Atriplex mollis Desf. Aerial Parts: Extraction Procedures, Secondary Metabolites and Color Analysis
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
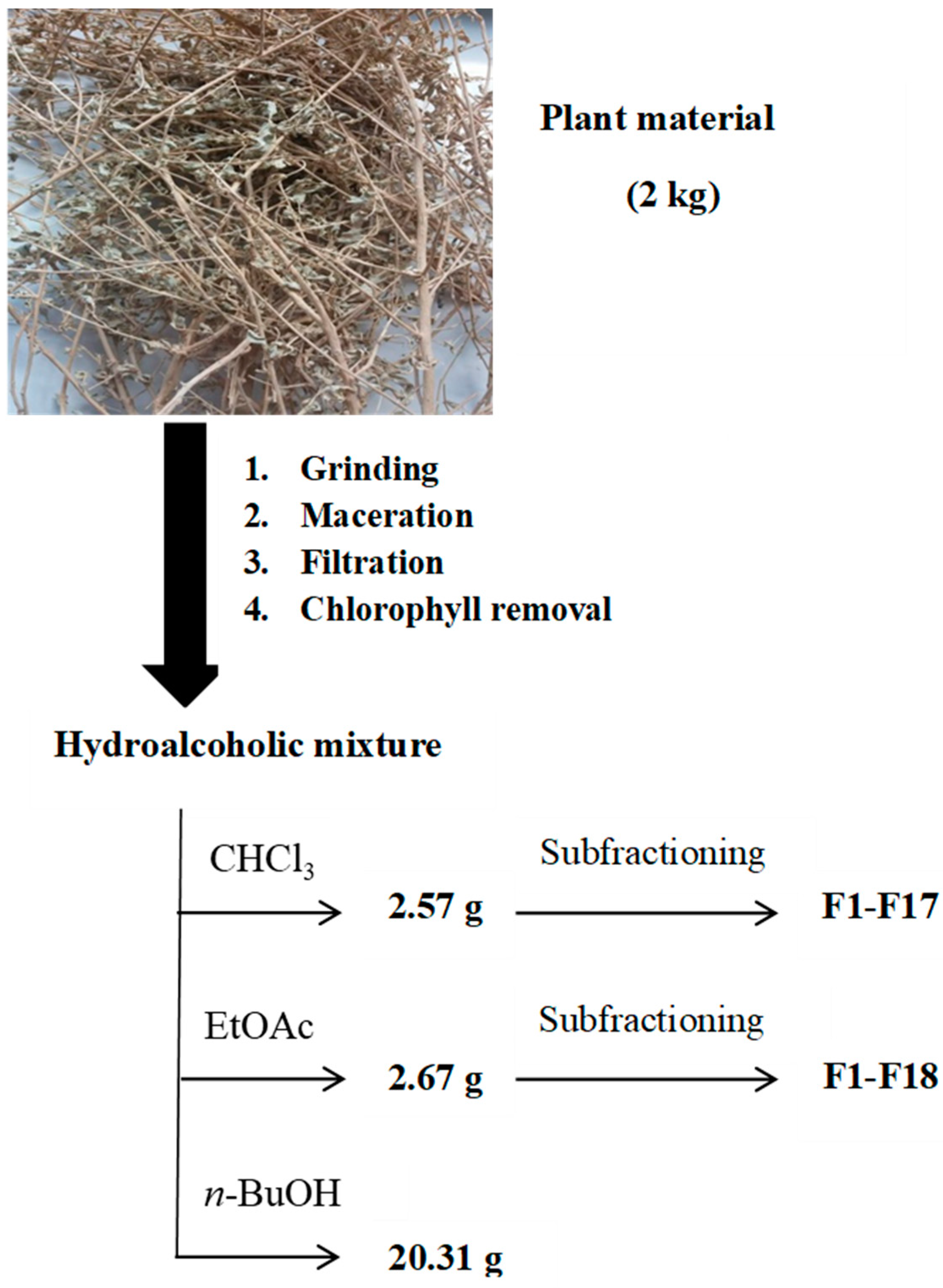
2.2. Plant Material
3. Extraction Techniques
3.1. Maceration and Further Fractionation
3.2. Supercritical Fluid Extraction (SFE)
3.3. Microwave-Assisted Extraction (MAE)
3.4. HPLC-PDA Method for the Analysis of Phenolic Compounds
4. Color Evaluation
5. Pigment Determination
6. Results and Discussion
6.1. HPLC Analysis of Hydroalcoholic Extract and Subfractions
6.2. HPLC Analysis of Supercritical Carbon Dioxide Extracts
6.3. HPLC Analysis of Microwave-Assisted Extracts
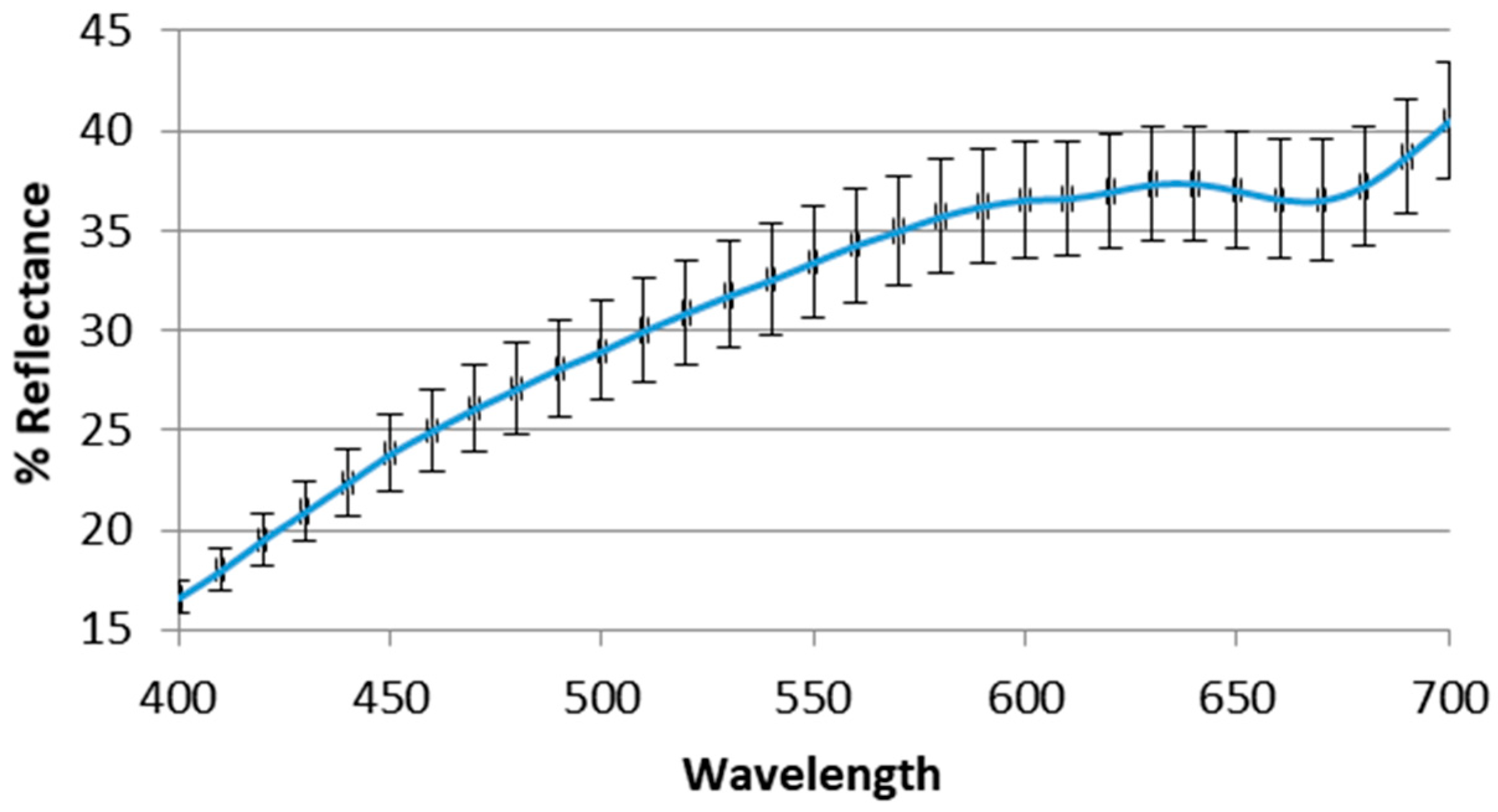
6.4. Color Analysis
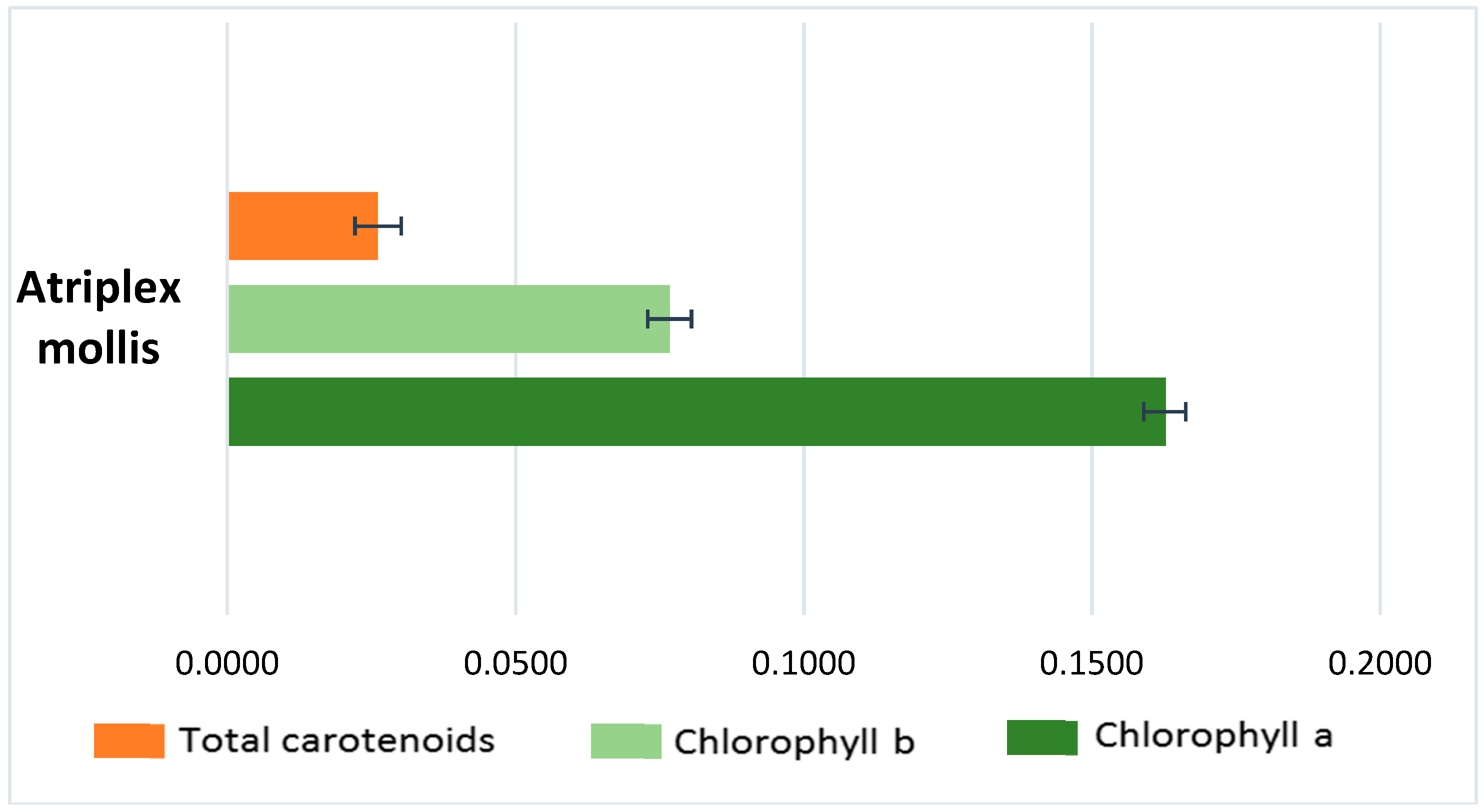
6.5. Pigments Determination (Total Carotenoids, Chlorophyll a and Chlorophyll b)
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Glenn, E.; Brown, J. Effects of soil salt levels on the growth and water use efficiency of Atriplex canescens (Chenopodiaceae) varieties in drying soil. Am. J. Bot. 1998, 85, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Smail-Saadoun, N. Anatomical adaptation of Algerian Sahara Chenopodiaceae to severe drought conditions. Sécheresse 2005, 16, 121–124. [Google Scholar]
- Ortíz-Dorda, J.; Martínez-Mora, C.; Correal, E.; Simón, B.; Cenis, J.L. Genetic structure of Atriplex halimus populations in the Mediterranean basin. Ann. Bot. 2005, 95, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Goldhirs, A.G.; Hankamer, B.; Lirs, S.H. Hydroxyproline and proline content and cell wall of sunflower, peanut and cotton under salt stress. Plant Sci. 1990, 69, 27–32. [Google Scholar] [CrossRef]
- Belkheiri, O.; Mulas, M. The effects of salt stress on growth, water relations and ion accumulation in two halophyte Atriplex species. Environ. Exp. Bot. 2013, 86, 17–28. [Google Scholar] [CrossRef]
- Hassine, A.B.; Lutts, S. Differential responses of saltbush Atriplex halimus L. exposed to salinity and water stress in relation to senescing hormones abscisic acid and ethylene. J. Plant Physiol. 2010, 167, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Glenn, E.P.; Nelson, S.G.; Ambrose, B.; Martinez, R.; Soliz, D.; Pabendinskas, V.; Hultine, K. Comparison of salinity tolerance of three Atriplex spp. in well-watered and drying soils. Environ. Exp. Bot. 2012, 83, 62–72. [Google Scholar] [CrossRef]
- Katembe, W.J.; Ungar, I.A.; Mitchell, J.P. Effect of salinity on germination and seedling growth of two Atriplex species (Chenopodiaceae). Ann. Bot. 1998, 82, 167–175. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Krishchenko, V.P.; Rotar, A.I.; Zadnipryanyi, Yu.F.; Kosorukov, M.L.; Pratov, U.; Anofrina, N.D. Chemical composition and nutritive value of plants of the family Chenopodiaceae of a Libyan rangeland massif. Izv. Timiryazevskoi Sel’skokhozyaistvennoi Akad. 1984, 4, 38–45. [Google Scholar]
- Ramani, B.; Zorn, H.; Papenbrock, J. Quantification and fatty acid profiles of sulfolipids in two halophytes and a glycophyte grown under different salt concentrations. Z. Naturforsch. 2004, 59, 835–842. [Google Scholar] [CrossRef]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gao, Y.; Wang, D.; Chen, J.; Zhang, F.; Zhou, J.; Yan, X.; Li, Y. Stoichiometric variation of halophytes in response to changes in soil salinity. Plant Biol. 2017, 19, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Rodriguez, A.; Lozano, E.A.; Duenez, J.; Aguilar, C.N.; Arevalo, J.R. The food habits of goats on rangelands with different amounts of four wing saltbush (Atriplex canescens) cover. J. Arid Environ. 2012, 84, 91–96. [Google Scholar] [CrossRef]
- Chemat, F.; Esveld, E. Microwave super-heated boiling of organic liquids: Origin, effect and application. Chem. Eng. Technol. 2001, 24, 735–744. [Google Scholar] [CrossRef]
- Mollica, A.; Locatelli, M.; Macedonio, G.; Carradori, S.; Sobolev, A.P.; De Salvador, R.F.; Monti, S.M.; Buonanno, M.; Zengin, G.; Angeli, A.; et al. Microwave-assisted extraction, HPLC analysis, and inhibitory effects on carbonic anhydrase I, II, VA, and VII isoforms of 14 blueberry Italian cultivars. J. Enzyme Inhib. Med. Chem. 2016, 31 (Suppl. 4), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Clydesdale, F.M.; Ahmed, E.M. Colorimetry-methodology and applications. CRC Crit. Rev. Food Sci. Nutr. 1978, 10, 243–301. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.E.; Chivkunova, O.B.; Merzlyak, M.N.; Reshetnikova, I.V. A spectrophotometric analysis of pigments in apples. Russ. J. Plant Physiol. 2001, 48, 693–700. [Google Scholar] [CrossRef]
- Boutaoui, N.; Zaiter, L.; Benayache, F.; Benayache, S.; Carradori, S.; Cesa, S.; Giusti, A.M.; Campestre, C.; Menghini, L.; Innosa, D.; et al. Qualitative and quantitative phytochemical analysis of different extracts from Thymus algeriensis aerial parts. Molecules 2018, 23, 463. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Locatelli, M.; Guglielmi, P.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Martinotti, S.; Brunetti, L.; et al. Protective effects induced by microwave-assisted aqueous Harpagophytum extract on rat cortex synaptosomes challenged with amyloid β-peptide. Phytother. Res. 2017, 31, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Ferrante, C.; Carradori, S.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Recinella, L.; Orlando, G.; Martinotti, S.; et al. Optimization of aqueous extraction and biological activity of Harpagophytum procumbens root on ex vivo rat colon inflammatory model. Phytother. Res. 2017, 31, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Gođevac, D.; Stanković, J.; Novaković, M.; Anđelković, B.; Dajić-Stevanović, Z.; Petrović, M.; Stanković, M. Phenolic compounds from Atriplex littoralis and their radiation-mitigating activity. J. Nat. Prod. 2015, 78, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Ben Nejma, A.; Znati, M.; Nguir, A.; Daich, A.; Othman, M.; Lawson, A.M.; Ben Jannet, H. Phytochemical and biological studies of Atriplex inflata f. Muell.: Isolation of secondary bioactive metabolites. J. Pharm. Pharmacol. 2017, 69, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Kamal, Z.; Ullah, F.; Ayaz, M.; Sadiq, A.; Ahmad, S.; Zeb, A.; Hussain, A.; Imran, M. Anticholinesterase and antioxidant investigations of crude extracts, subsequent fractions, saponins and flavonoids of Atriplex laciniata L.: Potential effectiveness in Alzheimer’s and other neurological disorders. Biol. Res. 2015, 48, 21. [Google Scholar] [CrossRef] [PubMed]
- Clauser, M.; Dall’Acqua, S.; Loi, M.C.; Innocenti, G. Phytochemical investigation on Atriplex halimus L. from Sardinia. Nat. Prod. Res. 2013, 27, 1940–1944. [Google Scholar] [CrossRef] [PubMed]
- Stanely Mainzen Prince, P.; Rajakumar, S.; Dhanasekar, K. Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur. J. Pharmacol. 2011, 688, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, M.C.; Um, J.Y.; Hong, S.H. The beneficial effect of vanillic acid on ulcerative colitis. Molecules 2010, 15, 7208–7217. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Kobayashi, M.; Tamesada, M.; Yagi, K. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin A-induced liver injury. Biol. Pharm. Bull. 2009, 32, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Selloum, L.; Bouriche, H.; Tigrine, C.; Boudoukha, C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp. Toxicol. Pathol. 2003, 54, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Alinejad, B.; Ghorbani, A.; Sadeghnia, H.R. Effects of combinations of curcumin, linalool, rutin, safranal, and thymoquinone on glucose/serum deprivation-induced cell death. Avicenna J. Phytomed. 2013, 3, 321–328. [Google Scholar] [PubMed]
- Sadeghnia, H.R.; Yousefsani, B.S.; Rashidfar, M.; Boroushaki, M.T.; Asadpour, E.; Ghorbani, A. Protective effect of rutin on hexachlorobutadiene-induced nephrotoxicity. Renal Fail. 2013, 35, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Janbaz, K.H.; Saeed, S.A.; Gilani, A.H. Protective effect of rutin on paracetamol-and CCl4-induced hepatotoxicity in rodents. Fitoterapia 2002, 73, 557–563. [Google Scholar] [CrossRef]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of processing effects on anthocyanin content and colour modifications of blueberry (Vaccinium spp.) extracts: Comparison between HPLC-DAD and CIELAB analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Cesa, S.; Casadei, M.A.; Cerreto, F.; Paolicelli, P. Infant milk formulas: Effect of storage conditions on the stability of powdered products towards autoxidation. Foods 2015, 4, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, L.A.; Ivanova, L.A.; Ronzhina, D.A.; Yudina, P.K. Changes in the chlorophyll and carotenoid contents in the leaves of steppe plants along a latitudinal gradient in South Ural. Russ. J. Plant Physiol. 2013, 60, 812–820. [Google Scholar] [CrossRef]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their derivatives used in food industry and medicine. Mini-Rev. Med. Chem. 2017, 17, 1194–1222. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.P.; Mannina, L.; Capitani, D.; Sanzò, G.; Ingallina, C.; Botta, B.; Fornarini, S.; Crestoni, M.E.; Chiavarino, B.; Carradori, S.; Locatelli, M.; et al. A multi-methodological approach in the study of Italian PDO “Cornetto di Pontecorvo” red sweet pepper. Food Chem. 2018, 255, 120–131. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Limited Atriplex mollis Desf. aerial parts samples are available from the authors of the Unité de recherché Valorisation des Ressources Naturelles, Molécules Bioactives et Analyses Physicochimiques et Biologiques, Université Frères Mentouri, Constantine 1, Route d’Aïn El Bey, 25000 Constantine, Algérie. |
| Identified Compound | Phenolics Content (µg/g DW ± SD) | ||||||
|---|---|---|---|---|---|---|---|
| F4 (CHCl3) | F7 (CHCl3) | F13 (CHCl3) | F6 (EtOAc) | F10 (EtOAc) | F16 (EtOAc) | n-BuOH | |
| Gallic acid | 0.23 ± 0.02 | 0.70 ± 0.07 | 1.5 ± 0.1 | 0.84 ± 0.07 | |||
| Catechin | 9.9 ± 0.9 | 0.94 ± 0.08 | 2.7 ± 0.1 | 34.0 ± 2.2 | 0.80 ± 0.06 | ||
| Chlorogenic acid | 1.0 ± 0.1 | ||||||
| p-Hydroxy-benzoic acid | 0.36 ± 0.04 | 115 ± 8 | 0.49 ± 0.05 | ||||
| Vanillic acid | 0.46 ± 0.06 | 7.9 ± 0.7 | 42.2 ± 1.3 | 1.3 ± 0.1 | 0.48 ± 0.05 | 7.7 ± 0.7 | |
| Epicatechin | 2.6 ± 0.1 | 3.2 ± 0.2 | 158 ± 10 | 2.2 ± 0.3 | 4.0 ± 0.3 | 8.6 ± 0.8 | 0.31 ± 0.02 |
| Syringic acid | 2.2 ± 0.1 | 0.37 ± 0.03 | |||||
| 3-Hydroxy benzoic acid | 0.19 ± 0.02 | 1.5 ± 0.1 | |||||
| 3-OH,4-MeO benzoic acid | 0.53 ± 0.05 | 1.9 ± 0.2 | 0.23 ± 0.02 | 6.0 ± 0.5 | 4.1 ± 0.4 | ||
| p-Coumaric acid | 24.9 ± 1.2 | 20.3 ± 2.3 | |||||
| Rutin | 9.1 ± 0.8 | 65.1 ± 5.3 | 1.0 ± 0.1 | 12.3 ± 0.2 | |||
| Sinapinic acid | 8.6 ± 0.8 | 1.8 ± 0.1 | |||||
| t-Ferulic acid | 1.8 ± 0.2 | 0.93 ± 0.08 | |||||
| Naringin | 0.17 ± 0.02 | 5.8 ± 0.3 | 6.3 ± 0.2 | 0.17 ± 0.01 | 9.6 ± 0.8 | ||
| 2,3-Dimethoxy-benzoic acid | 0.28 ± 0.02 | ||||||
| Benzoic acid | 2.3 ± 0.1 | 2.6 ± 0.3 | 12.6 ± 1.5 | 3.8 ± 0.4 | 0.25 ± 0.02 | 7.4 ± 0.5 | |
| o-Coumaric acid | 0.28 ± 0.02 | 0.17 ± 0.01 | 0.84 ± 0.07 | ||||
| Quercetin | 0.96 ± 0.08 | ||||||
| t-Cinnamic acid | 0.51 ± 0.04 | ||||||
| Naringenin | 0.16 ± 0.01 | 7.2 ± 0.7 | 0.11 ± 0.01 | ||||
| Carvacrol | 0.19 ± 0.01 | 0.44 ± 0.02 | 0.28 ± 0.02 | 0.15 ± 0.01 | |||
| Total | 31.9 | 29.8 | 241 | 157.5 | 80.1 | 76.3 | 17.1 |
| Identified Compound | Phenolics Content (µg/g DW ± SD) | |
|---|---|---|
| 40 °C, 10 MPa | 40 °C, 30 MPa | |
| Gallic acid | 0.04 ± 0.01 | 0.02 ± 0.01 |
| Catechin | 0.21 ± 0.05 | 0.32 ± 0.03 |
| Vanillic acid | 0.29 ± 0.06 | 1.75 ± 0.09 |
| Epicatechin | 0.76 ± 0.05 | |
| Syringic acid | 0.14 ± 0.02 | |
| 3-OH,4-MeO benzaldehyde | 0.25 ± 0.04 | 0.82 ± 0.03 |
| p-Coumaric acid | 0.24 ± 0.07 | 0.04 ± 0.01 |
| Rutin | 0.06 ± 0.01 | |
| Sinapinic acid | 0.07 ± 0.01 | 2.39 ± 0.08 |
| t-Ferulic acid | 0.33 ± 0.07 | 0.34 ± 0.04 |
| Naringin | 0.38 ± 0.06 | |
| 2,3-diMeO benzoic acid | 0.04 ± 0.01 | 0.003 ± 0.001 |
| Benzoic acid | 0.08 ± 0.01 | |
| Harpagoside | 0.20 ± 0.05 | |
| Naringenin | 0.06 ± 0.01 | |
| Total | 2.05 | 6.78 |
| Identified Compound | Phenolics Content (µg/g DW ± SD) | ||||||
|---|---|---|---|---|---|---|---|
| 40 °C, 10 min, Water | 60 °C, 10 min, Water | 80 °C, 10 min, Water | 100 °C, 10 min, Water | 120 °C, 10 min, Water | 80 °C, 5 min, Water | 80 °C, 15 min, Water | |
| Gallic acid | 59.6 ± 1.6 | 58.7 ± 1.8 | 21.9 ± 1.5 | 76.6 ± 5.2 | 63.9 ± 4.2 | ||
| Catechin | 55.5 ± 3.8 | ||||||
| Chlorogenic acid | 30.8 ± 2.4 | ||||||
| p-Hydroxy-benzoic acid | 34.7 ± 3.5 | 37.9 ± 1.8 | 19.8 ± 1.3 | 5.16 ± 0.09 | 6.18 ± 0.09 | ||
| Vanillic acid | 115 ± 12 | 8.62 ± 0.9 | 125 ± 13 | 16.4 ± 0.8 | 16.8 ± 0.9 | 137 ± 11 | |
| 3-OH,4-MeOH benzaldehyde | 21.8 ± 1.5 | ||||||
| p-Coumaric acid | 15.9 ± 1.1 | 12.7 ± 1.0 | 14.9 ± 1.1 | 16.3 ± 1.7 | 5.42 ± 0.08 | 6.23 ± 0.09 | |
| Rutin | 377 ± 29 | 411 ± 32 | 486 ± 39 | 520 ± 45 | 521 ± 40 | 440 ± 34 | 552 ± 48 |
| Sinapinic acid | 23.3 ± 1.6 | 15.6 ± 1.0 | 19.5 ± 1.3 | 26.5 ±2.1 | 18.3 ± 1.8 | 18.9 ± 1.2 | |
| t-Ferulic acid | 80.8 ± 5.7 | 81.6 ± 4.9 | 95.5 ± 8.1 | 109 ± 14 | 112 ± 10 | 94.3 ± 8.3 | 107 ± 11 |
| Naringin | 41.9 ± 2.5 | ||||||
| Benzoic acid | 27.9 ± 8.9 | ||||||
| Total | 762.89 | 613.50 | 832.56 | 761.58 | 805.94 | 558.17 | 784.63 |
| Color Parameters | Mean Value ± SD |
|---|---|
| L* | 64.1 ± 2.2 |
| a* | 1.0 ± 0.4 |
| b* | 14.2 ± 0.5 |
| C*ab | 14.2 ± 0.5 |
| hab | 85.9 ± 1.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutaoui, N.; Zaiter, L.; Benayache, F.; Benayache, S.; Cacciagrano, F.; Cesa, S.; Secci, D.; Carradori, S.; Giusti, A.M.; Campestre, C.; et al. Atriplex mollis Desf. Aerial Parts: Extraction Procedures, Secondary Metabolites and Color Analysis. Molecules 2018, 23, 1962. https://doi.org/10.3390/molecules23081962
Boutaoui N, Zaiter L, Benayache F, Benayache S, Cacciagrano F, Cesa S, Secci D, Carradori S, Giusti AM, Campestre C, et al. Atriplex mollis Desf. Aerial Parts: Extraction Procedures, Secondary Metabolites and Color Analysis. Molecules. 2018; 23(8):1962. https://doi.org/10.3390/molecules23081962
Chicago/Turabian StyleBoutaoui, Nassima, Lahcene Zaiter, Fadila Benayache, Samir Benayache, Francesco Cacciagrano, Stefania Cesa, Daniela Secci, Simone Carradori, Anna Maria Giusti, Cristina Campestre, and et al. 2018. "Atriplex mollis Desf. Aerial Parts: Extraction Procedures, Secondary Metabolites and Color Analysis" Molecules 23, no. 8: 1962. https://doi.org/10.3390/molecules23081962
APA StyleBoutaoui, N., Zaiter, L., Benayache, F., Benayache, S., Cacciagrano, F., Cesa, S., Secci, D., Carradori, S., Giusti, A. M., Campestre, C., Menghini, L., & Locatelli, M. (2018). Atriplex mollis Desf. Aerial Parts: Extraction Procedures, Secondary Metabolites and Color Analysis. Molecules, 23(8), 1962. https://doi.org/10.3390/molecules23081962










