Ultrasensitive (Co)polymers Based on Poly(methacrylamide) Structure with Fining-Tunable pH Responsive Value
Abstract
1. Introduction
2. Results
2.1. Synthesis of the (Co)polymers
2.2. pH Titration of Different Copolymers and Measurement of pKa
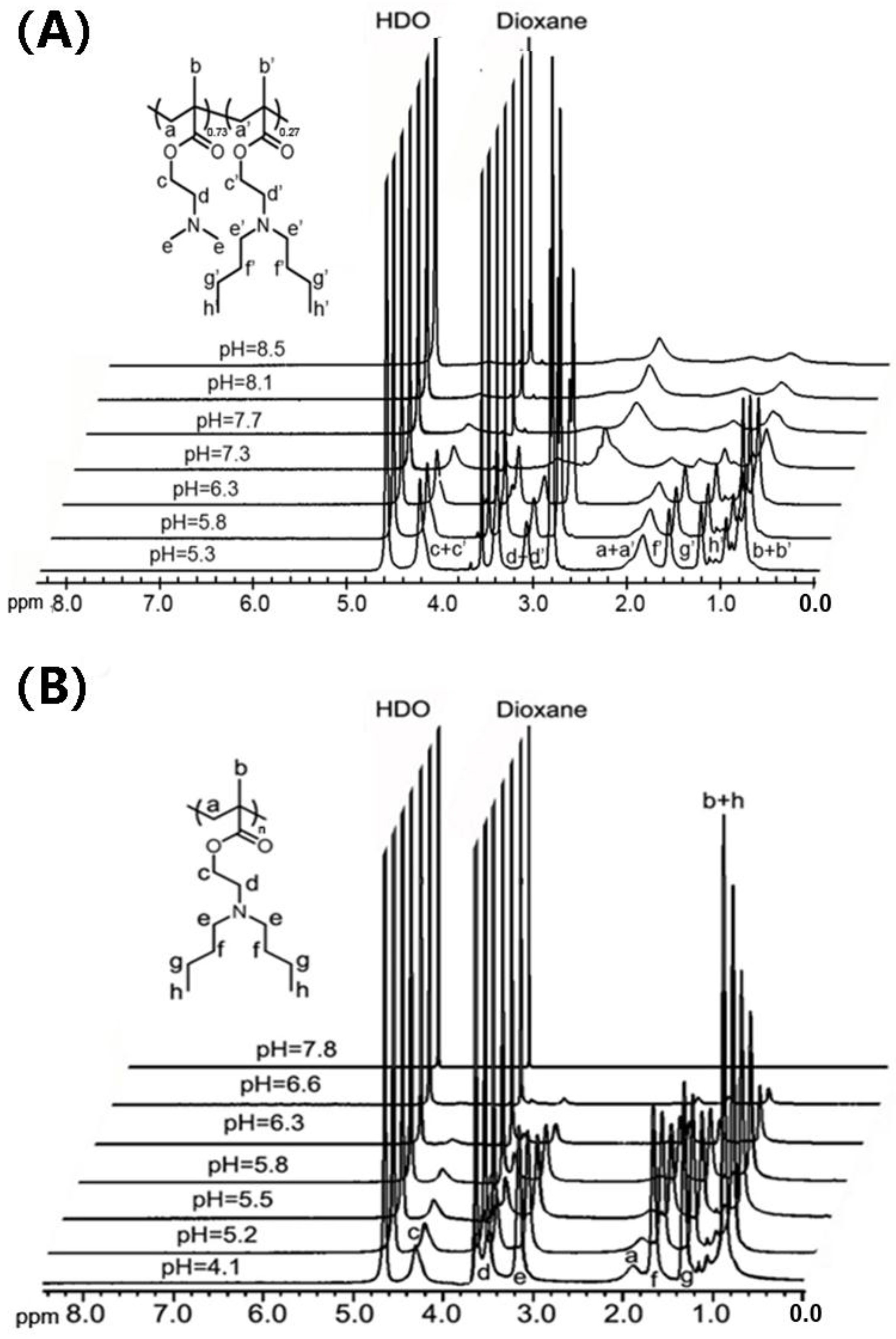
2.3. Phase Transition Behaviors of the Fine-Tuning pH Responsive Polymers
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the DBA Monomer
3.3. Synthesis of the (Co)polymers
3.4. Characterization of the (Co)polymers
3.5. pH Titration
3.6. Turbidity Measurements by UV/Vis Spectroscopy
3.7. 1H-NMR Measurements of pH-Dependent Phase Transition
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Greenfield, M.A.; Mata, A.; Palmer, L.C.; Bitton, R.; Mantei, J.R.; Conrado Aparicio, C.; Cruz, M.O.; Stupp, S.I. A self-assembly pathway to aligned monodomain gels. Nat. Mater. 2010, 9, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, E.G.; Wyrsta, M.D.; Pakstis, L.; Pochan, D.J.; Deming, T.J. Stimuli-responsive polypeptide vesicles by conformation-specific assembly. Nat. Mater. 2004, 3, 244–248. [Google Scholar] [CrossRef] [PubMed]
- So, M.K.; Xu, C.J.; Loening, A.M.; Gambhir, S.S.; Rao, J.H. Self-illuminating quantum dot conjugates for in vivo imaging. Nat. Biotechnol. 2006, 24, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Zhao, T.; Li, Y.; Huang, G.; White, M.A.; Gao, J.M. Investigation of Endosome and Lysosome Biology by Ultra pH-Sensitive Nanoprobes. Adv. Drug Deliv. Rev. 2017, 113, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Kopecek, J. Polymer-drug conjugates: Origins, progress to date and future directions. Adv. Drug Deliv. Rev. 2013, 65, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Huang, G.; Li, Y.; Yang, S.; Ramazani, S.; Lin, Z.; Wang, Y.; Ma, X.; Zeng, Z.; Luo, M.; et al. A transistor-like pH nanoprobe for tumour detection and image-guided surgery. Nat. Biomed. Eng. 2016, 1, 0006–0013. [Google Scholar] [CrossRef] [PubMed]
- Engin, K.; Leeper, D.B.; Cater, J.R.; Thistlethwaite, A.J.; Tupchong, L.; Mcfarlane, J.D. Extracellular pH distribution in human tumors. Int. J. Hyperth. 1995, 11, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Van Sluis, R.; Bhujwalla, Z.M.; Raghunand, N.; Ballesteros, P.; Alvarez, J.; Cerdan, S.; Galons, J.P.; Gillies, R.J. In vivo imaging of extracellular pH using H-1 MRSI. Magn. Reson. Med. 1999, 41, 743–750. [Google Scholar] [CrossRef]
- Yang, L.; Tang, H.L.; Sun, H. Progress in Photo-Responsive Polypeptide Derived Nano-Assemblies. Micromachines 2018, 9, 296–313. [Google Scholar] [CrossRef]
- Dai, Y.Q.; Sun, H.; Pal, S.; Zhang, Y.L.; Park, S.; Kabb, C.; Wei, D.; Sumerlin, B. Near-IR-induced dissociation of thermally-sensitive star polymers. Chem. Sci. 2017, 8, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kabb, C.; Dai, Y.Q.; Hill, M.; Ghiviriga, I.; Bapat, A.; Sumerlin, B. Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture. Nat. Chem. 2017, 9, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Alfurhood, J.; Sun, H.; Kabb, C.; Tucker, B.; Matthews, J.; Luesch, H.; Sumerlin, B. Poly(N-(2-hydroxypropyl)methacrylamide)—Valproic acid conjugates as block copolymer nanocarriers. Polym. Chem. 2017, 8, 4983–4987. [Google Scholar] [CrossRef] [PubMed]
- Yan, E.Y.; Ding, Y.; Chen, C.J.; Li, R.T.; Hu, Y.; Jiang, X.Q. Polymer/silica hybrid hollow nanospheres with pH-sensitive drug release in physiological and intracellular environments. Chem. Commun. 2009, 19, 2718–2720. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Subr, V. Polymeric anticancer drugs with pH-controlled activation. Adv. Drug Deliv. Rev. 2004, 56, 1023–1050. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of Environment-Sensitive Supramolecular Assemblies for Intracellular Drug Delivery: Polymeric Micelles that are Responsive to Intracellular pH Change. Angew. Chem. 2003, 115, 4788–4791. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, T.; Wang, C.S.; Lin, Z.Q.; Huang, G.; Sumer, B.D.; Gao, J.M. Molecular basis of cooperativity in pH-triggered supramolecular self-assembly. Nat. Commun. 2016, 7, 13214–13222. [Google Scholar] [CrossRef] [PubMed]
- Urano, Y.; Asanuma, D.; Hama, Y.; Koyama, Y.; Barrett, T.; Kamiya, M.; Nagano, T.; Watanabe, T.; Hasegawa, A.; Choyke, P.L.; et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat. Med. 2009, 15, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Hama, Y.; Urano, Y.; Koyama, Y.; Choyke, P.L.; Kobayashi, H. d-galactose receptor–targeted in vivo spectral fluorescence imaging of peritoneal metastasis using galactosamin-conjugated serum albumin-rhodamine green. J. Biomed. Opt. 2007, 12, 051501–051509. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Hama, Y.; Urano, Y.; Nguyen, D.M.; Choyke, P.L.; Kobayashi, H. Spectral fluorescence molecular imaging of lung metastases targeting HER2/neu. Clin. Cancer Res. 2007, 13, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Kono, K. Carboxylated hyperbranched poly(glycidol)s for preparation of pH-sensitive liposomes. J. Control. Release 2011, 149, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, H.; Jiang, X.; Zhang, C.; Ping, Q. Novel pH-sensitive chitosan-derived micelles loaded with paclitaxel. Carbohydr. Polym. 2010, 82, 432–439. [Google Scholar] [CrossRef]
- Kim, J.H.; Li, Y.; Kim, M.S.; Kang, S.W.; Jeong, J.H.; Lee, D.S. Synthesis and evaluation of biotin-conjugated pH-responsive polymeric micelles as drug carriers. Int. J. Pharm. 2012, 427, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Prajakta, D.; Ratnesh, J.; Chandan, K.; Suresh, S.; Grace, S.; Meera, V.; Vandana, P. Curcumin Loaded pH-Sensitive Nanoparticles for the Treatment of Colon Cancer. J. Biomed. Nanotechnol. 2009, 5, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Kushwah, V.; Sau, S.; Jain, S.; Iyer, A.K. PH responsive biodegradable nanogels for sustained release of bleomycin. Bioorg. Med. Chem. 2017, 25, 4595–4613. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Diaz-Gomez, L.; Cirillo, G.; Concheiro, A.; Iemma, F.; Alvarez-Lorenzo, C. PH/redox dual-sensitive dextran nanogels for enhanced intracellular drug delivery. Eur. J. Pharm. Biopharm. 2017, 117, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Achazi, K.; Haag, R. Boronate Cross-linked ATP- and pH-Responsive Nanogels for Intracellular Delivery of Anticancer Drugs. Adv. Healthc. Mater. 2015, 4, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Wang, C.S.; Li, Y.; Huang, G.; Zhao, T.; Ma, X.P.; Wang, Z.H.; Sumer, B.D.; White, M.A.; Gao, J.M. Digitization of Endocytic pH by Hybrid Ultra-pH-Sensitive Nanoprobes at Single-Organelle Resolution. Adv. Mater. 2016, 29, 1603794–1603802. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Miyata, K.; Oba, M.; Ishii, T.; Fukushima, S.; Han, M.; Koyama, H.; Nishiyama, N.; Kataoka, K. Charge-Conversion Ternary Polyplex with Endosome Disruption Moiety: A Technique for Efficient and Safe Gene Delivery. Angew. Chem. Int. Ed. 2008, 120, 5241–5244. [Google Scholar] [CrossRef]
- Weaver, J.V.M.; Williams, R.T.; Royles, B.J.L.; Findlay, P.H.; Cooper, A.I.; Rannard, S.P. PH-responsive branched polymer nanoparticles. Soft Matter 2008, 4, 985–992. [Google Scholar] [CrossRef]
- Bae, Y.; Nishiyama, N.; Kataoka, K. In vivo antitumor activity of the folate-conjugated pH-Sensitive polymeric micelle selectively releasing adriamycin in the intracellular acidic compartments. Bioconj. Chem. 2007, 18, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Pal, S. Synthesis of poly (ethylene glycol)-block-poly (acrylamide)-block-poly (lactide) amphiphilic copolymer through ATRP, ROP and click chemistry: Characterization, micellization and pH-triggered sustained release behaviour. Polymer 2017, 127, 150–158. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Q.; Tang, H.D.; Zhan, Y.H.; Van, K.; Murdoch, W.J. Degradable poly (β-amino ester) nanoparticles for cancer cytoplasmic drug delivery. Nanomedicine 2009, 5, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, R.; Yang, M.; Jiang, X.; Liu, B. Thermo and pH dualresponsive nanoparticles for anti-cancer drug delivery. Adv. Mater. 2007, 19, 2988–2992. [Google Scholar] [CrossRef]
- Topham, P.D.; Howse, J.R.; Mykhaylyk, O.O.; Armes, S.P.; Jones, R.A.L.; Ryan, A.J. Synthesis and solid state properties of a poly(methyl methacrylate)-block-poly(2-(diethylamino)ethyl methacrylate)-block-poly(methyl methacrylate) triblock copolymer. Macromolecules 2006, 39, 5573–5576. [Google Scholar] [CrossRef]
- Zhou, K.J.; Wang, Y.G.; Huang, X.N.; Luby-Phelps, K.; Sumer, B.D.; Gao, J.M. Tunable, Ultrasensitive pH-Responsive Nanoparticles Targeting Specific Endocytic Organelles in Living Cells. Angew. Chem. Int. Ed. 2011, 50, 6109–6114. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not Available. |
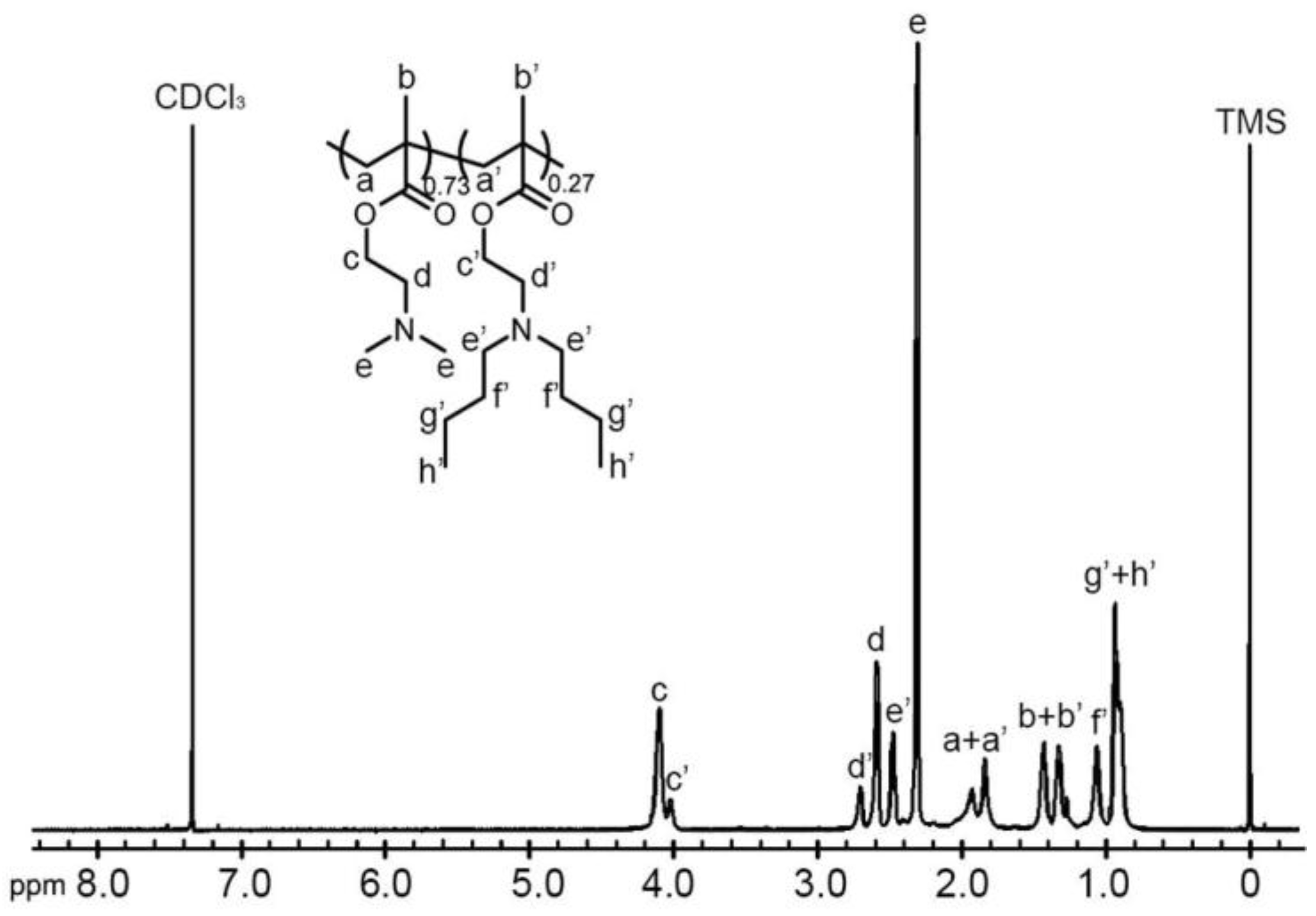
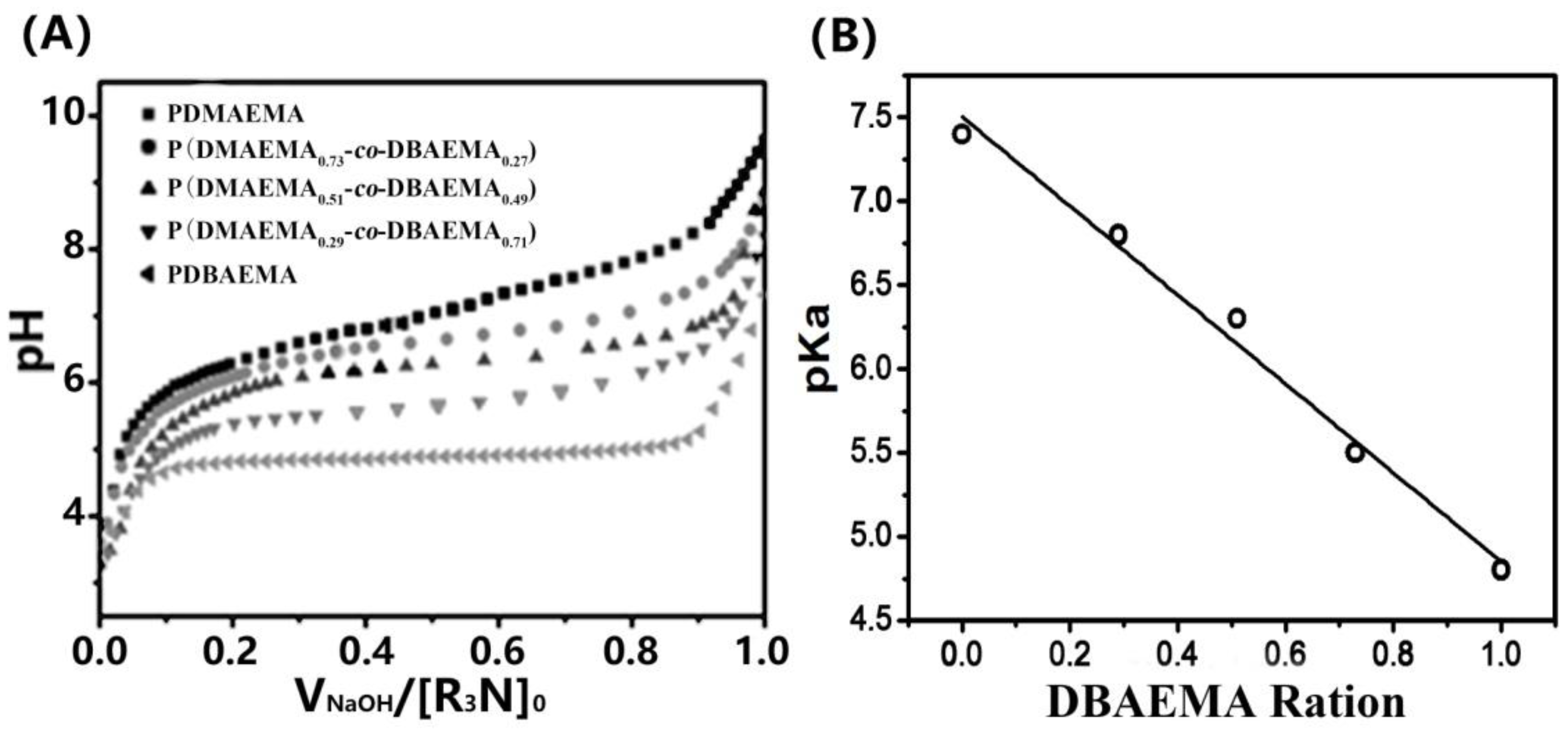

| Copolymer | DMA Content | Yield (%) | Mnb (×104) | Mwb (×104) | PDI | pKa c | |
|---|---|---|---|---|---|---|---|
| In Feed | In Polymer a | ||||||
| PDMAEMA | 100 | 100 | 73 | 1.21 | 1.85 | 1.53 | 7.4 |
| P(DMAEMA0.73-co-DBAEMA0.27) | 75 | 73 | 67 | 1.06 | 1.47 | 1.39 | 6.8 |
| P(DMAEMA0.51-co-DBAEMA0.49) | 50 | 51 | 62 | 1.01 | 1.84 | 1.82 | 6.3 |
| P(DMAEMA0.29-co-DBAEMA0.71) | 25 | 29 | 65 | 1.13 | 2.03 | 1.80 | 5.5 |
| PDBAEMA | 0 | 0 | 56 | 1.40 | 2.59 | 1.85 | 4.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Li, P.; Li, W.; Li, H.; Huang, X. Ultrasensitive (Co)polymers Based on Poly(methacrylamide) Structure with Fining-Tunable pH Responsive Value. Molecules 2018, 23, 1870. https://doi.org/10.3390/molecules23081870
Fan H, Li P, Li W, Li H, Huang X. Ultrasensitive (Co)polymers Based on Poly(methacrylamide) Structure with Fining-Tunable pH Responsive Value. Molecules. 2018; 23(8):1870. https://doi.org/10.3390/molecules23081870
Chicago/Turabian StyleFan, Haiming, Po Li, Wei Li, Hui Li, and Xiaonan Huang. 2018. "Ultrasensitive (Co)polymers Based on Poly(methacrylamide) Structure with Fining-Tunable pH Responsive Value" Molecules 23, no. 8: 1870. https://doi.org/10.3390/molecules23081870
APA StyleFan, H., Li, P., Li, W., Li, H., & Huang, X. (2018). Ultrasensitive (Co)polymers Based on Poly(methacrylamide) Structure with Fining-Tunable pH Responsive Value. Molecules, 23(8), 1870. https://doi.org/10.3390/molecules23081870







