Changes in Nutrient Profile and Antioxidant Activities of Different Fish Soups, Before and After Simulated Gastrointestinal Digestion
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Chemical Composition
2.2. Mineral Elements Analysis
2.3. Free Amino Acids Contents
2.4. Fatty Acids Profile
2.5. Antioxidant Activities
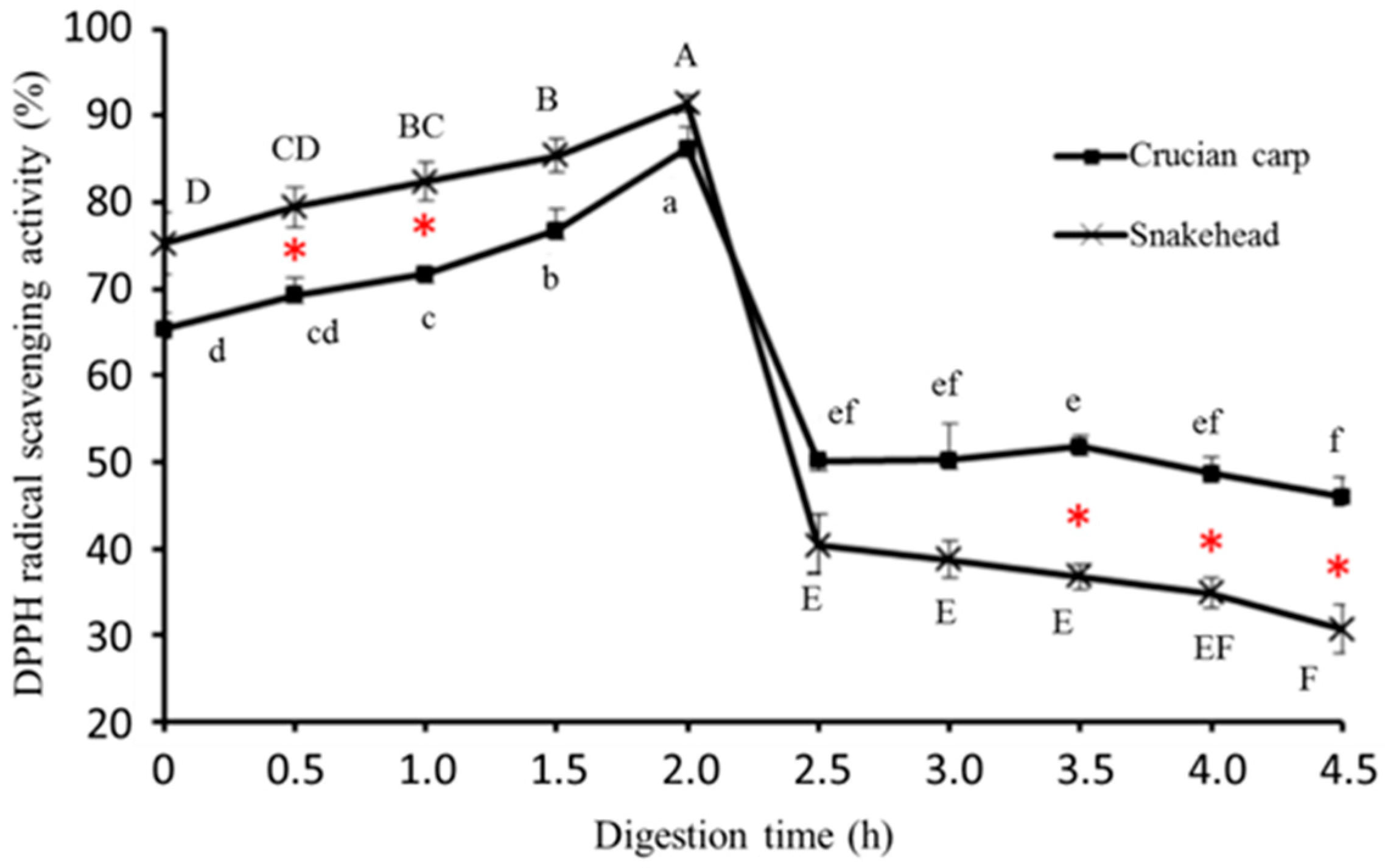
2.5.1. DPPH Radical-Scavenging Activity
2.5.2. Hydroxyl Radical-Scavenging Activity
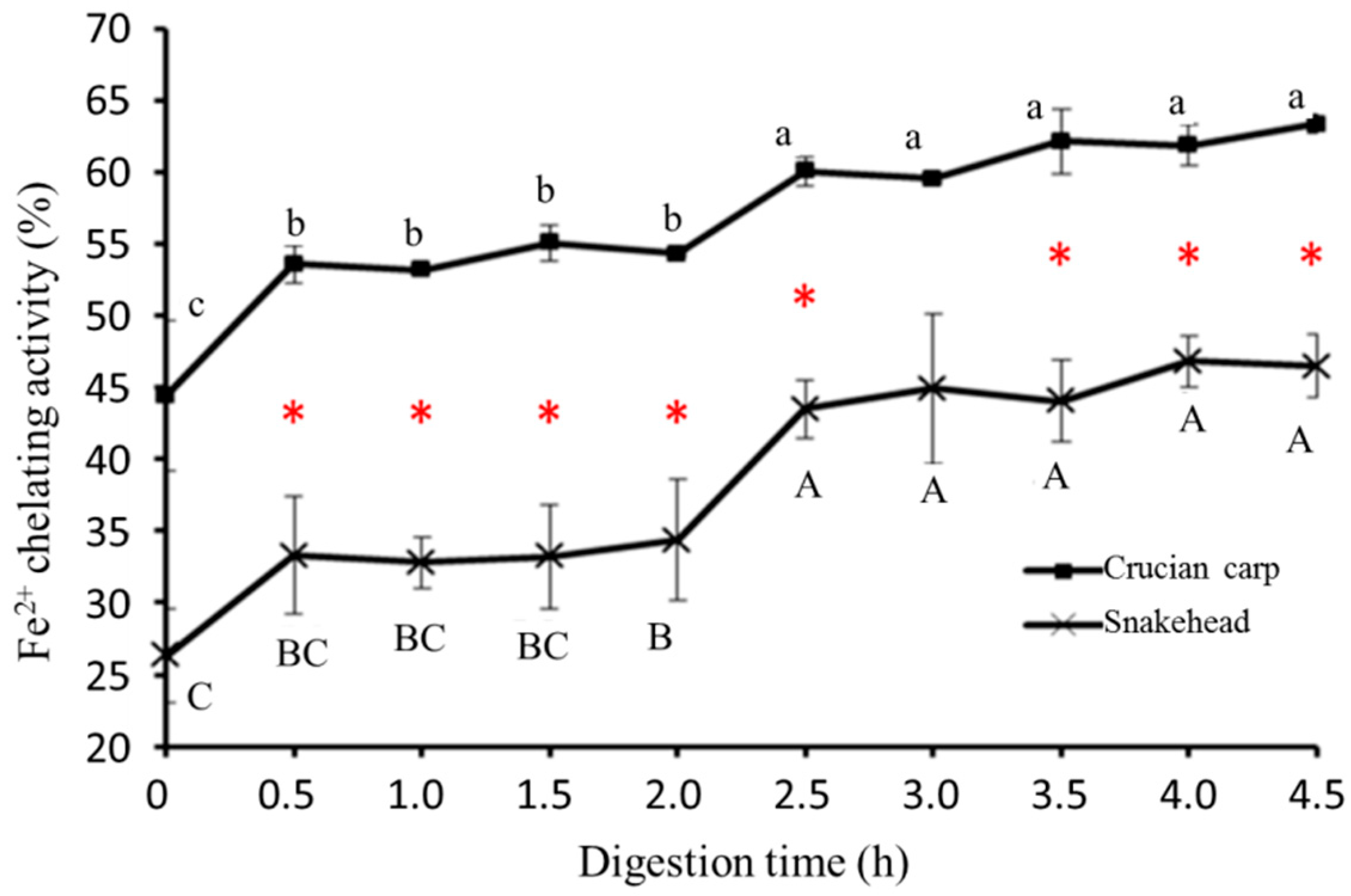
2.5.3. Ferrous Ion Chelating Activity
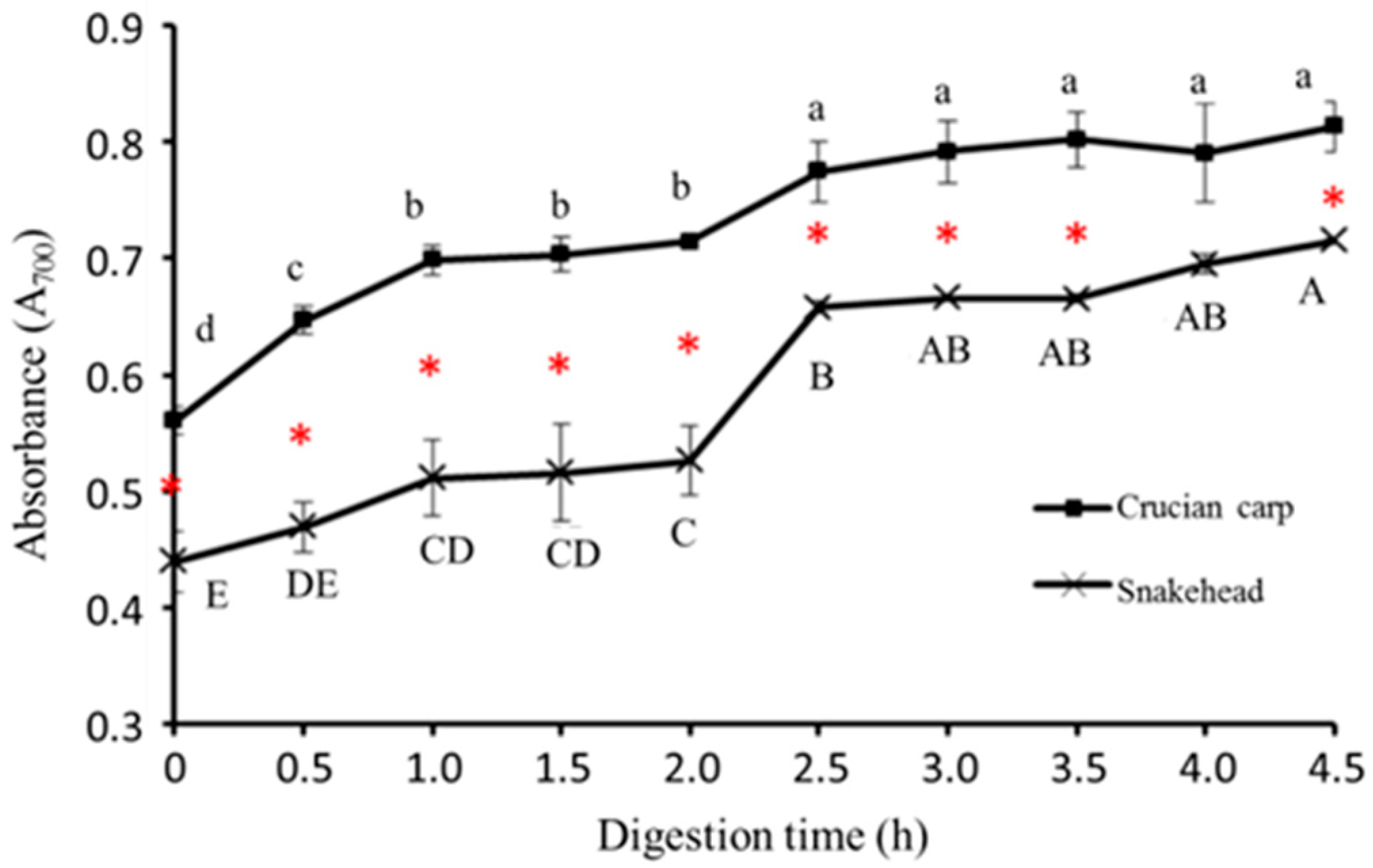
2.5.4. Reducing Power
3. Statistical Analysis
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of Fish Soup Samples
4.3. Simulated Gastrointestinal Digestion
4.4. Proximate Chemical Composition
4.5. Mineral Element Analysis
4.6. Free Amino Acid Analysis
4.7. Fatty Acid Analysis
4.8. Measurement of Antioxidant Activity
4.8.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical-Scavenging Activity
4.8.2. Hydroxyl Radical-Scavenging Activity
4.8.3. Fe2+ Chelating Activity
4.8.4. Reducing Power
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nishimura, T.; Goto, S.; Miura, K.; Takakura, Y.; Egusa, A.S.; Wakabayashi, H. Umami compounds enhance the intensity of retronasal sensation of aromas from model chicken soups. Food Chem. 2016, 196, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.W.; Hong, J.H.; Kim, K.O. Sensory characteristics and consumer acceptability of beef soup with added glutathione and/or MSG. J. Food Sci. 2010, 75, S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, M.; Xie, J.; Zhao, M.; Hou, L.; Liang, J.; Wang, S.; Cheng, J. Volatile flavor constituents in the pork broth of black-pig. Food Chem. 2017, 226, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Tu, Z.-C.; Zhang, L.; Lin, D.-R.; Sha, X.-M.; Zeng, K.; Wang, H.; Pang, J.-J.; Tang, P.-P. Characterization of volatile compounds in grass carp (ctenopharyngodon idellus) soup cooked using a traditional chinese method by GC–MS. J. Food Process. Preserv. 2016, 41, e12995. [Google Scholar] [CrossRef]
- Bertrais, S.; Galan, P.; Renault, N.; Zarebska, M.; Preziosi, P.; Hercberg, S. Consumption of soup and nutritional intake in French adults: Consequences for nutritional status. J. Hum. Nutr. Diet. 2001, 14, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Naser, M.N.; Abdullah, A.T.M.; Khan, N. Nutrient contents of some popular freshwater and marine fish species of Bangladesh. Bangl. J. Zool. 2015, 42, 251. [Google Scholar] [CrossRef]
- Das, U.N. Nutritional factors in the prevention and management of coronary artery disease and heart failure. Nutrition 2015, 31, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Saadin, K.; Bliden, K.P.; Harris, W.S.; Dinh, B.; Sharma, T.; Tantry, U.S.; Gurbel, P.A. Risk factors associated with plasma omega-3 fatty acid levels in patients with suspected coronary artery disease. Prostag. Leukotr. Ess. 2016, 113, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Borow, K.M.; Nelson, J.R.; Mason, R.P. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis 2015, 242, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.B.; Hashim, R.; Abdul Manaf, M.S.; Nor, S.A. Dietary Prebiotics and probiotics influence the growth performance, feed utilisation, and body indices of snakehead (channa striata) fingerlings. Trop. Life Sci. Res. 2016, 27, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.Q.; Hui-Qing, X.U. Cooking technology of thick crucian carp soup and its nutritional analysis. Cuisine J. Yangzhou Univ. 2005, 3, 24–26. [Google Scholar]
- Leeb, E.; Kulozik, U.; Cheison, S. Thermal pre-treatment of β-Lactoglobulin as a tool to steer enzymatic hydrolysis and control the release of peptides. Procedia Food Sci. 2011, 1, 1540–1546. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh, k.B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Selle, W.A. The effect of enteric-coated pancreatin on fat and protein digestion of depancreatized dogs. J. Nutr. 1937, 13, 15–28. [Google Scholar] [CrossRef]
- Koyyalamudi, S.R.; Jeong, S.-C.; Manavalan, S.; Vysetti, B.; Pang, G. Micronutrient mineral content of the fruiting bodies of Australian cultivated Agaricus bisporus white button mushrooms. J. Food Compos. Anal. 2013, 31, 109–114. [Google Scholar] [CrossRef]
- Sandstead, H.H.; Henriksen, L.K.; Greger, J.L.; Prasad, A.S.; Good, R.A. Zinc nutriture in the elderly in relation to taste acuity, immune response, and wound healing. Am. J. Clin. Nutr. 1982, 36, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Pena-Rivas, L.; Ortega, E.; Lopez-Martinez, C.; Olea-Serrano, F.; Lorenzo, M.L. Mineral element contents in commercially valuable fish species in Spain. Sci. World J. 2014, 2014, 949364. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Agren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Zhang, W.; Liu, Q. Lake fisheries in China: Challenges and opportunities. Fish. Res. 2013, 140, 66–72. [Google Scholar] [CrossRef]
- Thong, H.T.; Liebert, F. Amino acid requirement of growing pigs depending on amino acid efficiency and level of protein deposition. 2nd communication: Threonine. Arch. Anim. Nutr. 2004, 58, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Wakshlag, J.J.; Kallfelz, F.A.; Wakshlag, R.R.; Davenport, G.M. The effects of branched-chain amino acids on canine neoplastic cell proliferation and death. J. Nutr. 2006, 136, 2007S–2010S. [Google Scholar] [CrossRef] [PubMed]
- Monirujjaman, M.; Ferdouse, A. Metabolic and physiological roles of branched-chain amino acids. Adv. Mol. Biol. 2014, 2014, 364976. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Li, H.; Liang, M.; Yang, L. In vitro antioxidant activity of rice protein affected by alkaline degree and gastrointestinal protease digestion. J. Sci. Food Agric. 2016, 96, 4940–4950. [Google Scholar] [CrossRef] [PubMed]
- Karna, S.; Lim, W.B.; Ji, S.K.; Sang, W.K.; Bae, K.H.; Min, S.C.; Oh, H.K.; Kim, O.S.; Hong, R.C.; Kim, O.J. C16-Saturated fatty acid induced autophagy in A549 cells through topoisomerase i inhibition. Food Nutr. Sci. 2012, 3, 1220–1227. [Google Scholar]
- Kou, W.; Luchtman, D.; Song, C. Eicosapentaenoic acid (EPA) increases cell viability and expression of neurotrophin receptors in retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cells. Eur. J. Nutr. 2008, 47, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Delplanque, B.; Du, Q.; Leruyet, P.; Agnani, G.; Pages, N.; Gripois, D.; Ould-Hamouda, H.; Carayon, P.; Martin, J.C. Brain docosahexaenoic acid (DHA) levels of young rats are related to alpha-linolenic acid (ALA) levels and fat matrix of the diet: Impact of dairy fat. OCL 2011, 18, 293–296. [Google Scholar] [CrossRef]
- Fan, Y.; Meng, H.M.; Hu, G.R.; Li, F.L. Biosynthesis of nervonic acid and perspectives for its production by microalgae and other microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Anwar, S.; Basir, A. DPPH free radical scavenging activity and phenotypic difference in hepatoprotective plant (Silybum marianum L.). Toxicol. Ind. Health 2013, 29, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Bhaskar, N. In vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresour. Technol. 2008, 99, 9013–9016. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.A.; de Magalhães, J.P. A review and appraisal of the DNA damage theory of ageing. Mutat. Res. Rev. Mut. Res. 2011, 728, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.J.; Jung, W.K.; Byun, H.G.; Kim, S.K. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour. Technol. 2008, 99, 3365–3371. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Zafertel, A.; Kirecci, E. Antioxidant, antimicrobial, antifungal, and antiradical activities of cyclotrichium niveum (BOISS.) manden and scheng. Int. J. Food Prop. 2008, 11, 450–471. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Tang, X.Y.; Chen, J.; Li, G.G.; Zhu, L.J.; Dai, Y.F. Effect of processing conditions on th enutrition value of fish soup. Sci. Technol. Food Ind. 2008, 10, 248–251. [Google Scholar]
- Zheng, L.; Ren, J.; Su, G.; Yang, B.; Zhao, M. Comparison of in vitro digestion characteristics and antioxidant activity of hot- and cold-pressed peanut meals. Food Chem. 2013, 141, 4246–4252. [Google Scholar] [CrossRef] [PubMed]
- Baur, F.J.; Ensminger, L.G. AOAC—Association of Official Analytical Chemists Official Methods of Analysis, 16th ed.; AOAC: Washington, DC, USA, 1996. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Jiang, S.L.; Wu, J.G.; Feng, Y.; Yang, X.E.; Shi, C.H. Correlation analysis of mineral element contents and quality traits in milled rice (Oryza stavia L.). J. Agric. Food Chem. 2007, 55, 9608–9613. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, S.; Kawakami, K.; Morimoto, S. Changes in the free amino acid content of the shucked oyster crassostrea gigas stored in salt Water at 3. Fish. Aquat. Sci. 2013, 16, 63–69. [Google Scholar] [CrossRef]
- Hartman, L.G.; Lago, B.C.A. Rapid preparation of fatty acid methyl esters from lipids. Lab. Pract. 1973, 22, 475–477. [Google Scholar] [PubMed]
- Zhang, T.; Li, Y.; Miao, M.; Jiang, B. Purification and characterisation of a new antioxidant peptide from chickpea (Cicer arietium L.) protein hydrolysates. Food Chem. 2011, 128, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Component | Crucian Carp Soup | Snakehead Soup | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Moisture | 98.82 ± 0.10 b | 98.2 ± 0.25 c | 99.16 ± 0.12 a | 98.64 ± 0.28 b |
| Ash | 0.14 ± 0.01 c | 0.095 ± 0.00 d | 0.34 ± 0.01 b | 0.52 ± 0.02 a |
| Protein | 0.82 ± 0.11 b | 0.91 ± 0.05 a | 0.41 ± 0.02 d | 0.70 ± 0.01 c |
| Fat | 0.18 ± 0.06 a | 0.17 ± 0.01 b | 0.15 ± 0.09 c | 0.13 ± 0.01 d |
| Mineral Element | Crucian Carp Soup | Snakehead Soup | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Na (mg/g) | 0.13 ± 0.03 c | 0.69 ± 0.01 a | 0.21 ± 0.09 b | 0.69 ± 0.00 a |
| K (mg/g) | 0.79 ± 0.05 a | 0.79 ± 0.04 a | 0.75 ± 0.01 a | 0.87 ± 0.04 a |
| Ca (μg/g) | 0.13 ± 0.01 b | 0.14 ± 0.01 b | 0.81 ± 0.01 a | 0.82 ± 0.00 a |
| Mg (μg/g) | 6.12 ± 0.497 b | 6.12 ± 0.21 b | 6.45 ± 0.165 a | 6.01 ± 0.11 c |
| Zn (μg/g) | 0.07 ± 0.01 d | 0.16 ± 0.00 c | 0.25 ± 0.12 b | 0.37 ± 0.01 a |
| Fe (μg/g) | 0.12 ± 0.01 d | 0.17 ± 0.00 c | 0.35 ± 0.02 b | 0.39 ± 0.01 a |
| Amino Acid | Crucian Carp Soup | Snakehead Soup | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Asp # | 0.04 ± 0.01 d | 1.13 ± 0.01 a | 0.33 ± 0.01 c | 0.81 ± 0.01 b |
| Thr * | 1.10 ± 0.02 c | 1.05 ± 0.05 c | 2.04 ± 0.03 b | 5.01 ± 0.04 a |
| Ser | 0.66 ± 0.02 c | 1.43 ± 0.06 b | 0.54 ± 0.01 c | 1.87 ± 0.01 a |
| Glu # | 1.88 ± 0.01 a | 2.00 ± 0.08 a | 0.82 ± 0.06 b | 1.99 ± 0.01 a |
| Gly # | 35.48 ± 0.40 a | 28.75 ± 1.18 b | 22.92 ± 0.44 c | 21.44 ± 0.15 c |
| Ala # | 1.43 ± 0.01 d | 4 ± 0.18 c | 7.04 ± 0.18 b | 9.58 ± 0.22 a |
| Cys | 0.66 ± 0.06 c | 1.3 ± 0.07 b | 0.3 ± 0.22 d | 1.63 ± 0.10 a |
| Val * | 0.62 ± 0.01 b | 2.7 ± 0.13 a | 0.45 ± 0.01 b | 3.07 ± 0.01 a |
| Met | 0.52 ± 0.01 c | 1.16 ± 0.07 b | 0.27 ± 0.01 d | 1.57 ± 0.02 a |
| Ile * | 0.27 ± 0.01 c | 1.45 ± 0.07 a | 0.16 ± 0.02 c | 1.27 ± 0.01 a |
| Leu * | 0.96 ± 0.05 c | 14.42 ± 0.60 a | 0.58 ± 0.01 d | 10.69 ± 0.10 b |
| Tyr | 1.88 ± 0.04 b | 5.33 ± 0.23 a | 0.64 ± 0.21 c | 5.83 ± 0.23 a |
| Phe * | 0.68 ± 0.09 b | 15.69 ±0.67 a | 0.82 ± 0.12 b | 15.53 ± 0.08 a |
| Lys * | 0.81 ± 0.01 c | 22.07 ± 0.88 a | 2.23 ± 0.06 b | 20.31 ± 0.12 a |
| Pro | 0.84 ± 0.03 b | 0.83 ± 0.21 b | 1.16 ± 0.01 a | 1.07 ± 0.13 a |
| His | 34.58 ± 0.45 a | 29.09 ± 1.22 b | 6.9 ± 0.09 c | 6.58 ± 0.04 c |
| Arg # | 0.10 ± 0.02 d | 18.68 ± 0.72 b | 0.34 ± 0.01 c | 25.45 ± 0.21 a |
| Total Amino acid (TAA) | 82.51 ± 1.12 c | 146.87 ± 0.00 a | 47.54 ± 1.63 d | 133.13 ± 0.00 b |
| Total Essential Amino acid (TEAA) | 4.44 ± 0.03 c | 58.52 ± 2.49 a | 6.28 ± 0.02 b | 57.42 ± 0.37 a |
| EAA/TAA | 5.38% | 39.84% | 13.21% | 41.96% |
| Fatty Acid | Molecular Formula | Crucian Carp Soup | Snakehead Fish Soup | ||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| 2-Methylbutyric acid | C4:0 | 0.02 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.05 ± 0.01 a |
| Caproic acid | C6:0 | 0.01 ± 0.00 a | 0.05 ± 0.01 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b |
| Caprylic acid | C8:0 | / | 0.03±0.01 | / | / |
| Pelargonic acid | C9:0 | / | 0.06±0.01 | / | / |
| Anchoic acid | C9:0 | 0.10 ± 0.01 b | 0.37 ± 0.02 a | 0.12 ± 0.02 b | 0.04 ± 0.00 b |
| Lauric acid | C12:0 | 0.15 ± 0.02 b | 0.4 ± 0.02 a | 0.16 ± 0.04 b | 0.14 ± 0.01 b |
| Tridecanoic acid | C13:0 | 0.05 ± 0.01 b | 0.08 ± 0.01 a | 0.10 ± 0.03 a | 0.09 ± 0.01 a |
| 12-Methyl-tridecanoic acid | C13:0 | / | 0.06 ± 0.00 a | 0.10 ± 0.02 a | 0.07 ± 0.01 a |
| Myristic acid | C14:0 | 0.13 ± 0.01 c | 2.57 ± 0.21 b | 0.15 ± 0.04 c | 4.19 ± 0.29 a |
| Pentadecanoic acid | C15:0 | 0.61 ± 0.03 b | 0.85 ± 0.03 a | 0.62 ± 0.05 b | 0.92 ± 0.05 a |
| Palmitic acid | C16:0 | 23.56 ± 0.43 b | 21.47 ± 0.54 c | 25.31 ± 0.64 a | 23.42 ± 1.48 b |
| 14-Methylpalmitic acid | C16:0 | 0.13 ± 0.02 b | 1.3 ± 0.1 a | 0.20 ± 0.02 b | / |
| Heptadecanoic acid | C17:0 | 0.46 ± 0.05 c | 0.69 ± 0.01 b | 0.73 ± 0.07 b | 0.8 ± 0.02 a |
| Stearic acid | C18:0 | 7.22 ± 0.05 c | 8.15 ± 0.15 b | 8.61 ± 0.36 b | 9.48 ± 0.72 a |
| Nonadecanoic acid | C19:0 | 0.15 ± 0.01 b | 0.14 ± 0.01 b | 0.12 ± 0.03 b | 0.22 ± 0.01 a |
| Arachidic acid | C20:0 | 0.36 ± 0.04 b | 0.35 ± 0.01b | / | 0.46 ± 0.01 a |
| Heneicosanoic acid | C21:0 | / | 0.4 ± 0.02 a | 0.18 ± 0.04 b | 0.04 ± 0.01 c |
| Behenic acid | C22:0 | 0.10 ± 0.01 b | 0.13 ± 0.02 b | 0.06 ± 0.01 c | 0.18 ± 0.01 a |
| Tricosanoic acid | C23:0 | / | 0.02 ± 0.01 a | / | 0.03 ± 0.00 a |
| Lignoceric acid | C24:0 | / | 0.03 ± 0.01 a | 0.06 ± 0.01 a | 0.07 ± 0.01 a |
| Saturated fatty acid (SFA) | 37.60 ± 0.77 b | 37.17 ± 0.79 b | 40.73 ± 0.43 a | 40.21 ± 0.5 a | |
| Myristoleic acid | C14:1 | 0.13 ± 0.01 b | 0.11 ± 0.02 c | 0.15 ± 0.04 b | 0.18 ± 0.02 a |
| Palmitoleic acid | C16:1 | 9.39 ± 0.15 a | 7.59 ± 0.24 b | 9.57 ± 0.44 a | 9.07 ± 0.24 a |
| Oleic acid | C18:1n9 | 44.08 ± 0.08 b | 48.42 ± 1.22 a | 38.42 ± 0.33 c | 42.81 ± 0.57 b |
| Erucic acid | C22:1 | 0.62 ± 0.03 b | 0.98 ± 0.06 a | 0.14 ± 0.02 c | 1.15 ± 0.07 a |
| Nervonic acid | C24:1 | / | 0.01 ± 0.00 b | 0.07 ± 0.02 a | 0.08 ± 0.02 a |
| Monounsaturated fatty acid (MUFA) | 54.34 ± 0.22 b | 57.12 ± 1.07 a | 48.34 ± 0.83 c | 53.29 ± 0.31 b | |
| Linoleic acid | C18:2n6 | / | 0.42±0.03 a | 0.14 ± 0.03 b | 0.06 ± 0.01 c |
| Arachidonic acid | C20:4n6 | 1.52 ± 0.03 a | 1.25 ± 0.08 b | 1.83 ± 0.10 a | 1.11 ± 0.14 b |
| Cis-5,8,11,14,17-Eicosapentaenoic acid | C20:5n3 | 2.04 ± 0.06 b | 1.26 ± 0.06 c | 3.02 ± 0.09 a | 1.61 ± 0.19 c |
| Cis-8,11,14-Eicosatrienoic acid | C20:3 | 0.45 ± 0.03 a | 0.66 ± 0.09 a | / | / |
| Linolenic acid | C18:3 | 0.61 ± 0.01 a | 0.73 ± 0.01 a | 0.21±0.03 b | / |
| Cis-11,14-Eicosadienoic acid | C20:2 | / | / | / | 0.03 ± 0.00 |
| Docosahexenoic acid | C22:6n3 | 3.30 ± 0.03 b | 1.33 ± 0.12 c | 5.80 ± 0.27 a | 3.41 ± 0.22 b |
| Polyunsaturated fatty acids (PUFA) | 7.92 ± 0.12 b | 5.64 ± 0.08 d | 11.01 ± 0.12 a | 6.23 ± 0.23 c | |
| Unsaturated fatty acid (UFA) | 62.26 ± 0.34 a | 62.76 ± 1.01 a | 59.35 ± 0.71 b | 59.52 ± 0.30 b | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Zheng, S.; Feng, Y.; Shen, G.; Xiong, S.; Du, H. Changes in Nutrient Profile and Antioxidant Activities of Different Fish Soups, Before and After Simulated Gastrointestinal Digestion. Molecules 2018, 23, 1965. https://doi.org/10.3390/molecules23081965
Zhang G, Zheng S, Feng Y, Shen G, Xiong S, Du H. Changes in Nutrient Profile and Antioxidant Activities of Different Fish Soups, Before and After Simulated Gastrointestinal Digestion. Molecules. 2018; 23(8):1965. https://doi.org/10.3390/molecules23081965
Chicago/Turabian StyleZhang, Gaonan, Shujian Zheng, Yuqi Feng, Guo Shen, Shanbai Xiong, and Hongying Du. 2018. "Changes in Nutrient Profile and Antioxidant Activities of Different Fish Soups, Before and After Simulated Gastrointestinal Digestion" Molecules 23, no. 8: 1965. https://doi.org/10.3390/molecules23081965
APA StyleZhang, G., Zheng, S., Feng, Y., Shen, G., Xiong, S., & Du, H. (2018). Changes in Nutrient Profile and Antioxidant Activities of Different Fish Soups, Before and After Simulated Gastrointestinal Digestion. Molecules, 23(8), 1965. https://doi.org/10.3390/molecules23081965






