Determination of Novel Highly Effective Necrostatin Nec-1s in Rat Plasma by High Performance Liquid Chromatography Hyphenated with Quadrupole-Time-of-Flight Mass Spectrometry
Abstract
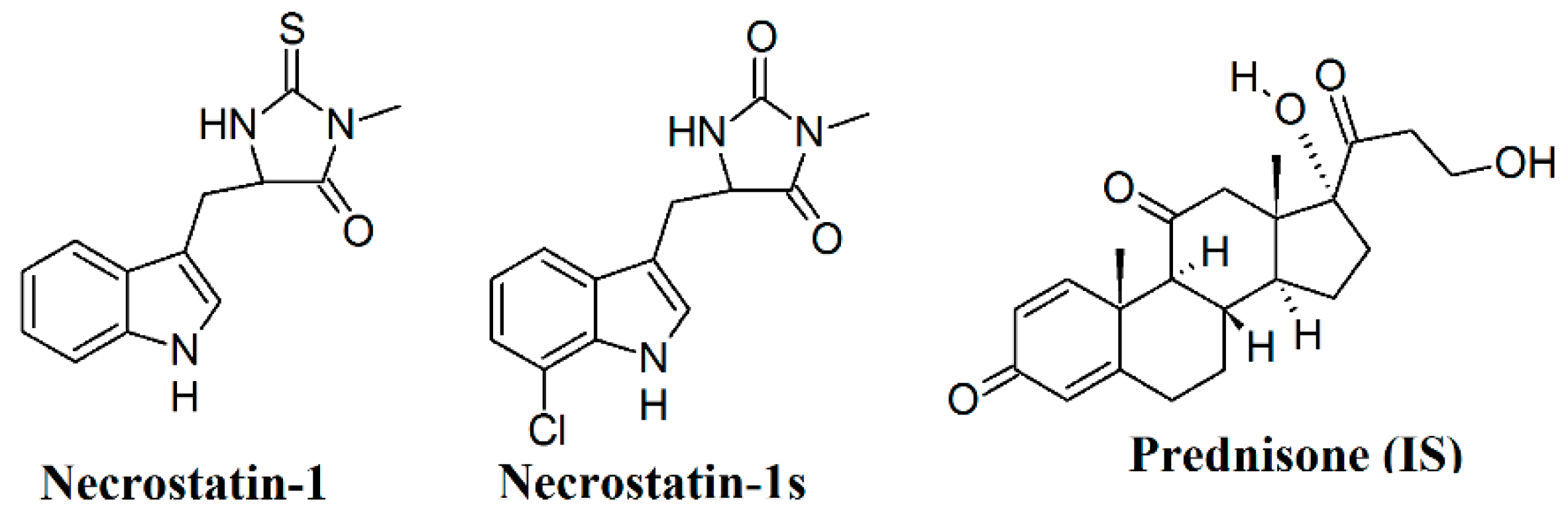
1. Introduction
2. Results and Discussion
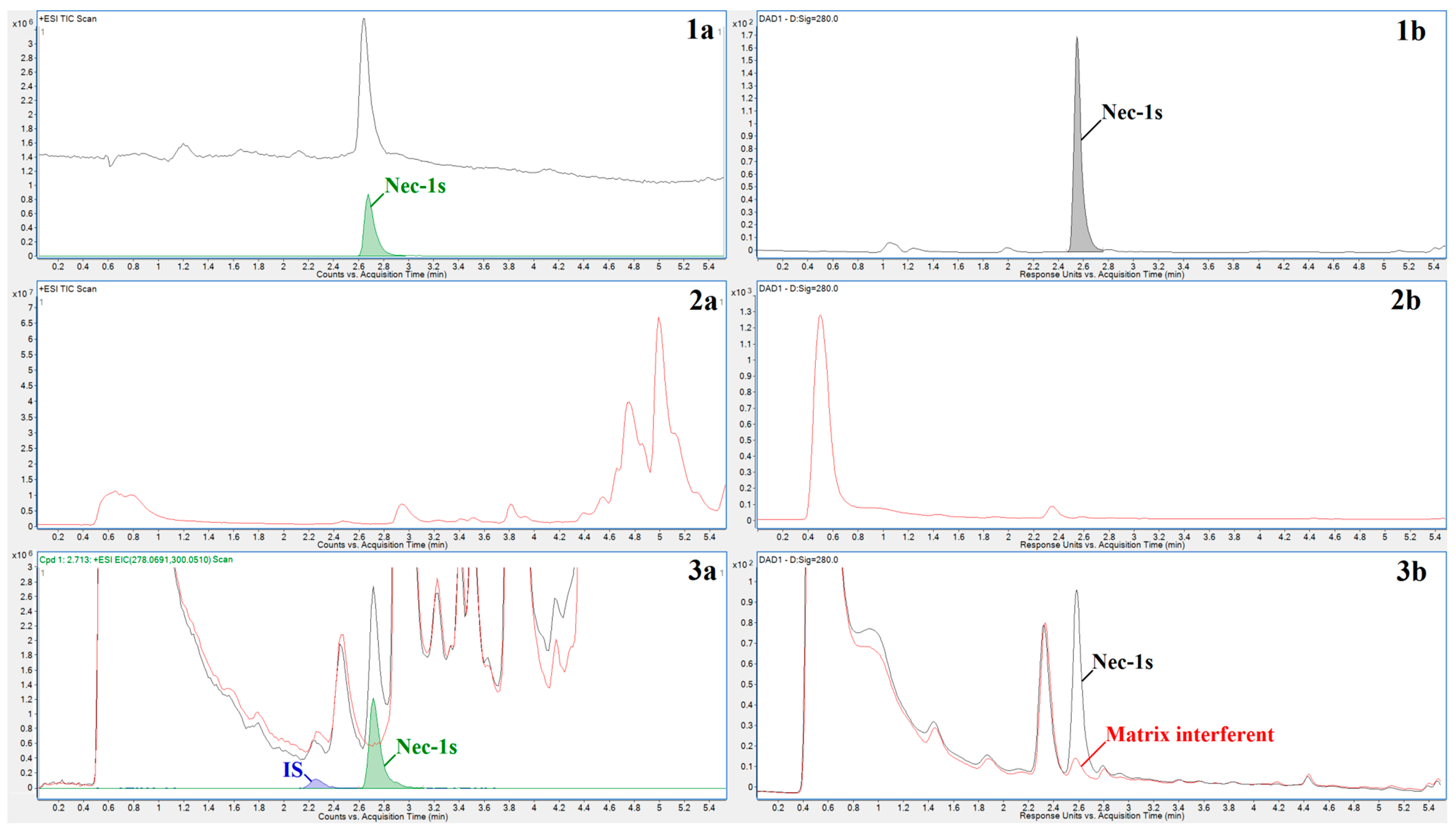
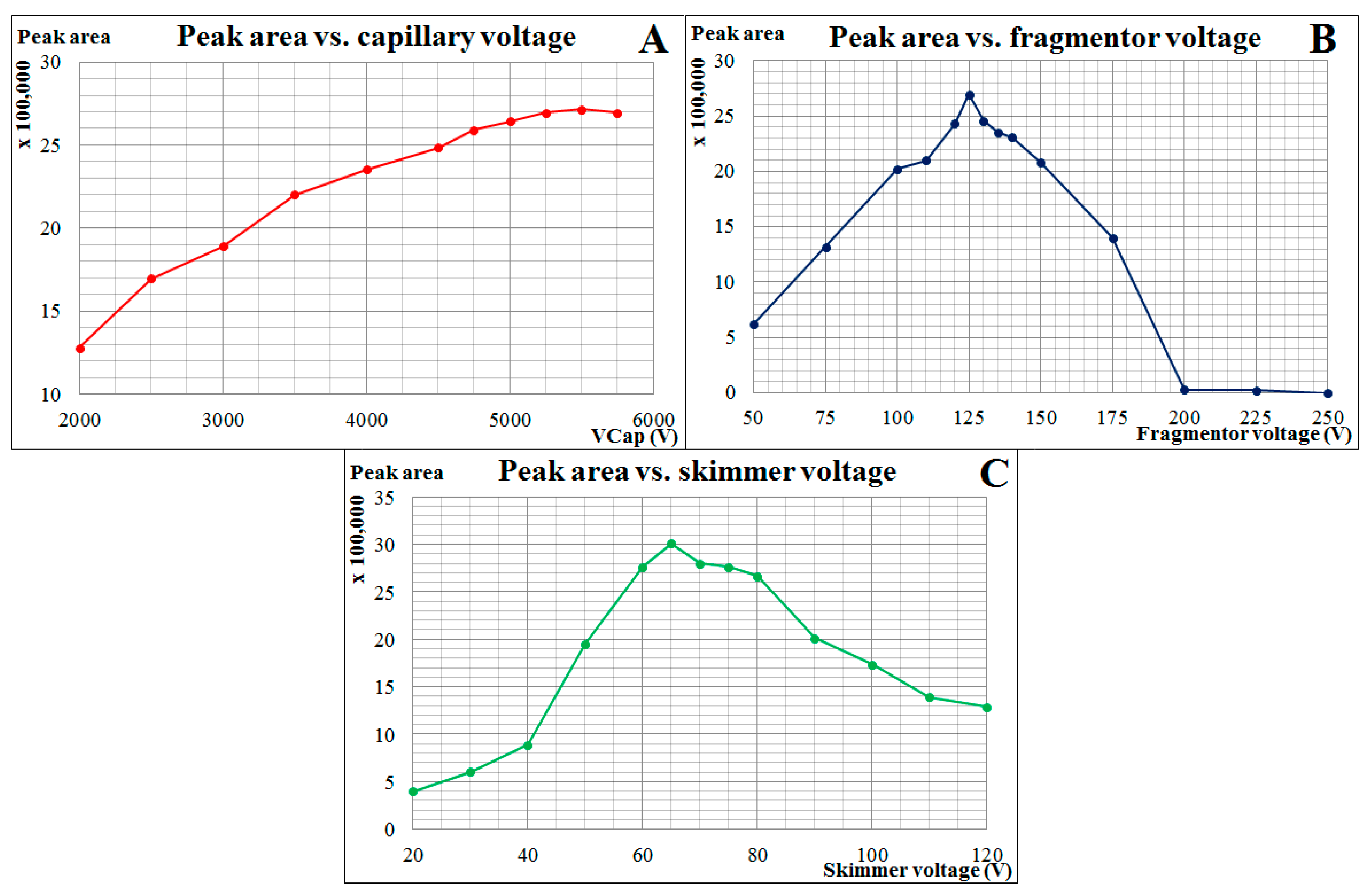
2.1. Method Development
2.2. Method Validation
2.3. Biomedical Application
3. Materials and Methods
3.1. Chemicals and Solutions
3.2. Plasma Sample Collection
3.3. Sample Preparation
3.4. Method Validation
3.5. LC-DAD/MS Apparatus
3.6. Chromatographic Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, S.; Tang, M.; Luo, H.; Shi, C.; Xu, Y. Necroptosis in neurodegenerative diseases: A potential therapeutic target. Cell Death Dis. 2017, 8, e2905. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Vandenabeele, P. Necroptosis and its role in inflammation. Nature 2015, 517, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jin, Y.; Yang, W.; Zhang, L.; Jin, X.; Liu, X.; He, Y.; Li, X. Necroptosis in cancer: An angel or a demon? Tumor Biol. 2017, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Adameova, A.; Goncalvesova, E.; Szobi, A.; Dhalla, N.S. Necroptotic cell death in failing heart: Relevance and proposed mechanisms. Heart Fail. Rev. 2016, 21, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Adameova, A.; Hrdlicka, J.; Szobi, A.; Farkasova, V.; Kopaskova, K.; Murarikova, M.; Neckar, J.; Kolar, F.; Ravingerova, T.; Dhalla, N.S. Evidence of necroptosis in hearts subjected to various forms of ischemic insults. Can. J. Physiol. Pharmacol. 2017, 95, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Hackl, M.J.; Kunzendorf, U.; Walczak, H.; Krautwald, S.; Jevnikar, A.M. Necroptosis in immunity and ischemia-reperfusion injury. Am. J. Transplant. 2013, 13, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Chan, F.K.-M.; Kroemer, G. Necroptosis: Mechanisms and relevance to disease. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 103–130. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Mediavilla-Varela, M.; Antonia, S. Indoleamine 2,3-Dioxygenase: Is it an immune suppressor? Cancer J. 2010, 16, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Duprez, L.; Grootjans, S.; Cauwels, A.; Nerinckx, W.; DuHadaway, J.B.; Goossens, V.; Roelandt, R.; Van Hauwermeiren, F.; Libert, C.; et al. Necrostatin-1 analogues: Critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012, 3, e437. [Google Scholar] [CrossRef] [PubMed]
- Szobi, A.; Rajtik, T.; Adameova, A. Effects of necrostatin-1, an inhibitor of necroptosis, and its inactive analogue Nec-1i on basal cardiovascular function. Physiol. Res. 2016, 65, 861–865. [Google Scholar] [PubMed]
- Wang, Q.; Zhou, T.; Liu, Z.; Ren, J.; Phan, N.; Gupta, K.; Stewart, D.M.; Morgan, S.; Assa, C.; Kent, K.C.; et al. Inhibition of receptor-interacting protein kinase 1 with necrostatin—1s ameliorates disease progression in elastase-induced mouse abdominal aortic aneurysm model. Sci. Rep. 2017, 7, 42159. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Yin, H.; Li, Z.; Li, Q.; He, C.; Wang, Z.; Yu, J. Quantitative analysis of necrostatin-1, a necroptosis inhibitor by LC–MS/MS and the study of its pharmacokinetics and bioavailability. Biomed. Pharmacother. 2017, 95, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Bioanalytical Method Validation. In Food and Drug Administration Guidance for Industry; U.S. Department Health Human Services: Washington, DC, USA, 2001.
- Teng, X.; Degterev, A.; Jagtap, P.; Xing, X.; Choi, S.; Denu, R.; Yuan, J.; Cuny, G.D. Structure–activity relationship study of novel necroptosis inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 5039–5044. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Maki, J.L.; Yuan, J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 2013, 20, 366. [Google Scholar] [CrossRef] [PubMed]
- Szobi, A.; Farkašová-Ledvényiová, V.; Lichý, M.; Muráriková, M.; Čarnická, S.; Ravingerová, T.; Adameová, A. Cardioprotection of ischaemic preconditioning is associated with inhibition of translocation of MLKL within the plasma membrane. J. Cell. Mol. Med. 2018, 1–14. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| RT (min) | RSDRT (%) | Linear Range (ng/mL) | Linearity r2 | LLOQ (ng/mL) | Regression Equation | |||
|---|---|---|---|---|---|---|---|---|
| b | SD b | a | SD a | |||||
| 2.72 | 0.57 | 20.3–2030.0 | 0.9991 | 20.3 | 4.062 | 0.028 | −0.014 | 0.023 |
| QC (ng/mL) | Intra-Day, n = 5 | Inter-Day, n = 15 | ||
|---|---|---|---|---|
| Precision (RSD %) | Accuracy (RE %) | Precision (RSD %) | Accuracy (RE %) | |
| 20.30 | 1.78 | 106.76 | 4.59 | 107.89 |
| 203.00 | 1.28 | 108.52 | 1.97 | 106.95 |
| 2030.00 | 0.97 | 99.07 | 1.83 | 97.45 |
| QC (ng/mL) | Extraction Recoveries (%) | Matrix Effects (%) |
|---|---|---|
| 20.30 | 89.82 ± 1.43 | 109.94 ± 3.60 |
| 203.00 | 102.58 ± 1.36 | 90.75 ± 0.43 |
| 2030.00 | 110.40 ± 0.65 | 97.20 ± 0.40 |
| Sample | Plasma Concentration (ng/mL) | Plasma Concentration (nM) | RSD% (n = 3) |
|---|---|---|---|
| N1 | 103.99 | 374.44 | 0.74 |
| N2 | 90.93 | 327.43 | 1.37 |
| N3 | 110.55 | 398.08 | 0.81 |
| N4 | 88.81 | 319.81 | 0.54 |
| N5 | 129.56 | 466.52 | 0.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikuš, P.; Pecher, D.; Rauová, D.; Horváth, C.; Szobi, A.; Adameová, A. Determination of Novel Highly Effective Necrostatin Nec-1s in Rat Plasma by High Performance Liquid Chromatography Hyphenated with Quadrupole-Time-of-Flight Mass Spectrometry. Molecules 2018, 23, 1946. https://doi.org/10.3390/molecules23081946
Mikuš P, Pecher D, Rauová D, Horváth C, Szobi A, Adameová A. Determination of Novel Highly Effective Necrostatin Nec-1s in Rat Plasma by High Performance Liquid Chromatography Hyphenated with Quadrupole-Time-of-Flight Mass Spectrometry. Molecules. 2018; 23(8):1946. https://doi.org/10.3390/molecules23081946
Chicago/Turabian StyleMikuš, Peter, Daniel Pecher, Drahomíra Rauová, Csaba Horváth, Adrián Szobi, and Adriana Adameová. 2018. "Determination of Novel Highly Effective Necrostatin Nec-1s in Rat Plasma by High Performance Liquid Chromatography Hyphenated with Quadrupole-Time-of-Flight Mass Spectrometry" Molecules 23, no. 8: 1946. https://doi.org/10.3390/molecules23081946
APA StyleMikuš, P., Pecher, D., Rauová, D., Horváth, C., Szobi, A., & Adameová, A. (2018). Determination of Novel Highly Effective Necrostatin Nec-1s in Rat Plasma by High Performance Liquid Chromatography Hyphenated with Quadrupole-Time-of-Flight Mass Spectrometry. Molecules, 23(8), 1946. https://doi.org/10.3390/molecules23081946






