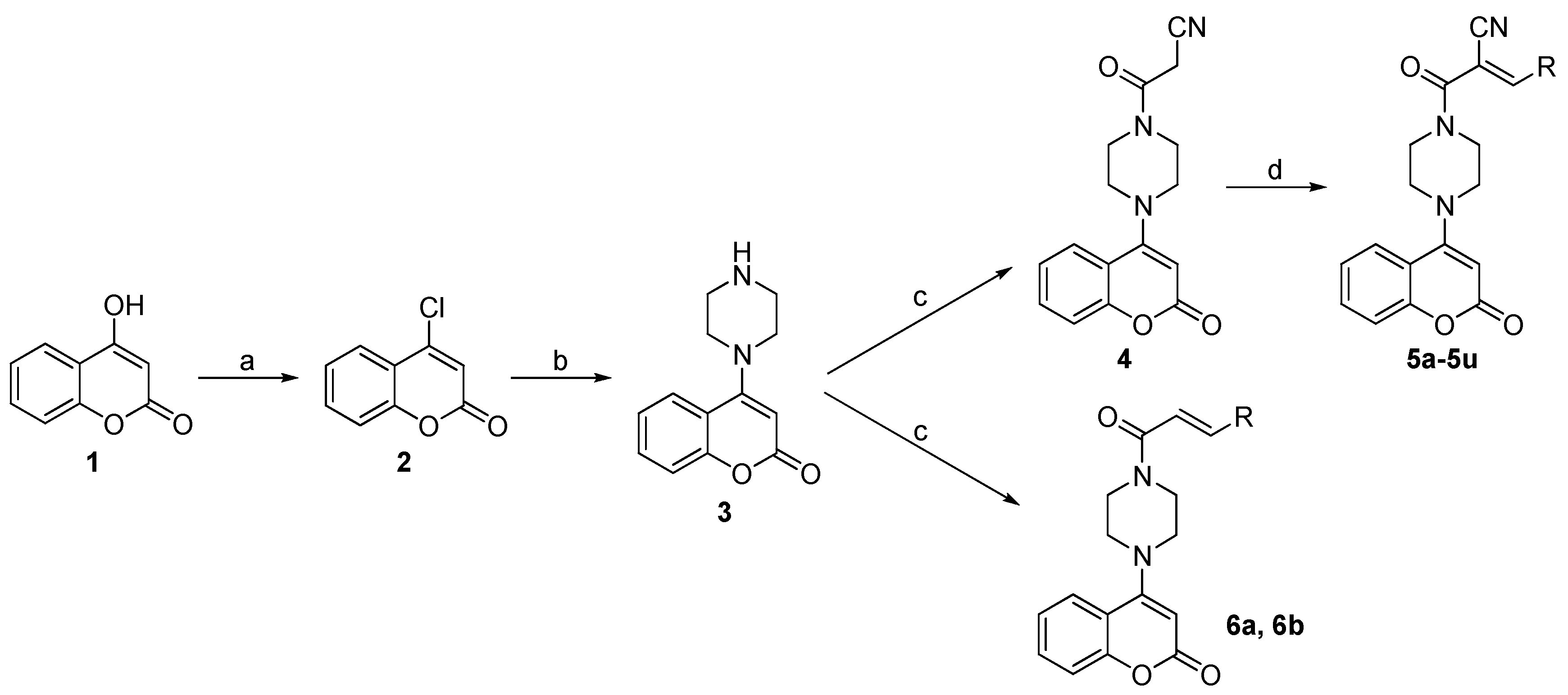
2.1. Chemistry
2.2. Antiproliferative Activity
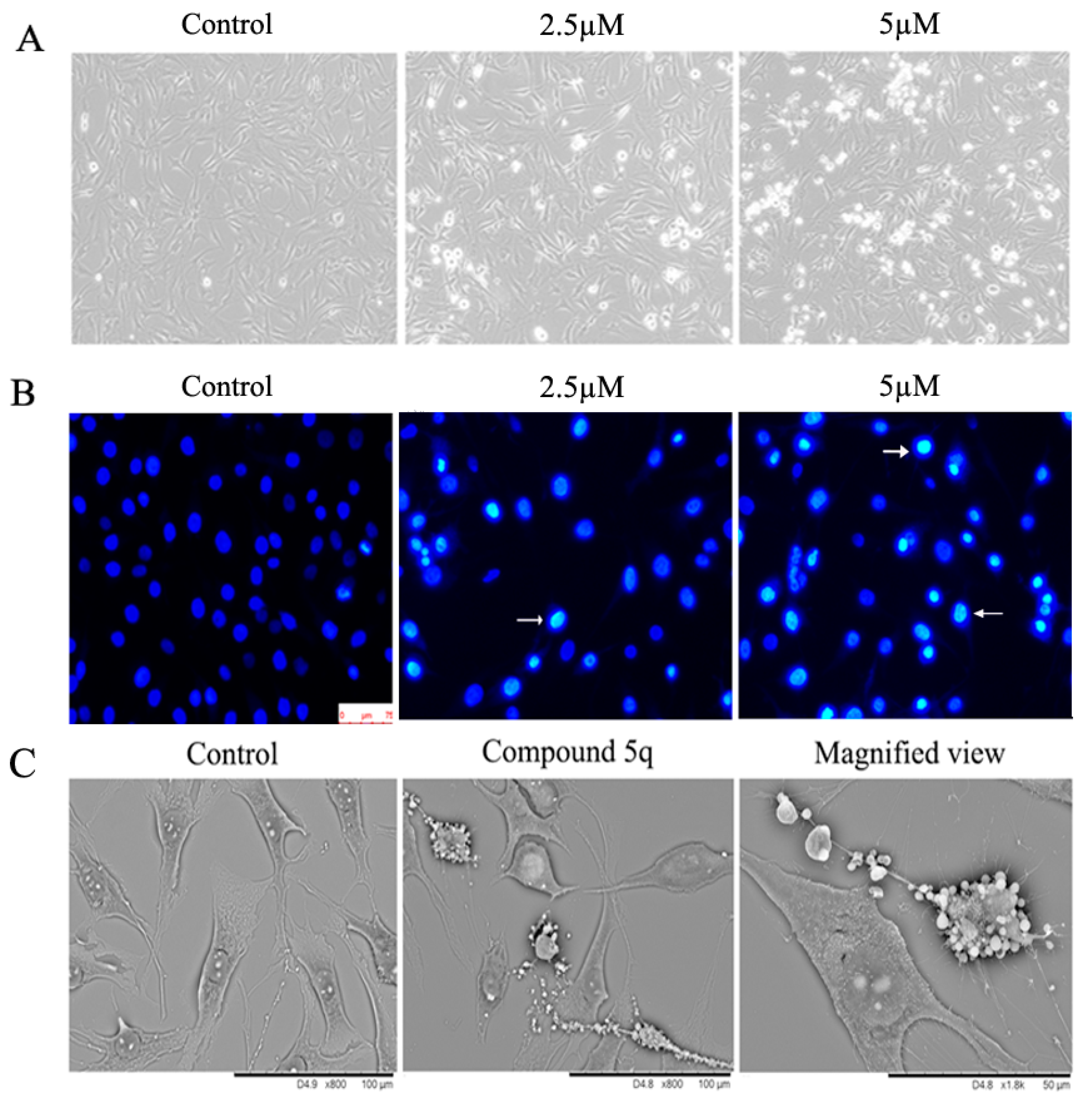
2.3. Compound 5q Induced Morphological Changes and Promoted Apoptosis of MG63 Cells
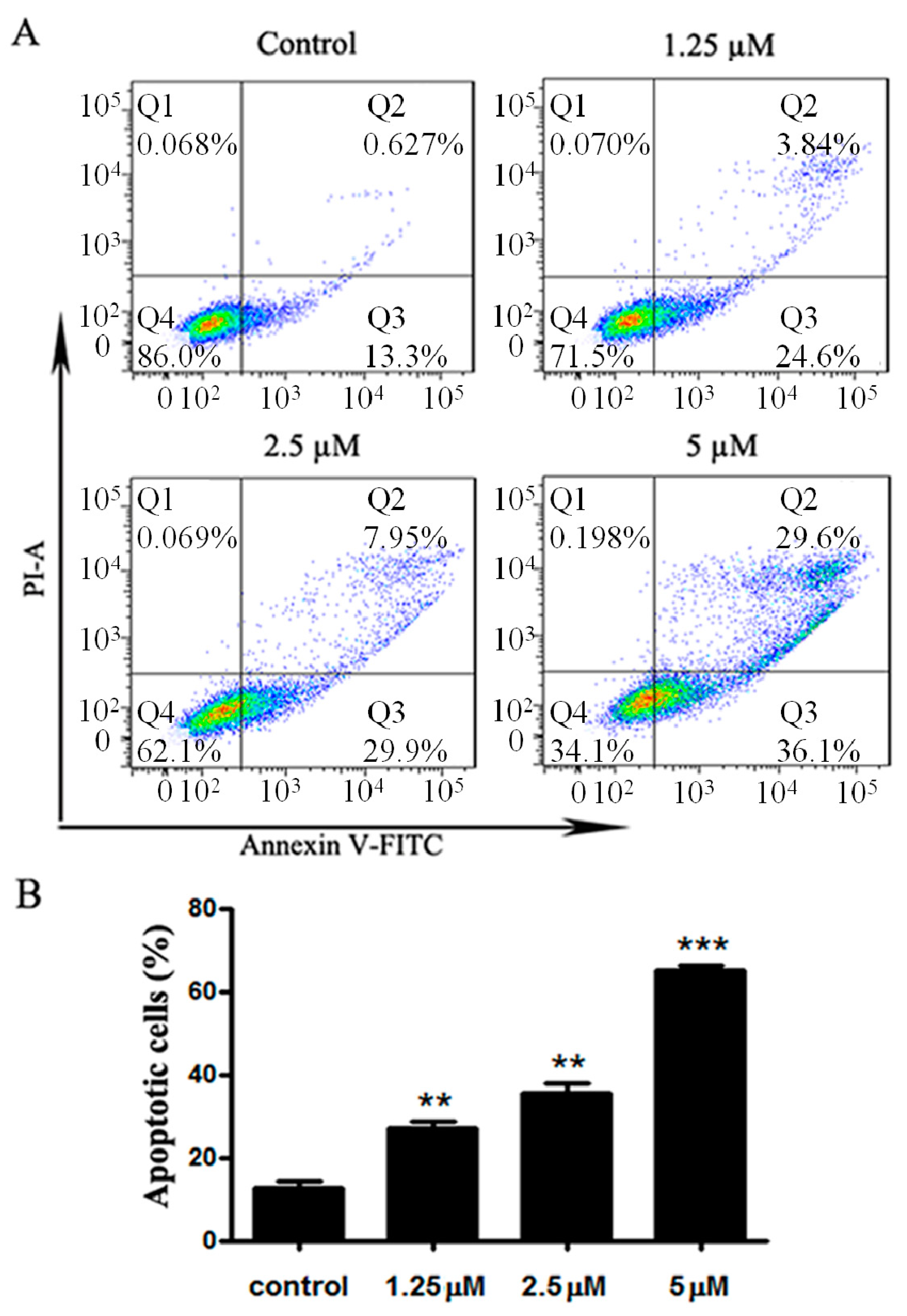
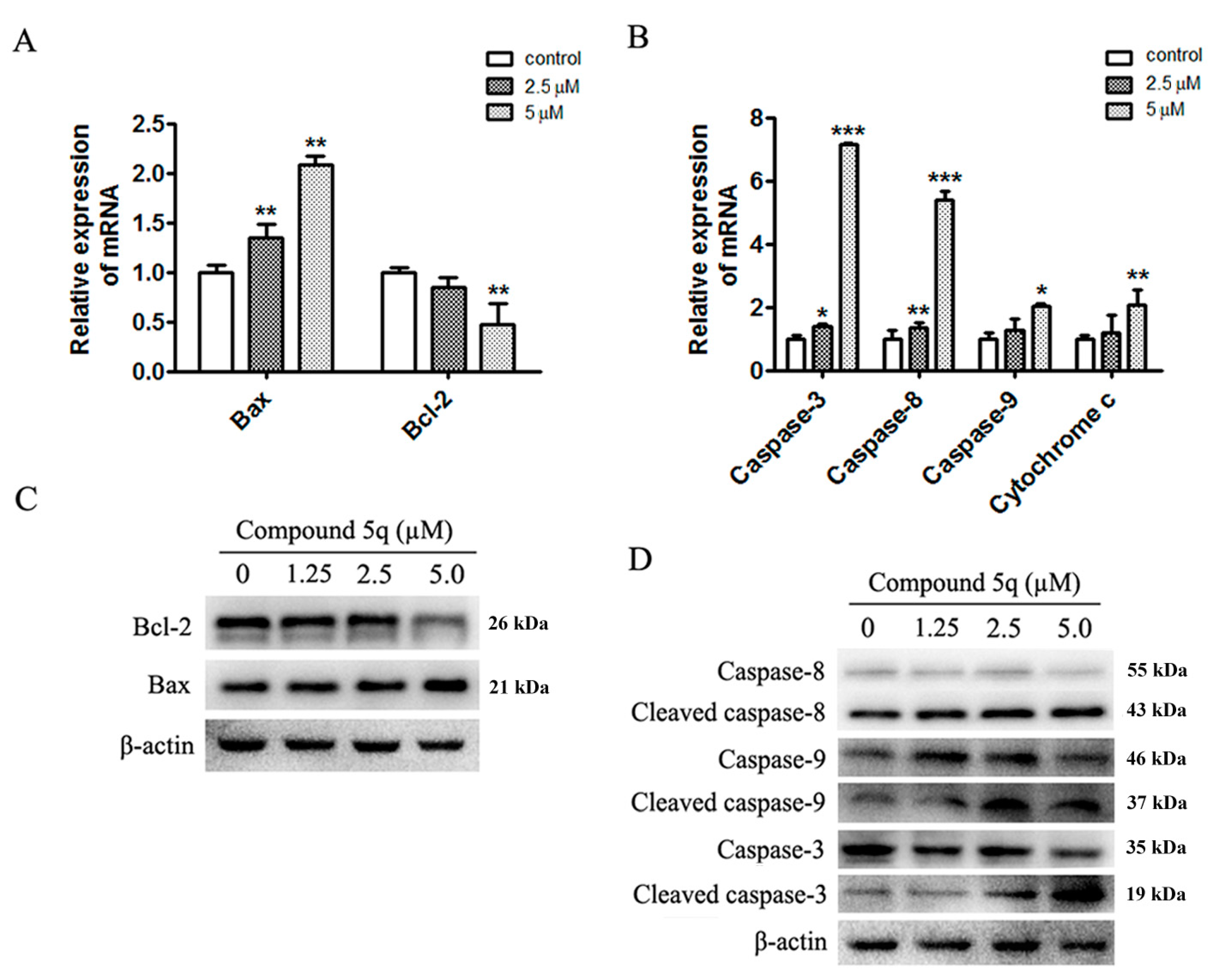
2.4. Apoptosis Induced by 5q Was Caspase-Dependent in MG63 Cells
3. Materials and Methods
3.1. Chemistry
3.1.1. 4-Chloro-2H-Chromen-2-One (2)
3.1.2. 3-Oxo-3-(4-(2-Oxo-2H-Chromen-4-yl)Piperazin-1-yl)Propanenitrile (4)
3.1.3. General Procedures for Synthesis of 5a–5u
3.1.4. General Procedures for Synthesis of 6a and 6b
3.2. Biological Evaluation Methods
3.2.1. Cell Line and Culture Condition
3.2.2. Cell Viability Assay
3.2.3. Microscopy and Photography
3.2.4. Electron Microscope Analysis
3.2.5. DNA Fragmentation Analysis with DAPI Staining
3.2.6. Apoptosis Analysis
3.2.7. Reverse Transcription Quantitative Real-Time PCR
3.2.8. Western Blotting Analysis
3.3. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, H. Cardiotoxicity of Anticancer Therapeutics. Front. Cardiovasc. Med. 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.S.A.; Su, Y.; Wang, P.; Sethi, T.; Agama, K.; Ravji, A.; Redon, C.E.; Kiselev, E.; Horzmann, K.A.; Freeman, J.L.; et al. Design and Synthesis of Chlorinated and Fluorinated 7-Azaindenoisoquinolines as Potent Cytotoxic Anticancer Agents That Inhibit Topoisomerase I. J. Med. Chem. 2017, 60, 5364–5376. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Symeonidis, T.; Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E. Synthesis and anti-inflammatory evaluation of novel angularly or linearly fused coumarins. Eur. J. Med. Chem. 2009, 44, 5012–5017. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Xu, Z.; Liu, M.L.; Feng, L.S.; Zhang, G.D. Recent Developments of Coumarin Hybrids as Anti-fungal Agents. Curr. Top. Med. Chem. 2017, 17, 3219–3231. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Martorell, M.; Capo, X.; Tur, J.A.; Pons, A.; Sureda, A. Coumarin and Derivates as Lipid Lowering Agents. Curr. Top. Med. Chem. 2017, 17, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Dadashpour, S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015, 102, 611–630. [Google Scholar] [CrossRef] [PubMed]
- Dandriyal, J.; Singla, R.; Kumar, M.; Jaitak, V. Recent developments of C-4 substituted coumarin derivatives as anticancer agents. Eur. J. Med. Chem. 2016, 119, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Combes, S.; Barbier, P.; Douillard, S.; McLeer-Florin, A.; Bourgarel-Rey, V.; Pierson, J.T.; Fedorov, A.Y.; Finet, J.P.; Boutonnat, J.; Peyrot, V. Synthesis and biological evaluation of 4-arylcoumarin analogues of combretastatins. Part 2. J. Med. Chem. 2011, 54, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Y.; Yan, W.; Wang, C.; Bai, P.; Wang, T.; Tang, M.; Wang, X.; Yang, Z.; Ma, B.; et al. Design, Synthesis, and Evaluation of in Vitro and in Vivo Anticancer Activity of 4-Substituted Coumarins: A Novel Class of Potent Tubulin Polymerization Inhibitors. J. Med. Chem. 2016, 59, 5721–5739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.; Zhou, M.; Wu, F.; Hou, X.; Luo, H.; Liu, H.; Han, X.; Yan, G.; Ding, Z.; et al. Synthesis and biological evaluation of 4-(1,2,3-triazol-1-yl)coumarin derivatives as potential antitumor agents. Bioorg. Med. Chem. Lett. 2014, 24, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Belluti, F.; Fontana, G.; Dal Bo, L.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorg. Med. Chem. 2010, 18, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yun, D.; Yao, J.; Fu, W.; Huang, F.; Chen, L.; Wei, T.; Yu, C.; Xu, H.; Zhou, X.; et al. Design, synthesis and QSAR study of novel isatin analogues inspired Michael acceptor as potential anticancer compounds. Eur. J. Med. Chem. 2018, 144, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.Z.; Sok, D.E. Michael Acceptor as a Tool for Anticancer Drug Design. Curr. Pharm. Des. 1996, 2, 247–262. [Google Scholar]
- Gersch, M.; Kreuzer, J.; Sieber, S.A. Electrophilic natural products and their biological targets. Nat. Prod. Rep. 2012, 29, 659–682. [Google Scholar] [CrossRef] [PubMed]
- Gyurkovska, V.; Stefanova, T.; Dimitrova, P.; Danova, S.; Tropcheva, R.; Ivanovska, N. Tyrosine kinase inhibitor tyrphostin AG490 retards chronic joint inflammation in mice. Inflammation 2014, 37, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Yang, Y.X.; Zhe, H.; He, Z.X.; Zhou, S.F. Bardoxolone methyl (CDDO-Me) as a therapeutic agent: An update on its pharmacokinetic and pharmacodynamic properties. Drug Des. Dev. Ther. 2014, 8, 2075–2088. [Google Scholar]
- Song, J.L.; Zhang, J.; Liu, C.L.; Liu, C.; Zhu, K.K.; Yang, F.F.; Liu, X.G.; Figueiró Longo, J.P.; Alexandre Muehlmann, L.; Azevedo, R.B.; et al. Design and synthesis of pregnenolone/2-cyanoacryloyl conjugates with dual NF-κB inhibitory and anti-proliferative activities. Bioorg. Med. Chem. Lett. 2017, 27, 4682–4686. [Google Scholar] [CrossRef] [PubMed]
- Kucuksayan, E.; Ozben, T. Hybrid Compounds as Multitarget Directed Anticancer Agents. Curr. Top. Med. Chem. 2017, 17, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Recent advances (2015–2016) in anticancer hybrids. Eur. J. Med. Chem. 2017, 142, 179–212. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, G. An overview of molecular hybrids in drug discovery. Expert Opin. Drug. Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.H.; Dicato, M.; Diederich, M. Hybrid curcumin compounds: A new strategy for cancer treatment. Molecules 2014, 19, 20839–20863. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D. Rings in drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Shaquiquzzaman, M.; Verma, G.; Marella, A.; Akhter, M.; Akhtar, W.; Khan, M.F.; Tasneem, S.; Alam, M.M. Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents. Eur. J. Med. Chem. 2015, 102, 487–529. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.K.; Syed, R.; Shin, H.S.; Patel, R.V. Piperazine derivatives for therapeutic use: A patent review (2010-present). Expert Opin. Ther. Pat. 2016, 26, 777–797. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Steuer, C.; Klein, C.D. Arylcyanoacrylamides as inhibitors of the Dengue and West Nile virus proteases. Bioorg. Med. Chem. 2011, 19, 7318–7337. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.; Odell, L.R.; Edwards, J.K.; Graham, M.E.; McGeachie, A.B.; Rusak, J.; Quan, A.; Abagyan, R.; Scott, J.L.; Robinson, P.J.; et al. Small molecule inhibitors of dynamin I GTPase activity: Development of dimeric tyrphostins. J. Med. Chem. 2005, 48, 7781–7788. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Mohamed, T.; Shakeri, A.; Rao, P.P.; Martínez-González, L.; Pérez, D.I.; Martínez, A.; Valente, M.J.; Garrido, J.; Uriarte, E.; et al. Development of Blood-Brain Barrier Permeable Nitrocatechol-Based Catechol O-Methyltransferase Inhibitors with Reduced Potential for Hepatotoxicity. J. Med. Chem. 2016, 59, 7584–7597. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Liby, K.T.; Yore, M.M.; Fu, L.; Lopchuk, J.M.; Gribble, G.W. New synthetic triterpenoids: Potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J. Nat. Prod. 2011, 74, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Suh, N.; Wang, Y.; Honda, T.; Gribble, G.W.; Dmitrovsky, E.; Hickey, W.F.; Maue, R.A.; Place, A.E.; Porter, D.M.; Spinella, M.J.; et al. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res. 1999, 59, 336–341. [Google Scholar] [PubMed]
- Los, M.; Wesselborg, S.; Schulze, O.K. The role of Caspases in development, immunity, and apoptotic signal transduction: Lessons from knockout mice. Immunity 1999, 10, 629–639. [Google Scholar] [CrossRef]
- Harvey, N.L.; Kumar, S. The role of caspases in apoptosis. Adv. Biochem. Eng. Biotechnol. 1998, 62, 107–128. [Google Scholar] [PubMed]
- Nuñez, G.; Benedict, M.A.; Hu, Y.; Inohara, N. Caspases: The proteases of the apoptotic pathway. Oncogene 1998, 17, 3237–3245. [Google Scholar] [CrossRef] [PubMed]
- Boatright, K.M.; Salvesen, G.S. Mechanisms of caspase activation. Curr. Opin. Cell. Biol. 2003, 15, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Luo, X.; Wang, X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001, 412, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 5a–5u, 6a and 6b are available from the authors. |
| Compound | R | Inhibition Ratio at 20 μM a | IC50 b (μM) |
|---|---|---|---|
| 3 | - | 1.28% | NT c |
| 4 | - | 2.31% | NT |
| 5a | 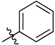 | 39.18% | NT |
| 5b | 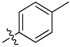 | 34.29% | NT |
| 5c |  | 26.67% | NT |
| 5d | 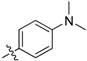 | 27.60% | NT |
| 5e | 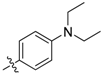 | 13.03% | NT |
| 5f | 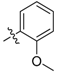 | 24.62% | NT |
| 5g | 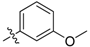 | 15.83% | NT |
| 5h | 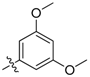 | 29.77% | NT |
| 5i | 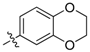 | 29.19% | NT |
| 5j | 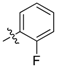 | 94.06% | 13.06 ± 0.76 |
| 5k |  | 74.79% | 18.92 ± 0.51 |
| 5l |  | 93.28% | 10.04 ± 0.25 |
| 5m | 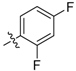 | 32.09% | NT |
| 5n |  | 95.65% | 8.37 ± 0.75 |
| 5o | 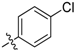 | 95.03% | 12.10 ± 0.33 |
| 5p | 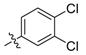 | 97.29% | 6.26 ± 0.07 |
| 5q | 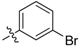 | 97.96% | 7.28 ± 0.57 |
| 5r | 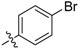 | 67.57% | 18.91 ± 1.21 |
| 5s | 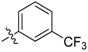 | 94.02% | 16.44 ± 0.70 |
| 5t | 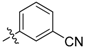 | 40.68% | NT |
| 5u | 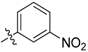 | 9.01% | NT |
| 6a | 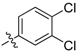 | 45.92% | NT |
| 6b | 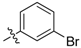 | 25.83% | NT |
| Doxorubicin | 98.7% | 1.12 ± 0.02 |
| Compound | H157 | HepG2 | MCF7 | MG63 | U2OS |
|---|---|---|---|---|---|
| 5j | >20 b | 12.84 ± 0.33 | >20 | >20 | >20 |
| 5k | >20 | >20 | >20 | >20 | >20 |
| 5l | 18.64 ± 2.13 | >20 | 10.98 ± 0.21 | 17.32 ± 0.33 | >20 |
| 5m | >20 | >20 | >20 | >20 | >20 |
| 5n | >20 | >20 | 18.75 ± 1.62 | 16.67 ± 0.34 | 9.84 ± 0.31 |
| 5o | 8.18 ± 0.50 | >20 | >20 | >20 | >20 |
| 5p | 6.09 ± 0.24 | 5.80 ± 0.26 | 13.02 ± 0.25 | 16.51 ± 0.33 | 19.60 ± 0.39 |
| 5q | 6.73 ± 0.04 | 13.32 ± 0.83 | 12.96 ± 0.11 | 5.06 ± 0.25 | 18.50 ± 0.46 |
| 5r | >20 | >20 | >20 | >20 | >20 |
| 5s | 15.88 ± 0.79 | 18.72 ± 1.43 | >20 | >20 | >20 |
| 6a | >20 | >20 | >20 | >20 | >20 |
| 6b | >20 | >20 | >20 | >20 | >20 |
| Doxorubicin | 2.88 ± 0.06 | 1.34 ± 0.02 | 1.22 ± 0.25 | 0.010 ± 0.001 | 0.012 ± 0.001 |
| Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Bax | AGCTGAGCGAGTGTCTCAAG | GTCCAATGTCCAGCCCATGA |
| Bcl-2 | GGTGAACTGGGGGAGGATTG | GGCAGGCATGTTGACTTCAC |
| Caspase-3 | TGTGAGGCGGTTGTAGAAGTT | GCTGCATCGACATCTGTACC |
| Caspase-9 | TTCCCAGGTTTTGTTTCCTG | CCTTTCACCGAAACAGCATT |
| Caspase-8 | CATCCAGTCACTTTGCCAGA | GCATCTGTTTCCCCATGTTT |
| cytochrome C | GAGATGAACAGGGGCTCGAAC | TGCTTCTGCCACATGATAACGAG |
| β-actin | GCCGCCAGCTCACCAT | TCGATGGGGTACTTCAGGGT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-Y.; Zhang, Q.-Q.; Song, J.-L.; Zhang, L.; Jiang, C.-S.; Zhang, H. Design, Synthesis, and Antiproliferative Evaluation of Novel Coumarin/2-Cyanoacryloyl Hybrids as Apoptosis Inducing Agents by Activation of Caspase-Dependent Pathway. Molecules 2018, 23, 1972. https://doi.org/10.3390/molecules23081972
Zhang Y-Y, Zhang Q-Q, Song J-L, Zhang L, Jiang C-S, Zhang H. Design, Synthesis, and Antiproliferative Evaluation of Novel Coumarin/2-Cyanoacryloyl Hybrids as Apoptosis Inducing Agents by Activation of Caspase-Dependent Pathway. Molecules. 2018; 23(8):1972. https://doi.org/10.3390/molecules23081972
Chicago/Turabian StyleZhang, Yu-Ying, Qian-Qian Zhang, Jia-Li Song, Liang Zhang, Cheng-Shi Jiang, and Hua Zhang. 2018. "Design, Synthesis, and Antiproliferative Evaluation of Novel Coumarin/2-Cyanoacryloyl Hybrids as Apoptosis Inducing Agents by Activation of Caspase-Dependent Pathway" Molecules 23, no. 8: 1972. https://doi.org/10.3390/molecules23081972
APA StyleZhang, Y.-Y., Zhang, Q.-Q., Song, J.-L., Zhang, L., Jiang, C.-S., & Zhang, H. (2018). Design, Synthesis, and Antiproliferative Evaluation of Novel Coumarin/2-Cyanoacryloyl Hybrids as Apoptosis Inducing Agents by Activation of Caspase-Dependent Pathway. Molecules, 23(8), 1972. https://doi.org/10.3390/molecules23081972






