A Sensitive and Rapid UPLC-MS/MS Method for Determination of Monosaccharides and Anti-Allergic Effect of the Polysaccharides Extracted from Saposhnikoviae Radix
Abstract
:1. Introduction
2. Results
2.1. Determination of Monosaccharide Composition and Content in SRPS
2.1.1. UPLC-MS/MS Conditions
2.1.2. Quantification and Validation
2.1.3. Determination of Monosaccharide Composition and Content in SRPS
2.2. The Effect of SRPS on Anti-Allergic Effect
2.2.1. The Effect of SRPS on Ear Swelling
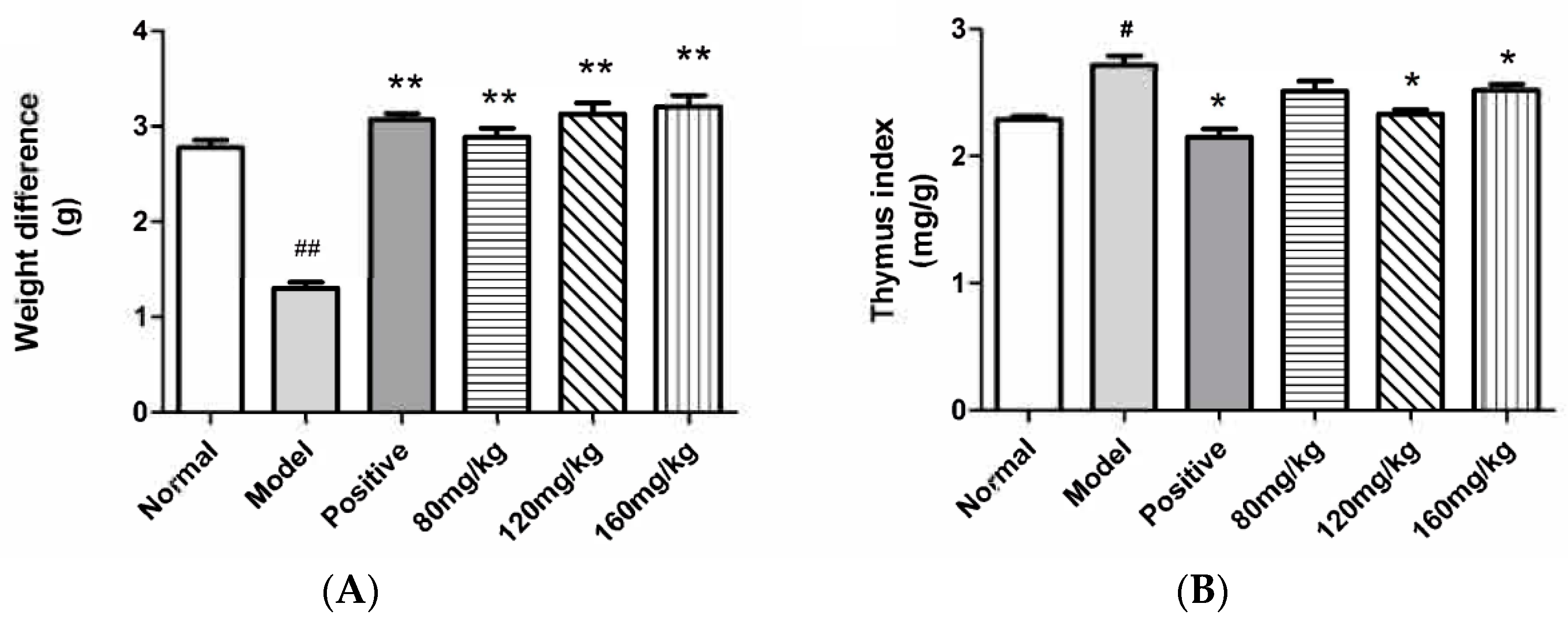
2.2.2. The Effect of SRPS on the Body Weight and the Relative Weight of the Organ
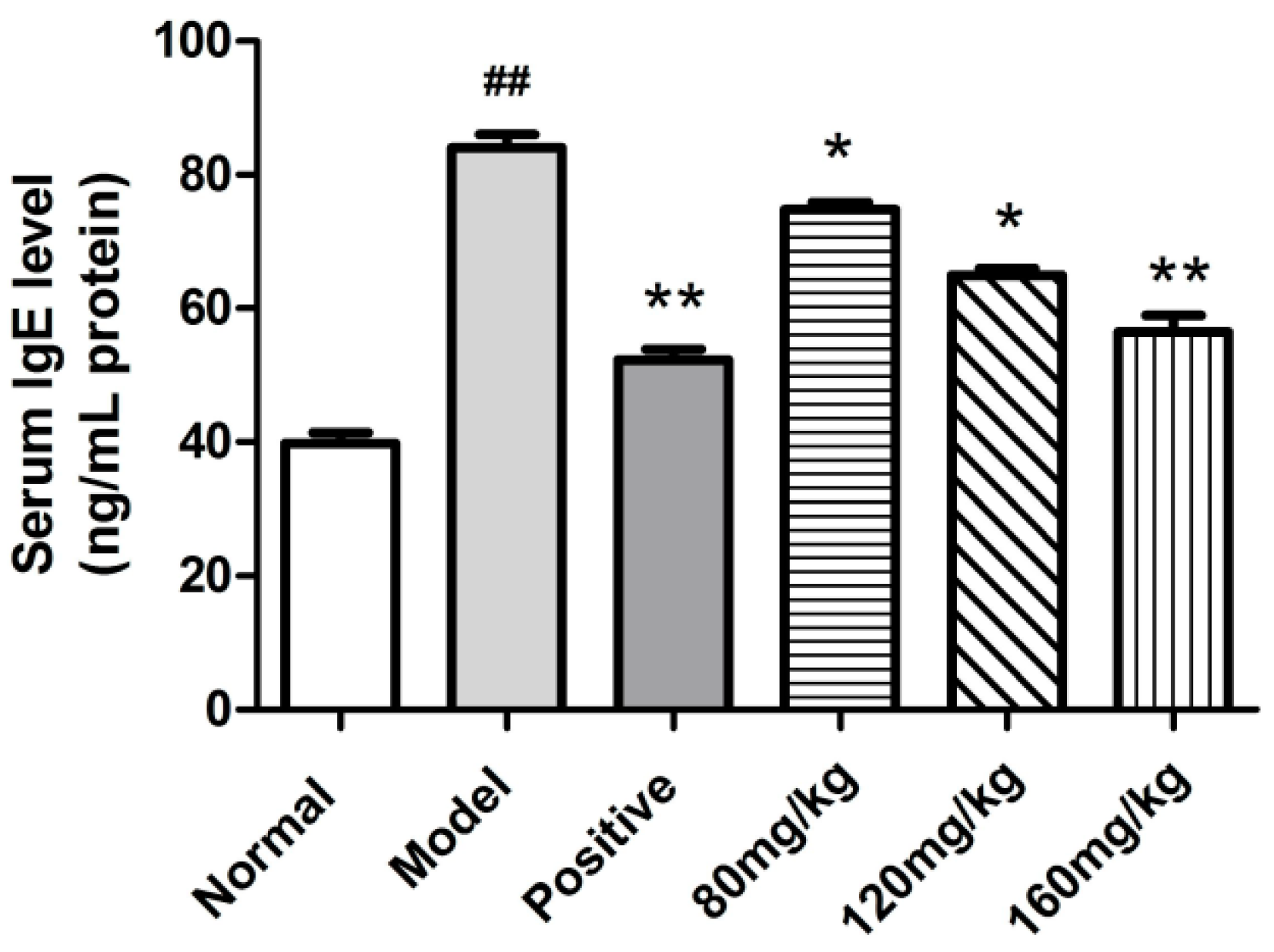
2.2.3. Determination of Serum Total IgE
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Chemistry
4.2.1. Preparation of Calibration Standards
4.2.2. Extraction and Purification of SRPS
4.2.3. Hydrolysis and Derivatization of Polysaccharides
4.2.4. Chromatographic and Mass Spectrometry Conditions
4.3. Anti-Allergic Activity of SRPS
4.3.1. Animals
4.3.2. DNFB-Induced DTH and Treatment with SRPS in Experimental Animals
4.3.3. Determination of Indicators
4.4. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SRPS | Polysaccharides extracted from the Saposhnikoviae Radix |
| UPLC-MS/MS | Ultra-high-performance liquid chromatography-tandem mass spectrometry |
| DNFB | 2,4-dinitrofluorobenzene |
| DTH | Delayed-type hypersensitivity |
| IS | Internal standard |
| PMP | 1-Pheny-3-Methyl-5-Pyrazolone |
| MRM | Multiple reaction monitoring |
| LOD | Limit of detection |
| LLOQ | Lower limits of quantitation |
| S/N | Signal-to-noise |
| BW | Body weight |
References
- Robinson, M.L.; Lanser, B.J. The role of baked egg and milk in the diets of allergic children. Immunol. Allergy Clin. N. Am. 2018, 38, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.; Coffman, A.; Erwin, E.; Mikhail, I. Diagnosis and treatment of food allergic reactions in pediatric emergency settings. Ann. Allergy Asthma Immunol. 2017, 119, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.V.; Dykewicz, M.S.; Oppenheimer, J.; Portnoy, J.M.; Lang, D.M. Pharmacologic treatment of seasonal allergic rhinitis: Synopsis of guidance from the 2017 joint task force on practice parameters. Ann. Intern. Med. 2017, 167, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Pfaar, O.; Akdis, C.A.; Ansotegui, I.J.; Durham, S.R.; Wijk, R.G.; Halken, S.; Larenas-Linnemann, D.; Pawankar, R.; Pitsios, C.; et al. EAACI guidelines on allergen immunotherapy: Allergic rhino conjunctivitis. Allergy 2018, 73, 765–798. [Google Scholar] [CrossRef] [PubMed]
- Belsito, D.V. The diagnostic evaluation, treatment, and prevention of allergic contact dermatitis in the new millennium. J. Allergy Clin. Immunol. 2000, 105, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Bellik, Y.; Boukraâ, L.; Alzahrani, H.A.; Bakhotmah, B.A.; Abdellah, F.; Hammoudi, S.M.; Iguer-Ouada, M. Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: An update. Molecules 2012, 18, 322–353. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.B.; Morais, T.C.; Carvalho, K.M.; Silva, C.R.; Andrade, G.M.; Brito, G.A.; Veras, M.L.; Pessoa, O.D.; Rao, V.S.; Santos, F.A. Topical anti-inflammatory potential of Physalin E from Physalis angulate on experimental dermatitis in mice. Phytomedicine 2010, 17, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.H.; Lim, H.; Kwon, S.Y.; Son, K.H.; Kim, H.P. Anti-inflammatory activity of the total flavonoid fraction from Broussonetia papyrifera in combination with Lonicera japonica. Biomol Ther. 2010, 18, 197–204. [Google Scholar] [CrossRef]
- Chun, J.M.; Lee, A.R.; Kim, H.S.; Lee, A.Y.; Gu, G.J.; Moon, B.C.; Kwon, B.I. Peucedanum japonicum extract attenuates allergic airway inflammation by inhibiting Th2 cell activation and production of pro-inflammatory mediators. J. Ethnopharmacol. 2018, 211, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia, 1st ed.; China Medical Science Press: Beijing, China, 2015; Volume 1, p. 149. ISBN 9787506773379. [Google Scholar]
- Liu, S.L.; Jiang, C.X.; Zhao, Y.; Xu, Y.H.; Wang, Z.; Zhang, L.X. Advance in study on chemical constituents of Saposhnikoviae divaricate and their pharmacological effects. Chin. Tradit. Herbal Drugs. 2017, 48, 2146–2152. [Google Scholar]
- Wu, X.B.; Jin, S.Y.; Li, S.M.; Jin, X.G.; Li, T.F.; Wang, M.Y.; Zhu, H.Y. Effect of Saposhnikoviae Radix extract on PAR-2 expression and related cytokine secretion of mast cells. Chin. J. Exp. Tradit. Med. Form. 2016, 22, 123–126. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, C.F.; Lin, N.; Fan, G.Q.; Zhang, C.; Xiao, Y.Q. Effect of chromones from Saposhnikovia divaricate on collagen-induced arthritis in rats. Chin. J. Exp. Tradit. Med. Form. 2009, 15, 52–56. [Google Scholar] [CrossRef]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl5-pyrazolone derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Z.; Huang, L.; Bai, Q.; Jia, J. An improved PMP derivatization method for analyzing monosaccharide composition. Chem. J. Chin. Univ. 2006, 27, 1456–1458. [Google Scholar]
- Phanuphak, P.; Moorhead, J.W.; Claman, H.N. Tolerance and contact sensitivity to DNFB in mice. I. In vivo detection by ear swelling and correlation with in vitro cell stimulation. J. Immunol. 1974, 112, 115–123. [Google Scholar] [PubMed]
- Sakai, H.; Yabe, S.; Sato, K.; Kai, Y.; Sato, F.; Yumoto, T.; Inoue, Y.; Narita, M.; Matsumoto, K.; Kato, S.; et al. ELR+ chemokine-mediated neutrophil recruitment is involved in 2,4,6-trinitrochlorobenzene-induced contact hypersensitivity. Clin. Exp. Pharmacol. Physiol. 2018, 45, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Masuda, M.; Naruto, S.; Murata, K.; Matsuda, H. Antiallergic activity of unripe Citrus hassaku fruits extract and its flavanone glycosides on chemical substance-induced dermatitis in mice. J. Nat. Med. 2009, 63, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.Y.; Cao, N.; Liu, Z.H.; Cui, Y.D. Immunosuppressive effect of kaempferol on delayed type hypersensitivity in mice. Chin. J. Biol. 2013, 26, 848–850, 855. [Google Scholar] [CrossRef]
- Liu, J.W.; Li, S.P.; Gao, L.Y.; Li, Y.Q.; Gao, Y.L. Comparison of analgesic and anti-inflammatory effects of Saposhnikoviae Radix and wild Saposhnikoviae Radix in Ningxia. Chin. J. Ethnomed. Ethnopharm. 2009, 18, 38–39. [Google Scholar]
- Ren, Z.; He, C.; Fan, Y.; Si, H.; Wang, Y.; Shi, Z.; Zhao, X.; Zheng, Y.; Liu, Q.; Zhang, H. Immune-enhancing activity of polysaccharides from Cyrtomium macrophyllum. Int. J. Biol. Macromol. 2014, 70, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, Y.N.; Paulsen, B.S.; Kiyohara, H.; Ciz, M.; Ognyanov, M.H.; Vasicek, O.; Rise, F.; Denev, P.N.; Yamada, H.; Lojek, A.; et al. The common lavender (Lavandula angustifolia Mill.) pectic polysaccharides modulate phagocytic leukocytes and intestinal Peyer’s patch cells. Carbohydr. Polym. 2017, 174, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cao, J.; Chen, G.; Xu, Y.; Lu, J.; Fang, F.; Chen, K. Anti-tumor nigricans on CT26 tumor-bearing mice. Int. Immunopharmacol. 2016, 36, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, X.Y.; Yang, R.L.; Qin, T.; Wang, X.Y.; Zhang, K.Y.; Fei, C.Z.; Li, Y.; Hu, Y.L.; Xue, F.Q. Cordyceps militaris polysaccharides can enhance the immunity and antioxidation activity in immunosuppressed mice. Carbohyd. Polym. 2012, 89, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Jiang, L.; Zhao, W.; Xi-Nan, M.; Huang, S.; Yang, J.; Hu, T.; Chen, H. Immunomodulatory and antioxidant effects of total flavonoids of Spatholobus suberectus Dunn on PCV2 infected mice. Sci. Rep. 2017, 7, 8676. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Che, H.L.; Ha, D.; Wei, Y.P.; Zheng, S.Y. Characterization and anti-allergic effect of a polysaccharide from the flower buds of Lonicera japonica. Carbohydr. Polym. 2012, 90, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, A.M.; Schulman, E.S.; Peters, S.P.; MacGlashan, D.W.; Newball, H.H.; Schleimer, R.P.; Lichtenstein, L.M. Immunoglobulin E-mediated degranulation of isolated human lung mast cells. Lab. Investig. 1985, 53, 45–56. [Google Scholar] [PubMed]
- Hammerberg, B.; Olivry, T.; Orton, S.M. Skin mast cell histamine release following stem cell factor and high-affinity immunoglobulin E receptor crosslinking in dogs with atopic dermatitis. Vet. Dermatol. 2001, 12, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Sismanopoulos, N.; Delivans, D.A.; Alysandratos, K.D.; Angelidou, A.; Therianou, A.; Kalogeromitros, D.; Theoharides, T.C. Mast cells in allergic and inflammatory diseases. Curr. Pharm. Des. 2012, 18, 2261–2277. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, Y.N.; Ognyanov, M.H.; Kiyohara, H.; Batsalova, T.G.; Dzhambazov, B.M.; Ciz, M.; Denev, P.N.; Yamada, H.; Paulsen, B.S.; Vasicek, O.; et al. Acidic polysaccharide complexes from purslane, silver linden and lavender stimulate Peyer’s patch immune cells through innate and adaptive mechanisms. Int. J. Biol. Macromol. 2017, 105, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Tang, G.; Zeng, G.; Yang, Y.; Cai, X.; Li, D.; Liu, H.; Zhou, N. Purification and characterization of an antitumor polysaccharide from Portulaca oleracea L. Carbohydr Polym. 2013, 93, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Shi, S.; Jiang, L. Evaluation of antioxidant and immuno-enhancing activities of Purslane polysaccharides in gastric cancer rats. Int. J. Biol. Macromol. 2014, 68, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, X.; Qian, T.; Sun, G.; Guo, Y.; Chang, F.; Zhou, S.; Sun, X. Antitumor and immunomodulatory activity of arabinoxylans: A major constituent of wheat bran. Int. J. Biol. Macromol. 2011, 48, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.W.; Feng, M.; Zhai, X.; Hu, M.; You, L.; Luo, W.; Zhao, M. Optimization for the extraction of polysaccharides from Ganoderma lucidum and their antioxidant and antiproliferative activities. J. Taiwan Inst. Chem. 2013, 44, 886–894. [Google Scholar] [CrossRef]
- Alam, N.; Gupta, P.C. Structure of a water-soluble polysaccharide from the seeds of Cassia angustifolia. Planta Med. 1986, 52, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Wang, J.L.; Yuan, J.; Quan, Q.H.; Ji, R.F.; Tan, P.; Han, J.; Liu, Y.G. Sugar composition analysis of Fuzi polysaccharides by HPLC-MSn and their protective effects on Schwann Cells exposed to high glucose. Molecules 2016, 21, 1496. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Katase, M.; Tsumura, K.; Saito, M.; Matsuda, T. Inhibitory effects of dietary soyasaponin on 2,4-dinitrofluorobenzene-induced contact hypersensitivity in mice. Exp. Dermatol. 2017, 26, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Vadas, M.A.; Miller, J.F.; Gamble, J.; Whitelaw, A. A radioisotopic method to measure delayed type hypersensitivity in the mouse I. studies in sensitized and normal mice. Int. Arch. Allergy Appl. Immunol. 1975, 49, 670–692. [Google Scholar] [CrossRef] [PubMed]
- Abeywardane, A.; Caviness, G.; Choi, Y.; Cogan, D.; Gao, A.; Goldberg, D.; Heim-Riether, A.; Jeanfavre, D.; Klein, E.; Kowalski, J.A.; et al. N-Arylsulfonyl-alpha-amino carboxamides are potent and selective inhibitors of the chemokine receptor CCR10 that show efficacy in the murine DNFB model of contact hypersensitivity. Bioorg. Med. Chem. Lett. 2016, 26, 5277–5283. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the compound SRPS is available from the authors. |
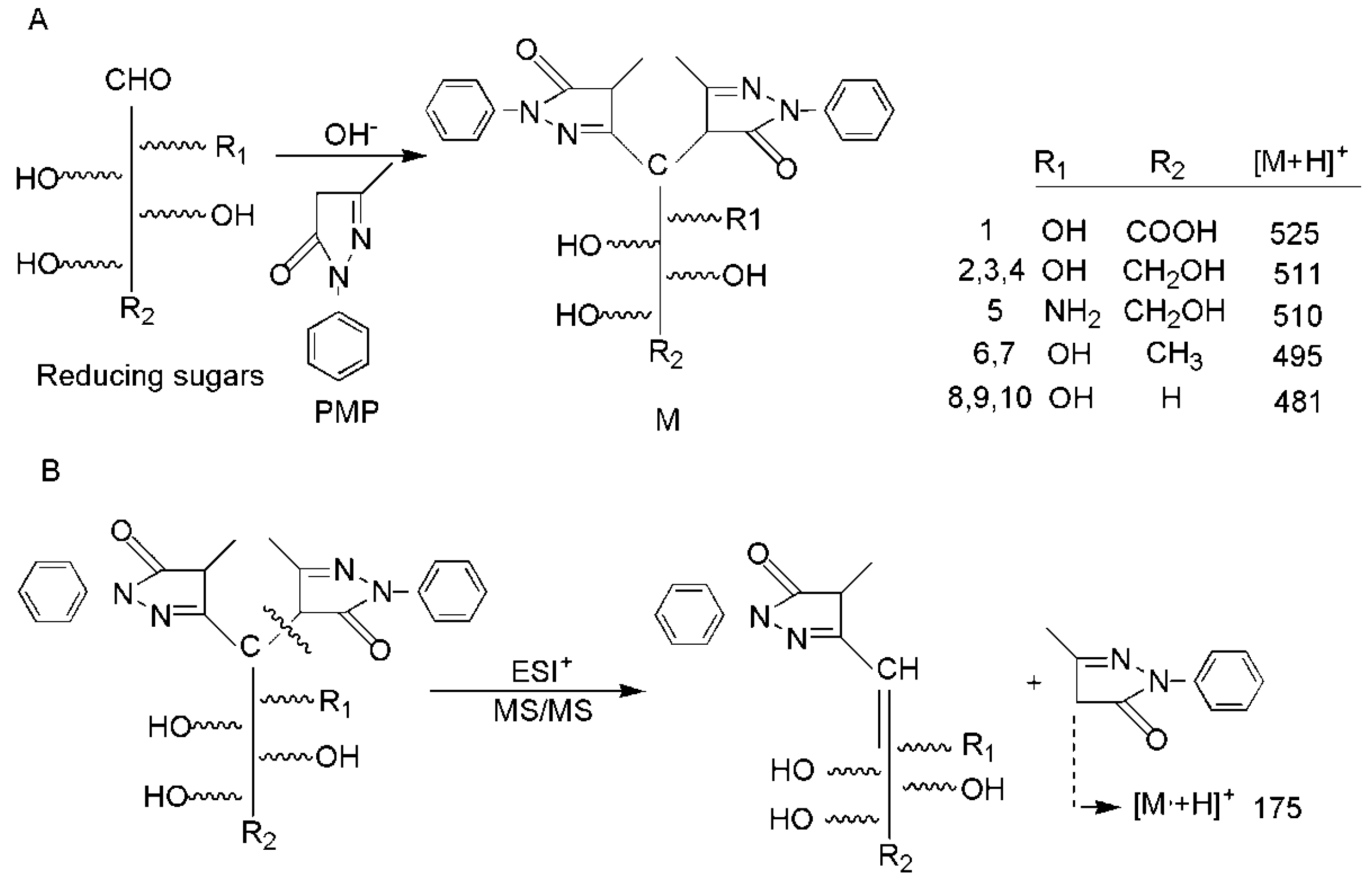
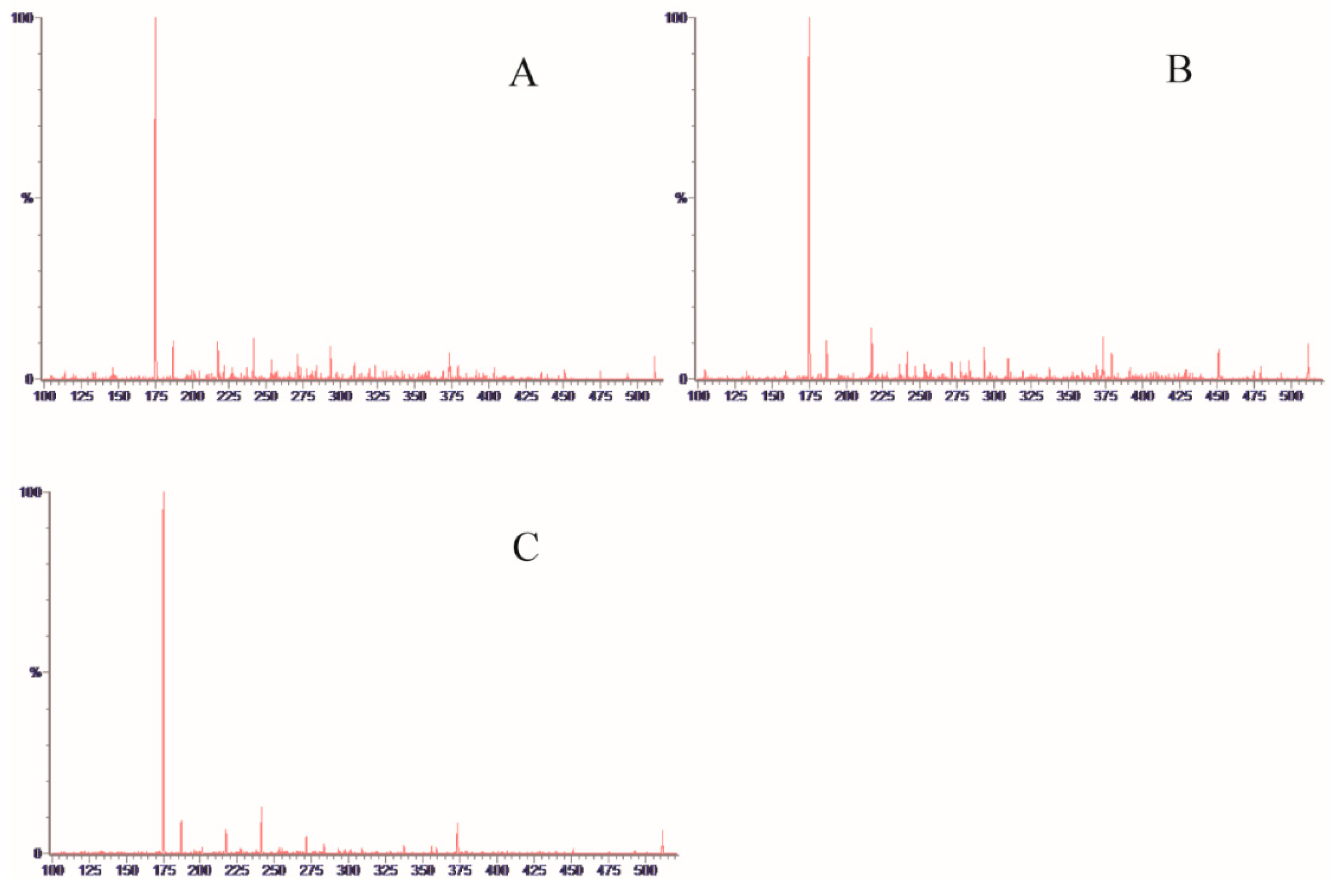
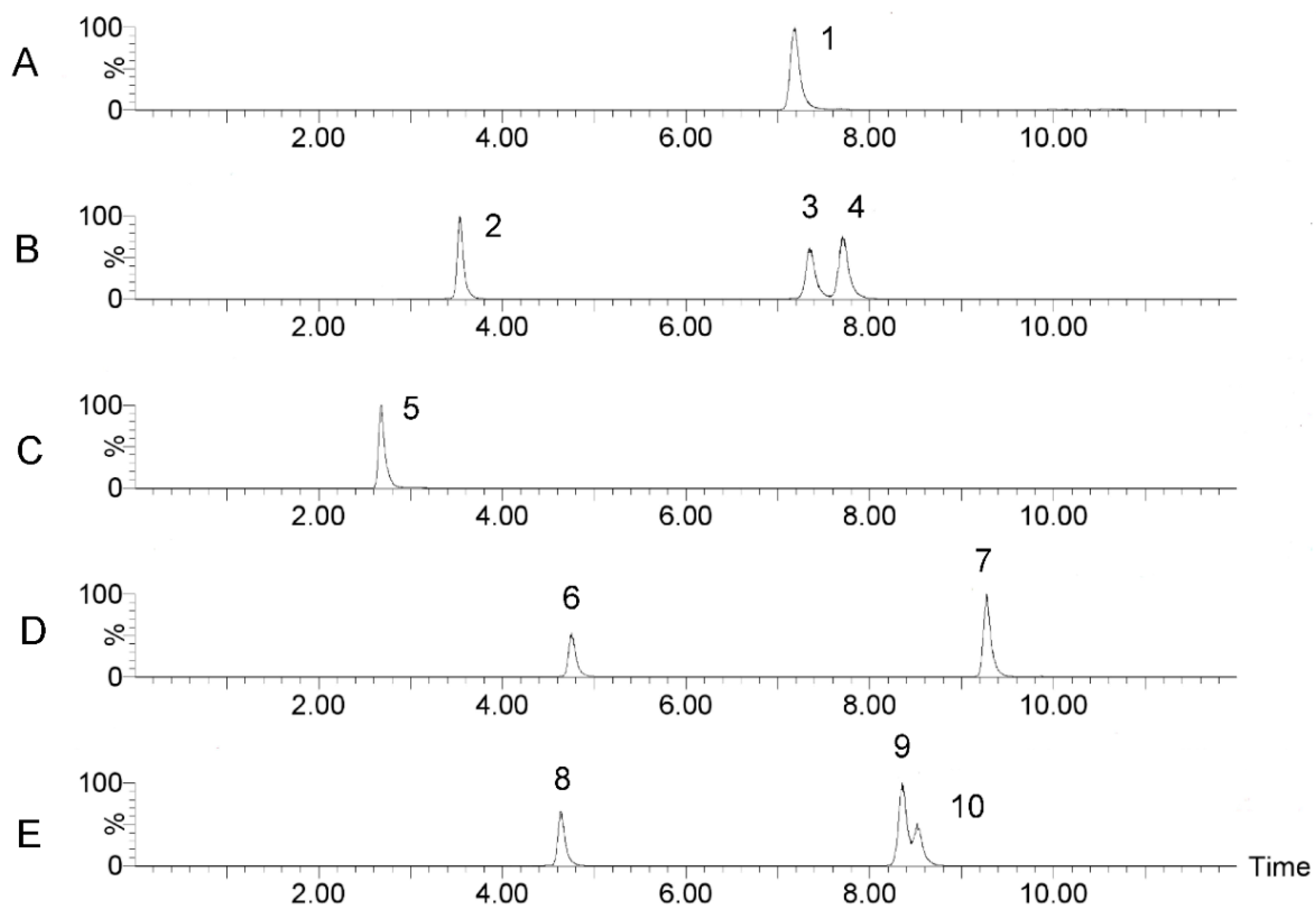
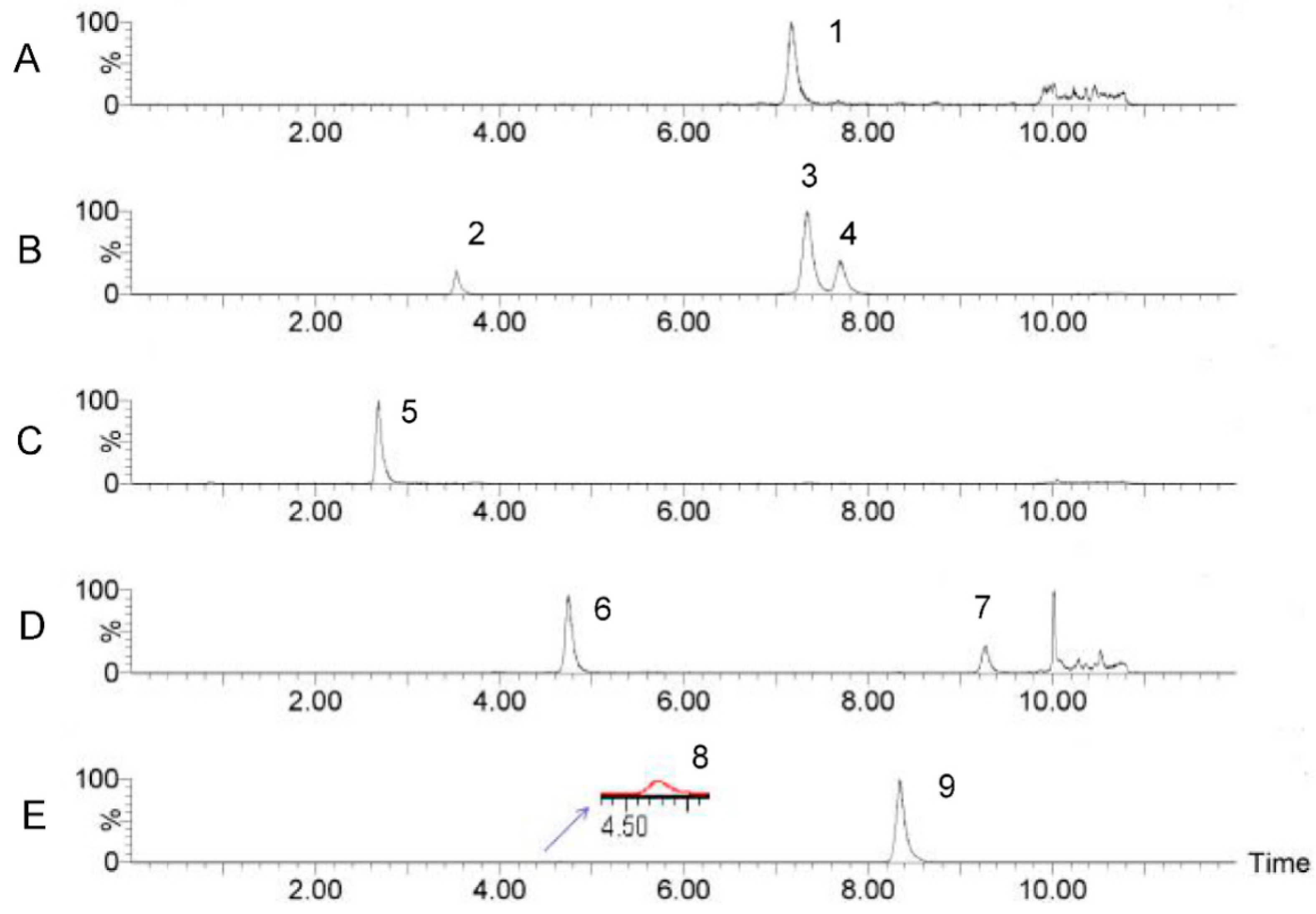
| No. | Compound Name | tR (min) | Q1 | Q2 | CV | CE |
|---|---|---|---|---|---|---|
| 1 | Gal-A | 7.75 | 525.223 | 175.125 | 22 | 22 |
| 2 | Man | 3.84 | 511.259 | 175.123 | 6 | 24 |
| 3 | Glu | 7.84 | 511.259 | 175.123 | 28 | 22 |
| 4 | Gal | 8.20 | 511.259 | 175.123 | 36 | 22 |
| 5 | D-Glu | 3.04 | 510.323 | 175.123 | 20 | 32 |
| 6 | Rha | 5.16 | 495.312 | 175.124 | 30 | 22 |
| 7 | Fuc | 9.64 | 495.312 | 175.124 | 26 | 20 |
| 8 | Rib | 5.04 | 481.169 | 175.129 | 26 | 22 |
| 9 | Ara | 8.80 | 481.169 | 175.129 | 26 | 22 |
| 10 | Xyl | 8.96 | 481.169 | 175.129 | 4 | 22 |
| Compound Name | Regression Equation | Linear Range (ng/mL) | Correlation Coefficient | LODs (ng/mL) | LOQs (ng/mL) |
|---|---|---|---|---|---|
| Gal-A | y = 4439.12x − 1286.93 | 4.85–194 | 0.9972 | 2.49 | 8.21 |
| Man | y = 5646.84x + 859.335 | 4.50–180 | 0.9992 | 2.04 | 6.74 |
| Glu | y = 1401.36x + 35412.6 | 4.50–540 | 0.9980 | 1.95 | 6.43 |
| Gal | y = 319.006x + 48035.9 | 4.50–540 | 0.9998 | 2.02 | 6.67 |
| Rha | y = 3945.84x + 2279.57 | 4.10–164 | 0.9988 | 1.85 | 6.12 |
| Fuc | y = 3619.60x − 1191.29 | 4.10–164 | 0.9988 | 1.66 | 5.49 |
| Rib | y = 5312.12x − 3594.90 | 3.75–150 | 0.9970 | 1.74 | 5.74 |
| Ara-Xyl | y = 5840.51x + 111844 | 3.75–750 | 0.9980 | 1.50 | 4.94 |
| Compound Name | Precision (n = 6) | Repeatability (n = 6) | Recovery (n = 3) | ||
|---|---|---|---|---|---|
| RSD (%) | RSD (%) | 40 μM (%) | 80 μM (%) | 120 μM (%) | |
| Gal-A | 2.37 | 2.85 | 104.07 | 96.52 | 94.65 |
| Man | 1.88 | 2.71 | 113.11 | 102.19 | 102.39 |
| Glu | 1.24 | 2.28 | 102.38 | 100.76 | 103.20 |
| Gal | 2.15 | 3.37 | 102.59 | 101.08 | 100.16 |
| Rha | 1.09 | 2.68 | 115.80 | 113.18 | 105.25 |
| Fuc | 2.08 | 4.44 | 105.12 | 108.64 | 107.50 |
| Rib | 2.66 | 2.99 | 114.43 | 108.80 | 103.50 |
| Ara-Xyl | 2.09 | 2.09 | 114.58 | 111.30 | 103.19 |
| Compound Name | Content (ng/mL) | Quality Percentage | Molar Percentage |
|---|---|---|---|
| Gal-A | 85.088 | 5.18% | 4.42% |
| Man | 140.336 | 8.55% | 7.86% |
| Glu | 423.062 | 25.76% | 23.69% |
| Gal | 215.414 | 13.12% | 12.06% |
| Rha | 50.411 | 3.07% | 3.10% |
| Fuc | 7.396 | 0.45% | 0.45% |
| Rib | 10.597 | 0.65% | 0.71% |
| Ara | 709.718 | 43.22% | 47.70% |
| Groups | Dose (mg/kg) | Administration Route | Swelling (%) | Inhibition (%) |
|---|---|---|---|---|
| Normal | - | Oral | 2.82 ± 2.20 | - |
| Model | - | Oral | 90.32 ± 3.39 ## | - |
| Positive | 3 | Oral | 36.56 ± 2.12 | 59.52 |
| 80 | Oral | 75.06 ± 3.82 ** | 16.90 | |
| SRPS | 120 | Oral | 57.10 ± 3.19 ** | 36.78 |
| 160 | Oral | 46.06 ± 3.13 ** | 49.01 |
| Groups | Dose (mg/kg) | Spleen Index (mg/kg) | Kidney Index (mg/g) | Adrenal Index (mg/g) |
|---|---|---|---|---|
| Normal | - | 3.11 ± 0.17 | 5.72 ± 0.31 | 0.124 ± 0.006 |
| Model | - | 3.05 ± 0.18 | 5.71 ± 0.29 | 0.127 ± 0.010 |
| Positive | 3 | 3.07 ± 0.18 | 5.65 ± 0.34 | 0.123 ± 0.008 |
| 80 | 3.03 ± 0.24 | 5.70 ± 0.24 | 0.129 ± 0.008 | |
| SRPS | 120 | 3.08 ± 0.25 | 5.68 ± 0.16 | 0.121 ± 0.007 |
| 160 | 3.12 ± 0.13 | 5.69 ± 0.29 | 0.128 ± 0.016 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.-Y.; Jiang, Y.; Chen, G.-C.; Li, S.-S.; Yang, F.; Ma, Q. A Sensitive and Rapid UPLC-MS/MS Method for Determination of Monosaccharides and Anti-Allergic Effect of the Polysaccharides Extracted from Saposhnikoviae Radix. Molecules 2018, 23, 1924. https://doi.org/10.3390/molecules23081924
Gao Y-Y, Jiang Y, Chen G-C, Li S-S, Yang F, Ma Q. A Sensitive and Rapid UPLC-MS/MS Method for Determination of Monosaccharides and Anti-Allergic Effect of the Polysaccharides Extracted from Saposhnikoviae Radix. Molecules. 2018; 23(8):1924. https://doi.org/10.3390/molecules23081924
Chicago/Turabian StyleGao, Yan-Yan, Yue Jiang, Guo-Chao Chen, Shuang-Shuang Li, Fei Yang, and Qun Ma. 2018. "A Sensitive and Rapid UPLC-MS/MS Method for Determination of Monosaccharides and Anti-Allergic Effect of the Polysaccharides Extracted from Saposhnikoviae Radix" Molecules 23, no. 8: 1924. https://doi.org/10.3390/molecules23081924
APA StyleGao, Y.-Y., Jiang, Y., Chen, G.-C., Li, S.-S., Yang, F., & Ma, Q. (2018). A Sensitive and Rapid UPLC-MS/MS Method for Determination of Monosaccharides and Anti-Allergic Effect of the Polysaccharides Extracted from Saposhnikoviae Radix. Molecules, 23(8), 1924. https://doi.org/10.3390/molecules23081924





