A Promising Copper(II) Complex as Antifungal and Antibiofilm Drug against Yeast Infection
Abstract
:1. Introduction
2. Results
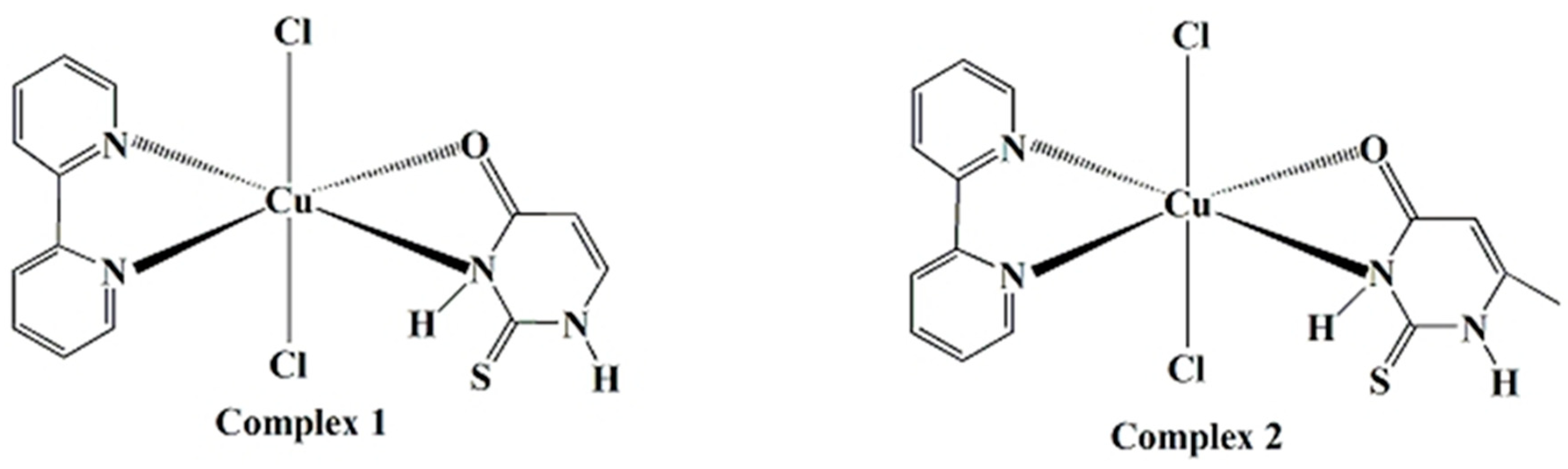
2.1. Chemistry
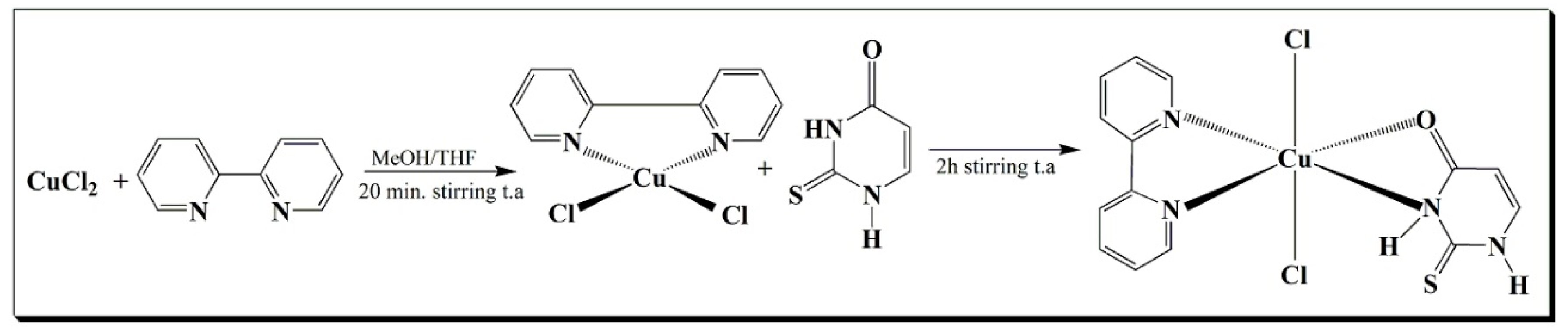
2.1.1. Syntheses of Complex
2.1.2. Vibrational Spectroscopy
2.1.3. ESI-HRMS Spectrometry
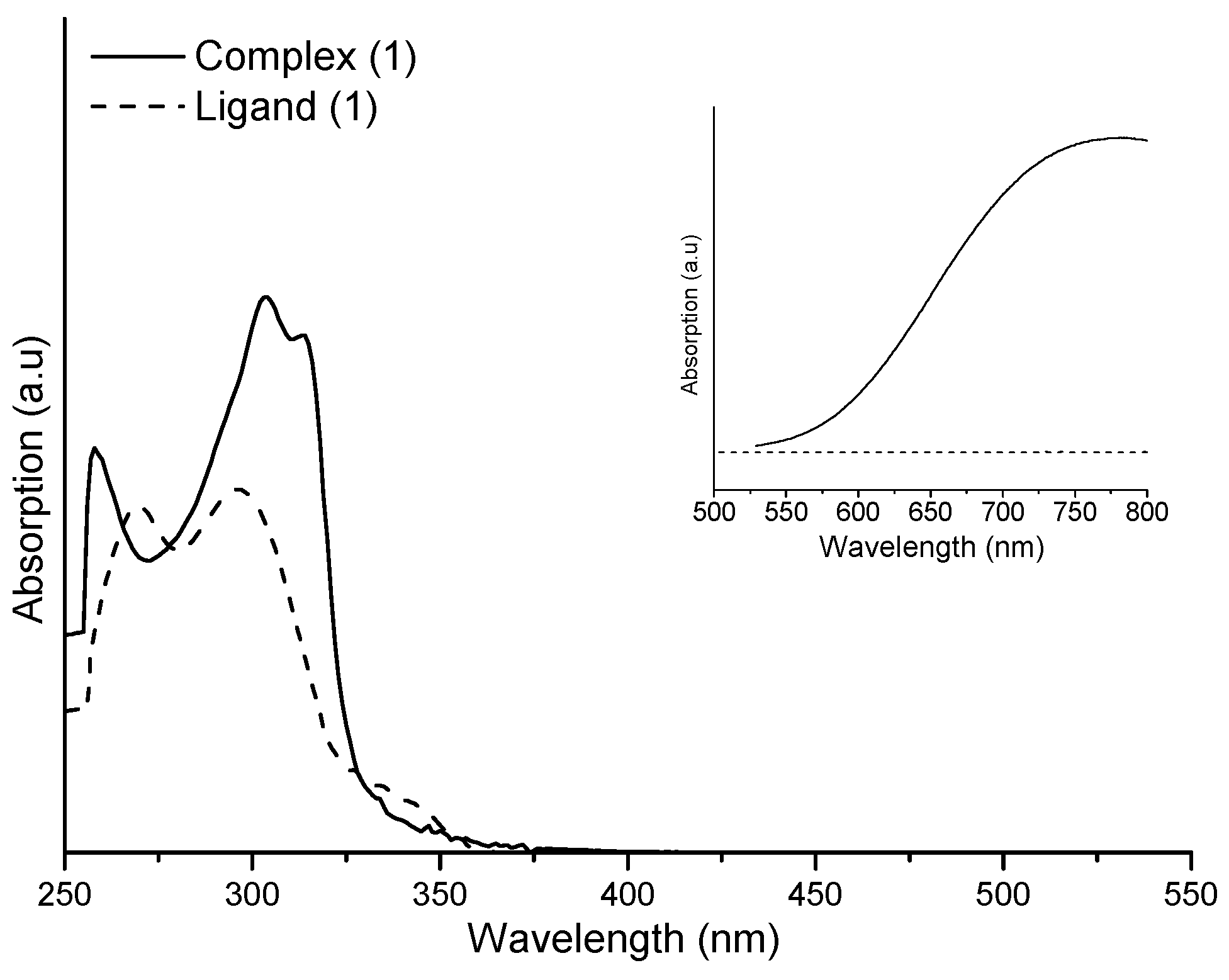
2.2. UV-Vis Studies and Stability Evaluation
2.3. Antifungal Activity
2.4. Biofilm Formation
2.5. Mutagenic Activity
3. Discussion
4. Materials and Methods
4.1. Chemical and Measurements
4.2. Preparation of Complexes
4.3. Microorganisms and Culture Conditions
4.4. Antifungal Activity Screening
4.5. Biofilm Formation
4.5.1. Effect of the Complex 1 on Biofilm Formation
4.5.2. Effect of the Complex 1 on Preformed Biofilms
4.6. Mutagenic Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garbee, D.D.; Pierce, S.S.; Manning, J. Manning. Opportunistic Fungal Infections in Critical Care Units. Crit. Care Nurs. Clin. N. Am. 2017, 29, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Giacomazzi, J.; Baethgen, L.; Carneiro, L.C.; Millington, M.A.; Denning, D.W.; Colombo, A.L.; Pasqualotto, A.C. The burden of serious human fungal infections in Brazil. Mycoses 2015, 59, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Doi, A.M.; Pignatari, A.C.C.; Edmond, M.B.; Marra, A.R.; Camargo, L.F.A.; Siqueira, R.A.; Mota, V.P.; Colombo, A.L. Epidemiology and Microbiologic Characterization of Nosocomial Candidemia from a Brazilian National Surveillance Program. PLoS ONE 2016, 11, e0146909. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Candida biofilms associated with CVC and medical devices. Mycoses 2012, 55, 46–57. [Google Scholar] [CrossRef]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes, G.M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.; Henriques, M.; Silva, S. Portrait of Candida Species Biofilm Regulatory Net work Genes. Trends Microbiol. 2017, 25, 62–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, J.V.; Mitchel, A.P.; Andes, D.R. Fungal Biofilms, Drug Resistance, and Recurrent Infection. Cold Spring Harb. Perspect. Med. 2014, 4, a019729. [Google Scholar] [CrossRef] [PubMed]
- Casteli, M.V.; Derita, M.G.; López, S.N. Novel antifungal agents: A patent review (2013–present). J. Expert Opin. Ther. Pat. 2017, 27, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R. Is there an emerging need for new antifungals? Expert Opin. Emerg. Drugs 2016, 21, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Altintop, M.D.; Oozdemir, A.; Zitouni-Turan, G.; Ilgin, S.; Atli, O.; Demirel, R.; Kaplancıkl, Z.A. A novel series of thiazolyl-pyrazoline derivatives: Synthesis and evaluation of antifungal activity, cytotoxicity and genotoxicity. Eur. J. Med. Chem. 2015, 92, 242–352. [Google Scholar] [CrossRef] [PubMed]
- Monte, C.; Carradori, S.; Bizzarria, B.; Bolasco, A.; Caprara, F.; Mollica, A.; Rivanera, D.; Mari, E.; Zicari, A.; Akdemir, A.; et al. Anti-Candida activity and cytotoxicity of a large library of new N-substituted-1,3-thiazolidin-4-one derivatives. Eur. J. Med. Chem. 2016, 107, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Salci, T.P.; Negri, M.; Abadio, A.K.R.; Bonfim-Mendonça, P.; Capoci, I.; Caparroz-Assef, S.M.; Donati, L.; Felipe, M.S.S.; Kioshima, E.S.; Svidzinsk, T.I.E. A new small-molecule KRE2 inhibitor against invasive Candida parapsilosis infection. Future Microbiol. 2017, 12, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Scăețeanu, G.V.; Chifiriuc, M.C.; Bleotu, C.; Kamerzan, C.; Măruţescu, L.; Daniliuc, C.G.; Maxim, C.; Calu, L.; Olar, R.; Badea, M. Synthesis, Structural Characterization, Antimicrobial Activity, and In Vitro Biocompatibility of New Unsaturated Carboxylate Complexes with 2,2′-Bipyridine. Molecules 2018, 23, 157. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.P.; Lima, G.M.; Paniago, E.B.; Takahashi, J.A.; Krambrock, K.; Pinheiro, C.B.; Wardell, J.L.; Visentin, L.C. Synthesis, characterization, structural and biological aspects of copper(II) dithiocarbamate complexes—Part II, [Cu{S2CN(Me)(R1)}2], [Cu{S2CN(Me)(R2)}2] and [Cu{S2CN(R3)(R4)}2] {R1 = –CH2CH(OMe)2, R2 = 2-methyl-1,3-dioxolane, R3 = –CH2(CH2)2N = CHPhOCH2Ph and R4 = –CH2CH2OH}. J. Mol. Struct. 2013, 1048, 357–366. [Google Scholar] [CrossRef]
- Kuzovlev, A.S.; Savinkina, E.V.; Chernyshev, V.V.; Grigoriev, M.S.; Volov, A.N. Copper and palladium complexes with substituted pyrimidine-2-thiones and 2-thiouracils: Syntheses, spectral characterization, and X-ray crystallographic study. J. Coord. Chem. 2016, 69, 508–521. [Google Scholar] [CrossRef]
- Palafox, M.A.; Rastogi, V.K.; Tanwarb, R.P.; Mittal, L. Vibrational frequencies and structure of 2-thiouracil by Hartree-Fock, post-Hartree-Fock and density functional methods. Spectrochim. Acta Mol. Biomol. Spectrosc. 2003, 59, 2473–2486. [Google Scholar] [CrossRef]
- Ma, L.Y.; Zheng, Y.C.; Wang, S.Q.; Wang, B.; Wang, Z.R.; Pang, L.P.; Zhang, M.; Wang, J.W.; Ding, L.; Li, J.; et al. Design, Synthesis, and Structure–Activity Relationship of Novel LSD1 Inhibitors Based on Pyrimidine–Thiourea Hybrids As Potent, Orally Active Antitumor Agents. J. Med. Chem. 2015, 58, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Pahont, E.; Paraschivescu, C.; Ilies, D.C.; Poirier, D. Oprean, Synthesis and Characterization of Novel Cu(II), Pd(II) and Pt(II) Complexes with 8-Ethyl-2-hydroxytricyclo(7.3.1.02,7)tridecan-13-one-thiosemicarbazone: Antimicrobial and in Vitro Antiproliferative Activity. Molecules 2016, 21, 674–692. [Google Scholar] [CrossRef] [PubMed]
- Kamalakannan, P.; Venkappayya, D. Spectral, Thermal, and Antimicrobial Studies on the Copper(II), Zinc(II), Cadmium(II), and Mercury(II) Chelates of a New Antimetabolite–5-Dimethylaminomethyl-2-Thiouracil. Russ. J. Coord. Chem. 2002, 28, 423–433. [Google Scholar] [CrossRef]
- Scorzoni, L.; Sangalli-Leite, F.; Singulani, J.L.; Silva, A.C.; Costa-Orlandi, C.B.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. Searching new antifungals: The use of in vitro and in vivo methods for evaluation of natural compounds. J. Microbiol. Methods 2016, 123, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ferle, A.; Pizzuti, L.; Inglez, S.D.; Caires, A.R.L.; Lang, E.S.; Back, D.F.; Flores, A.F.C.; Junior, A.M.; Deflon, V.M.; Casagrande, G.A. The first gold(I) complexes based on pyrazoline ligands: Synthesis, structural characterization and photophysical properties. Polyhedron 2013, 63, 9–14. [Google Scholar] [CrossRef]
- Favarin, L.R.V.; Rosa, P.P.; Pizzuti, L.; Junior, A.M.; Caires, A.R.L.; Bezerra, L.S.; Pinto, L.M.C.; Maia, G.; Gato, C.C.; Back, D.F.; et al. Synthesis and structural characterization of new heteroleptic copper(I) complexes based on mixed phosphine/thiocarbamoyl-pyrazoline ligands. Polyhedron 2017, 121, 185–190. [Google Scholar] [CrossRef]
- Wei-Da, Y.; Yin-Ping, H. Copper Complex of 2-Thiouracil. Polyhedron 1990, 9, 2747–2750. [Google Scholar] [CrossRef]
- Preda, N.; Mihut, L.; Baibarac, M.; Baltog, I.; Husano, M. The intercalation of PbI2 with 2,2′-bipyridine evidenced by photoluminescence, FT-IR and Raman spectroscopy. Rom. J. Phys. 2008, 54, 667–675. [Google Scholar] [CrossRef]
- Adelaide, O.M.; Abidemi, O.O.; Olubunmi, A.D. Synthesis, characterization and antibacterial studies of some copper(II) complexes of 2,2′-bipyridine and 1.10-phenanthroline. J. Chem. Pharm. Res. 2013, 5, 69–73. [Google Scholar]
- Lavanant, H.; Virelizier, H.; Hoppiliard, Y. Reduction of Copper(ii) Complexes by Electron Capture in an Electrospray Ionization Source. J. Am. Soc. Mass Spectrom. 1998, 9, 1217–1221. [Google Scholar] [CrossRef]
- Gianelli1, L.; Amendola, V.; Fabbrizzi, L.; Pallavicini, P.; Mellerio, G.G. Investigation of reduction of Cu(II) complexes in positive-ion mode electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 2347–2353. [Google Scholar] [CrossRef]
- Henderson, W.; Mcldoe, J.S. Mass Spectrometry of Inorganic and Organometallic Compounds; John Wiley & Sons Ltd.: New Jersey, NJ, USA, 2005; p. 292. ISBN 978-0-470-85015-2. [Google Scholar]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2016, 7, 2173. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; Pappas, P.G. Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2016, 30, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Mukherjee, P.K. Candida Biofilms: Development, Architecture, and Resistance. Microbiol. Spectr. 2015, 3, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Ferreira, I.C.F.R.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. In Vivo Anti-Candida Activity of Phenolic Extracts and Compounds: Future Perspectives Focusing on Effective Clinical Interventions. BioMed Res. Int. 2015, 2015, 247382. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.C.O.; Freires, I.A.; Lazarinia, J.G.; Infant, J.; Alencar, S.M. Unexplored endemic fruit species from Brazil: Antibiofilm properties, insights into mode of action, and systemic toxicity of four Eugenia spp. Microb. Pathog. 2017, 105, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Awad, S.M.; Ahmed, M. Synthesis and antimicrobial evaluation of some6-aryl-5-cyano-2-thiouracil derivatives. Acta Pharm. 2011, 6, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Youns, M.M.; Ahmed, N.M. Synthesis, antimicrobial, antioxidant activities of novel 6-aryl-5-cyanothiouracil derivatives. Eur. J. Med. Chem. 2013, 69, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Masoud, M.S.; Soayed, A.A.; Husseiny, A.F.E. Coordination modes, spectral, thermal and biological evaluation of hetero-metal copper containing 2-thiouracil complexes. Spectrochim. Acta Mol. Biomol. Spectrosc. 2012, 99, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- García-Santamarina, S.; Thiele, D.J. Copper at the Fungal Pathogen-Host Axis. J. Biol. Chem. 2015, 290, 18945–18953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA. International Conference on Harmonisation (ICH). In Guidance on S2(R1) Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use; Food and Drug Administration: Silver Spring, MD, USA, 2012. [Google Scholar]
- Escobar, P.A.; Kemper, R.A.; Tarca, J.; Nicolette, J.; Kenyon, M.; Glowienke, S.; Sawant, S.G.; Christensen, J.; Johnson, T.E.; McKnight, C.; et al. Bacterial mutagenicity screening in the pharmaceutical industry. Mutat. Res. 2013, 752, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Larone, D.H. Medically Important Fungi: A Guide to Identification, 5rd ed.; ASM Press: Washington, DC, USA, 2011; ISBN 1555816606. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Approved Standard; CLSI Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 28, pp. 1–13. [Google Scholar]
- Yang, Y.L.; Wang, A.H.; Wang, C.W.; Cheng, W.T.; Li, S.Y.; Lo, H.J. Suscetibilities to amphotericin B and fluconazole of Candida species in Taiwan surveillance of antimicrobial resistance of yeasts 2006. Diagn. Microbiol. Infect. Dis. 2008, 61, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Capoci, I.R.G.; Bonfim-Mendonça, P.S.; Arita, G.S.; Pereira, R.R.A.; Consolarom, M.E.L.; Bruschim, M.L.; Negri, M.; Svidzinski, T.I.E. Propolis Is an Efficient Fungicide and Inhibitor of Biofilm Production by Vaginal Candida albicans. Evid.-Based Complement. Altern. Med. 2015, 2015, 287693. [Google Scholar] [CrossRef] [PubMed]
- Kado, N.Y.; Langley, D.; Eisenstadt, E. A simple modification of the Salmonella liquid-incubation assay. Increased sensitivity for detecting mutagens in human urine. Mutat. Res. 1983, 121, 25–32. [Google Scholar] [CrossRef]
- Bernstein, L.; Kaldor, J.; McCann, J.; Pike, M.C. An empirical approach to the statistical analysis of mutagenesis data from the Salmonella test. Mutat. Res. 1982, 97, 267–281. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Frequency Vibrations (cm−1) | |||||
|---|---|---|---|---|---|
| Ligand (1) | Ligand (2) | Bipy | Complex (1) | Complex (2) | |
| ν (N-H) | 3086-3046 | 3110-3006 | ----- | 3109-3089 | 3109-3090 |
| ν(C=O, C=C) | 1707, 1616 | 1636, ----- | ----- | 1683, 1602 | 1656, 1604 |
| thioamide I | 1567 | 1557 | ----- | 1557 | 1568 |
| thioamide II | 1216 | 1200 | ----- | 1207 | 1193 |
| thioamide III | 1173 | 1167 | ----- | 1173 | 1167 |
| thioamide IV | 833 | 837 | ----- | 832 | 835 |
| ν(C-H)Ar | ----- | ----- | 3084-3051 | 3051-3035 | 3051-3034 |
| ν(C-H) | 2921 | 2928 | ----- | 2928 | 2930 |
| ν((ring) + δ(C-H)) | ----- | ----- | 1453 | 1444 | 1441 |
| ν(C=N + C-N) | ----- | ------ | 1583 | ----- | ----- |
| δ(C-H)Ar | ----- | ----- | 754 | 771 | 777 |
| Isolate | Source | MIC a | MFC b | FLC c | AmB d |
|---|---|---|---|---|---|
| C. albicans CA1 | Sputum | 62.5 | 62.5 | 0.5 | 0.5 |
| C. albicans CA2 | Sputum | 62.5 | 125 | 0.25 | 0.25 |
| C. albicans CA3 | Vaginal | 62.5 | 62.5 | 0.5 | 0.5 |
| C. albicans CA4 | Vaginal | 62.5 | 62.5 | 0.5 | 0.5 |
| C. albicans CA5 | Vaginal | 62.5 | 62.5 | 0.5 | 0.5 |
| C. albicans CA6 | Vaginal | 62.5 | 62.5 | 0.5 | 0.5 |
| C. albicans CA7 | Nasal swab | 125 | 250 | 0.25 | 0.5 |
| C. albicans CA8 | Urine | 125 | 125 | 0.25 | 0.5 |
| C. albicans CA9 | Vaginal | 125 | 125 | 0.25 | 0.5 |
| C. albicans CA10 | Vaginal | 125 | 125 | 0.5 | 0.5 |
| C. glabrata CG1 | Urine | 62.5 | 62.5 | 16 e | 0.03 |
| C. glabrata CG2 | Urine | 62.5 | 62.5 | 1 | 0.03 |
| C. glabrata CG3 | Urine | 62.5 | 125 | 16 e | 0.5 |
| C. glabrata CG4 | Urine | 62.5 | 125 | 1 | 0.03 |
| C. glabrata CG5 | Urine | 62.5 | 62.5 | 32 e | 0.03 |
| C. glabrata CG6 | Urine | 125 | 250 | 16 e | 0.5 |
| C. krusei CK1 | Rectal swab | 31.25 | 31.25 | 0.5 | 0.03 |
| C. parapsilosis CP1 | Blood | 62.5 | 125 | 0.25 | 0.25 |
| C. parapsilosis CP2 | Catheter tip | 125 | 125 | 0.25 | 0.03 |
| C. parapsilosis CP3 | Urine | 125 | 250 | 0.25 | 0.03 |
| C. tropicalis CT1 | Sputum | 62.5 | 125 | 2 | 0.5 |
| Treatment (µg/plate) | TA98 | TA100 | ||
|---|---|---|---|---|
| −S9 | +S9 | −S9 | +S9 | |
| 0.0 a | 42 ± 2 | 15.00 ± 2 | 192 ± 6 | 191 ± 8 |
| 15 | 31 ± 1 (0, 7) | 15 ± 2 (1, 0) | 169 ± 9 (0, 9) | 176 ± 8 (0, 9) |
| 50 | 31 ± 4 (0, 7) | 16 ± 1 (1, 0) | 229 ± 7 (1, 2) * | 201 ± 7 (1, 0) |
| 150 | 34 ± 3 (0, 8) | 17 ± 1 (1, 1) | 265 ± 9 (1, 4) ** | 190 ± 7 (1, 0) |
| 500 | 36 ± 1 (0, 8) | 14 ± 2 (1, 0) | 226 ± 6 (1, 2) * | 241 ± 6 (1, 2) ** |
| 1500 | 34 ± 2 (0, 8) | 16 ± 3 (1, 1) | 249 ± 5 (1, 3) ** | 241 ± 8 (1, 3) ** |
| 5000 | 36 ± 5 (0, 8) | 18 ± 2 (1, 2) | 286 ± 7 (1, 5) ** | 238 ± 9 (1, 4) ** |
| C+ | 246 ± 6 b | 227 ± 9 c | 990 ± 9 d | 979 ± 4 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes da Silva Dantas, F.; Araújo de Almeida-Apolonio, A.; Pires de Araújo, R.; Regiane Vizolli Favarin, L.; Fukuda de Castilho, P.; De Oliveira Galvão, F.; Inez Estivalet Svidzinski, T.; Antônio Casagrande, G.; Mari Pires de Oliveira, K. A Promising Copper(II) Complex as Antifungal and Antibiofilm Drug against Yeast Infection. Molecules 2018, 23, 1856. https://doi.org/10.3390/molecules23081856
Gomes da Silva Dantas F, Araújo de Almeida-Apolonio A, Pires de Araújo R, Regiane Vizolli Favarin L, Fukuda de Castilho P, De Oliveira Galvão F, Inez Estivalet Svidzinski T, Antônio Casagrande G, Mari Pires de Oliveira K. A Promising Copper(II) Complex as Antifungal and Antibiofilm Drug against Yeast Infection. Molecules. 2018; 23(8):1856. https://doi.org/10.3390/molecules23081856
Chicago/Turabian StyleGomes da Silva Dantas, Fabiana, Adriana Araújo de Almeida-Apolonio, Renata Pires de Araújo, Lis Regiane Vizolli Favarin, Pamella Fukuda de Castilho, Fernanda De Oliveira Galvão, Terezinha Inez Estivalet Svidzinski, Gleison Antônio Casagrande, and Kelly Mari Pires de Oliveira. 2018. "A Promising Copper(II) Complex as Antifungal and Antibiofilm Drug against Yeast Infection" Molecules 23, no. 8: 1856. https://doi.org/10.3390/molecules23081856
APA StyleGomes da Silva Dantas, F., Araújo de Almeida-Apolonio, A., Pires de Araújo, R., Regiane Vizolli Favarin, L., Fukuda de Castilho, P., De Oliveira Galvão, F., Inez Estivalet Svidzinski, T., Antônio Casagrande, G., & Mari Pires de Oliveira, K. (2018). A Promising Copper(II) Complex as Antifungal and Antibiofilm Drug against Yeast Infection. Molecules, 23(8), 1856. https://doi.org/10.3390/molecules23081856





