Purification and Characterization of Lipase Produced by Leuconostoc mesenteroides Subsp. mesenteroides ATCC 8293 Using an Aqueous Two-Phase System (ATPS) Composed of Triton X-100 and Maltitol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Triton X-100 and Maltitol on Lipase Activity
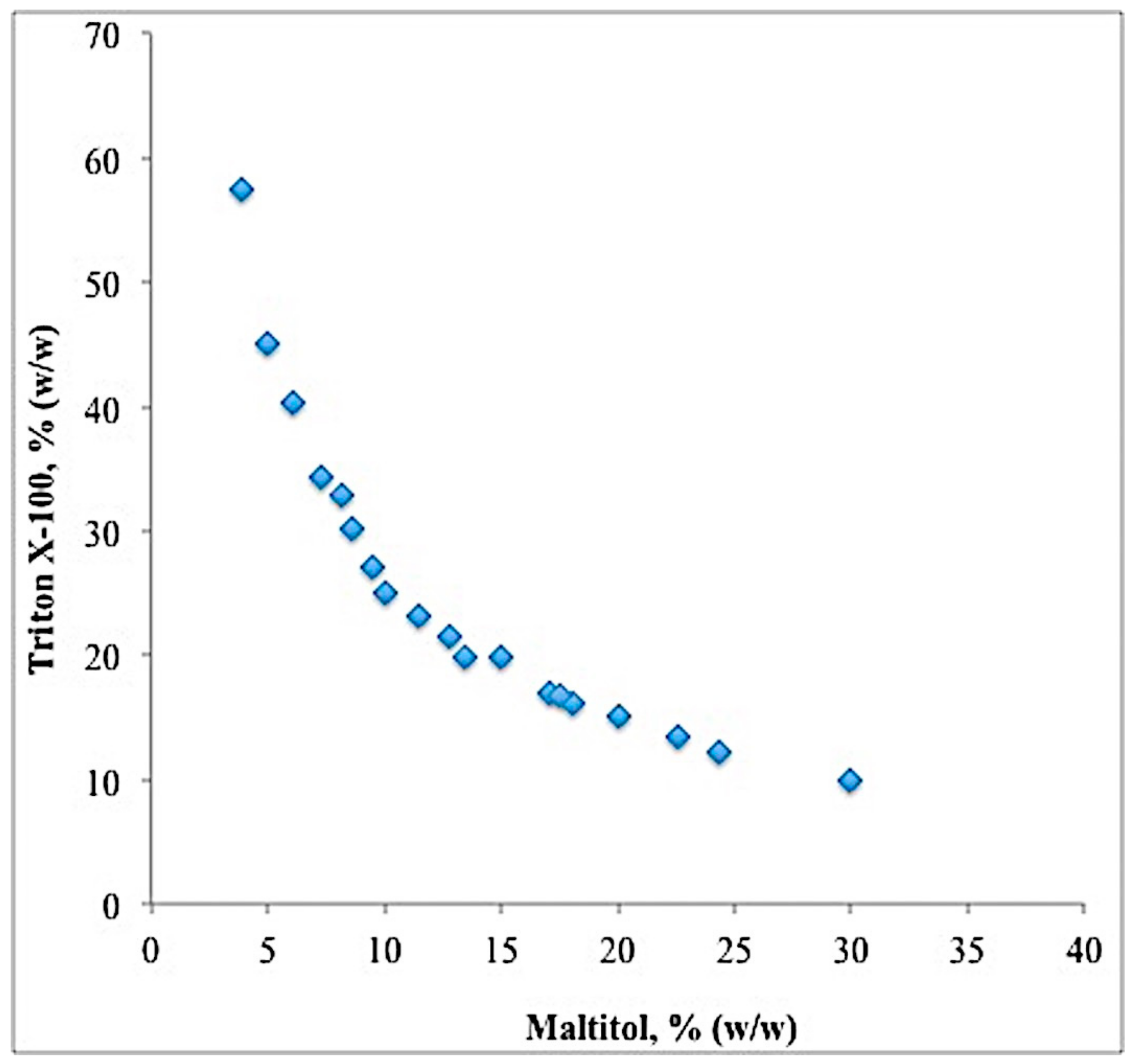
2.2. Binodal Curve and TLL
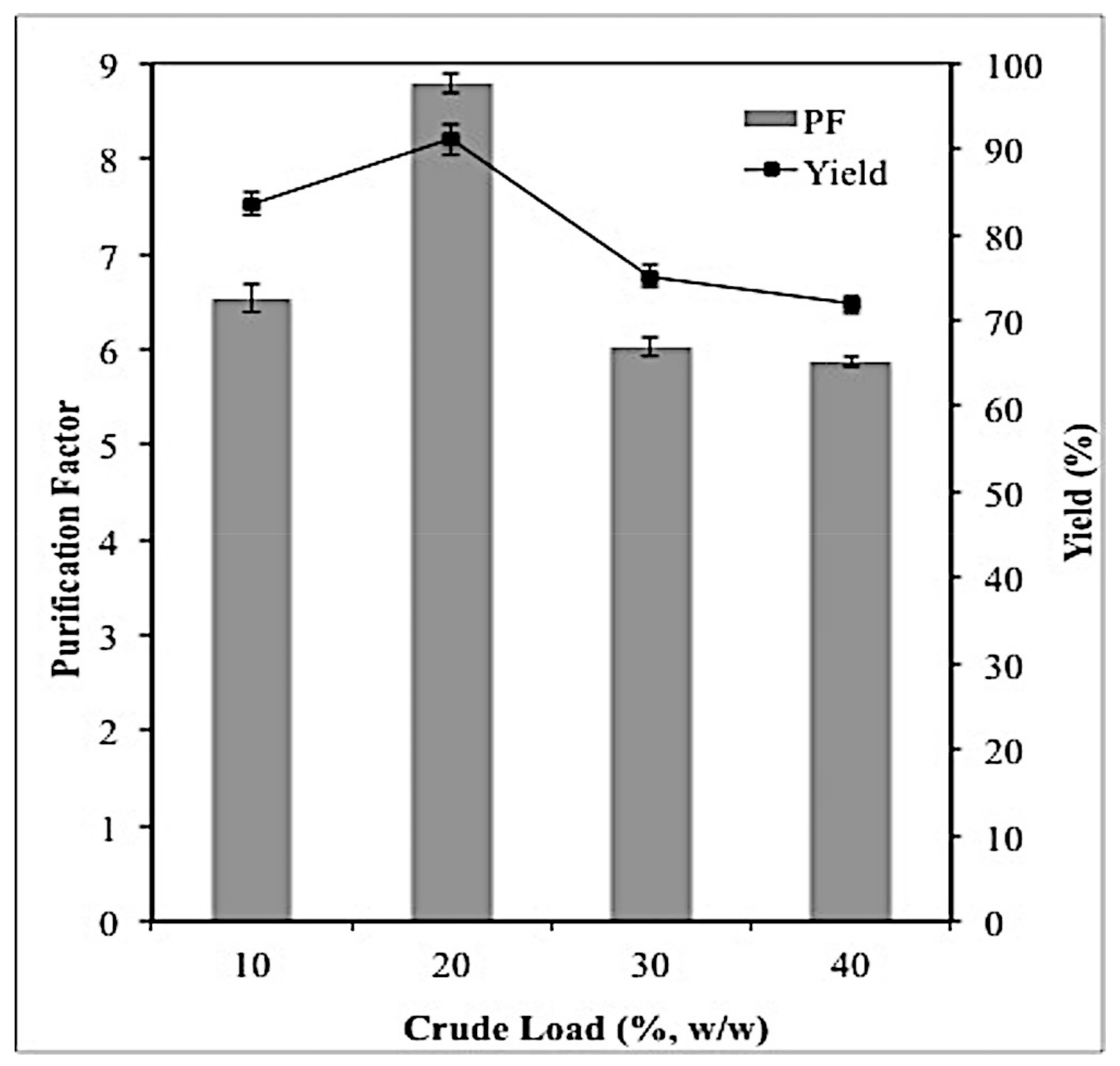
2.3. Effect of Crude Feedstock on Lipase Partitioning
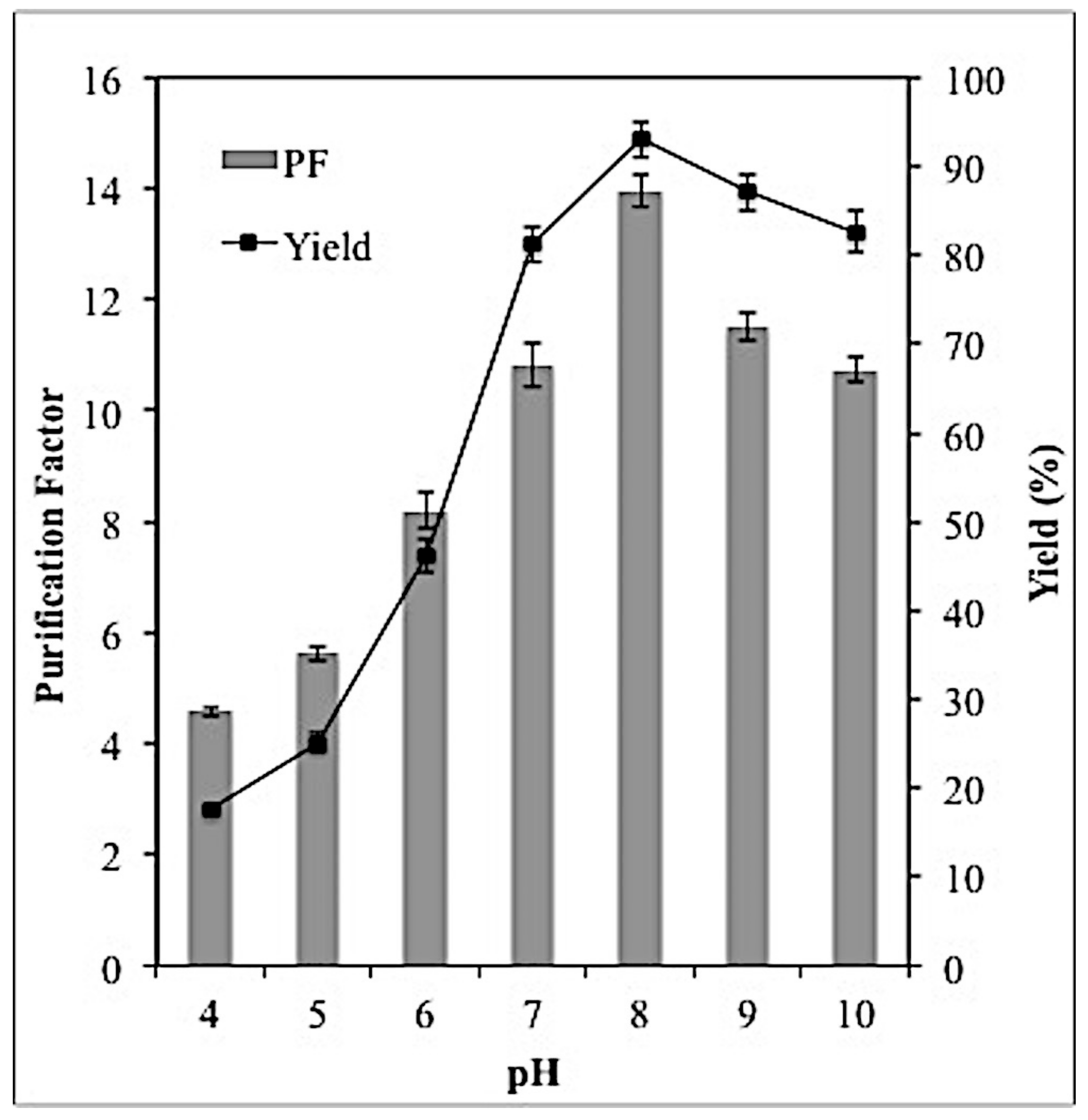
2.4. Effect of PH on Lipase Partitioning
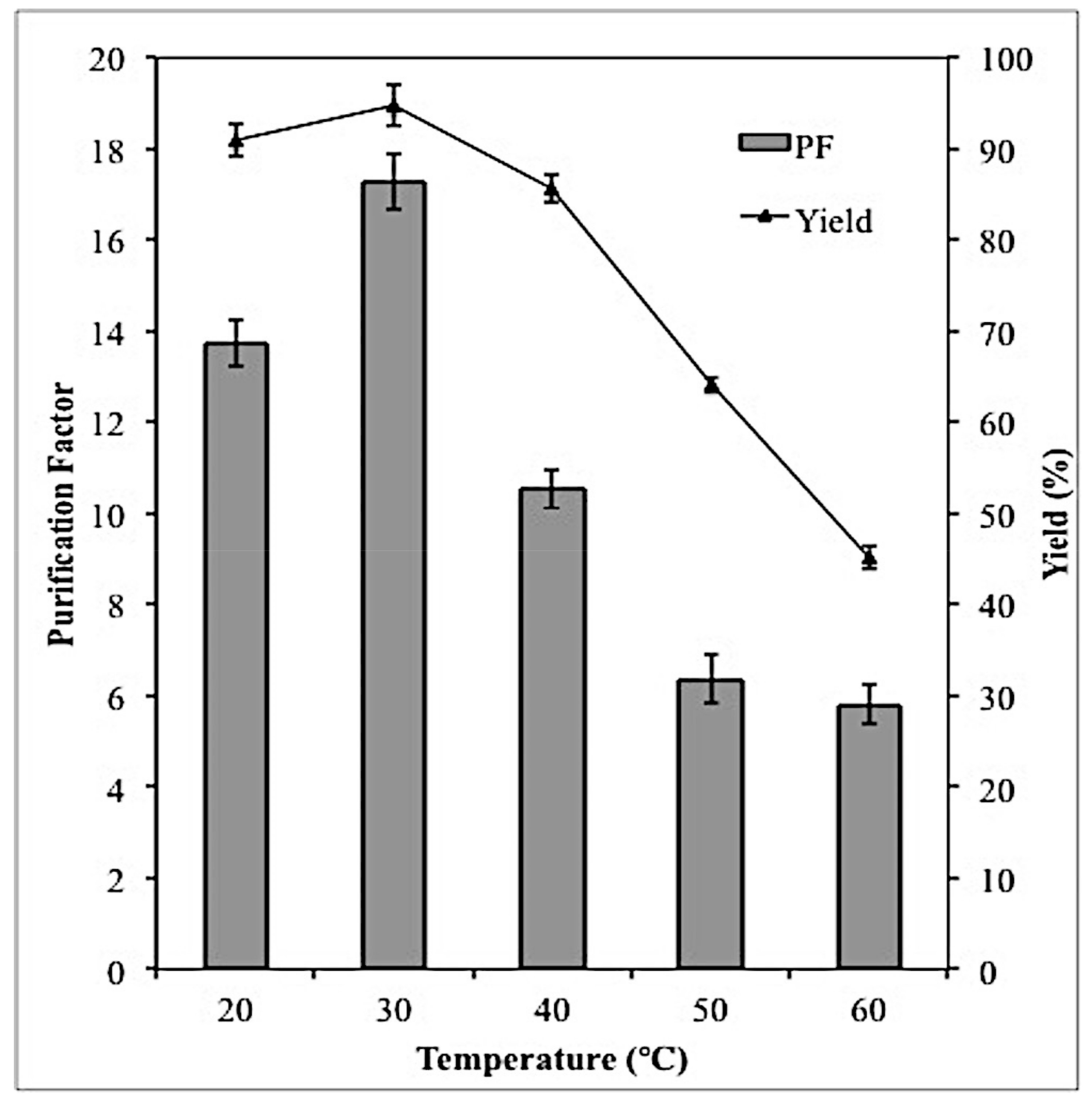
2.5. Effect of Temperature on Lipase Partitioning
2.6. Lipase Recovery
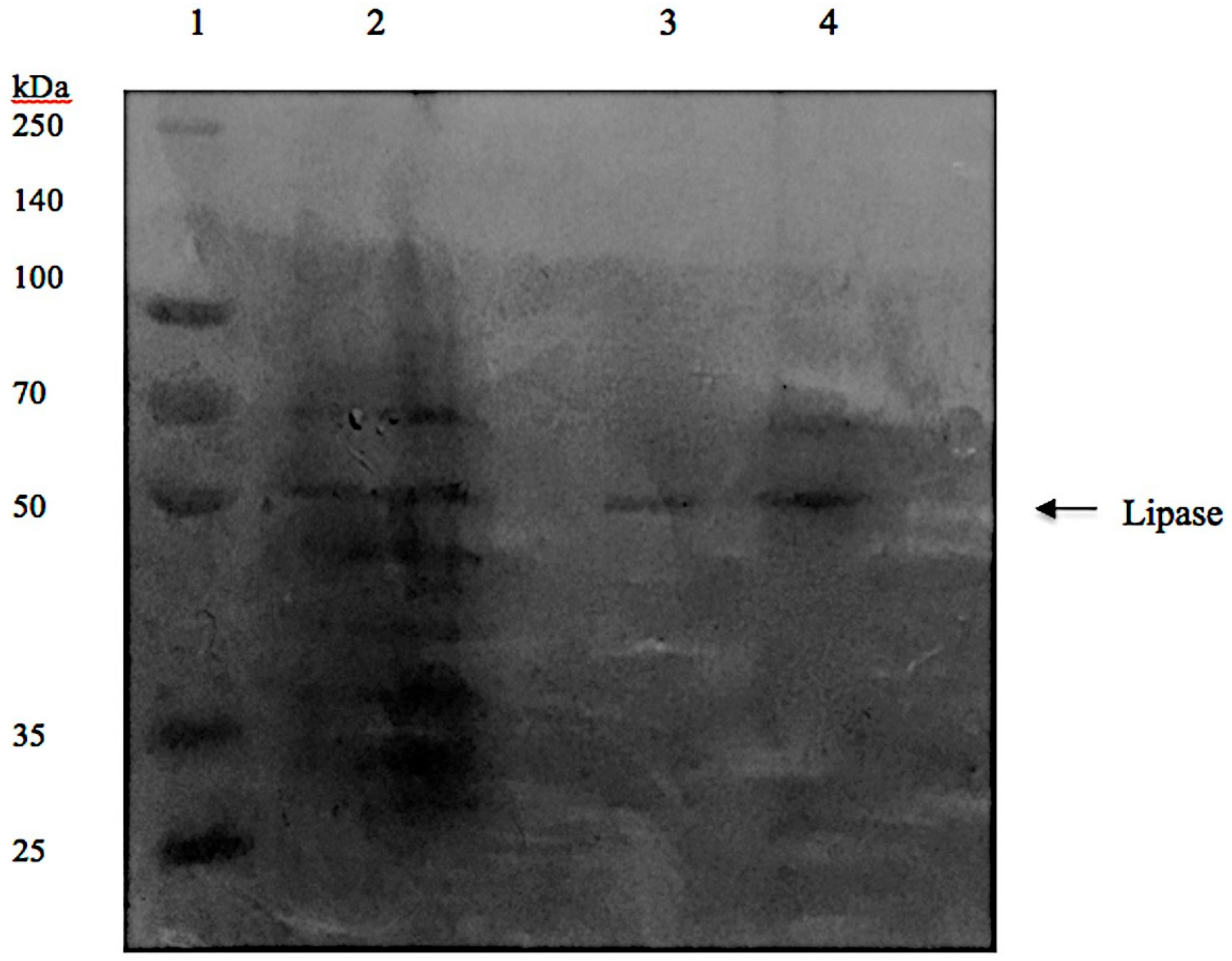
2.7. Characterization of Purified Lipase
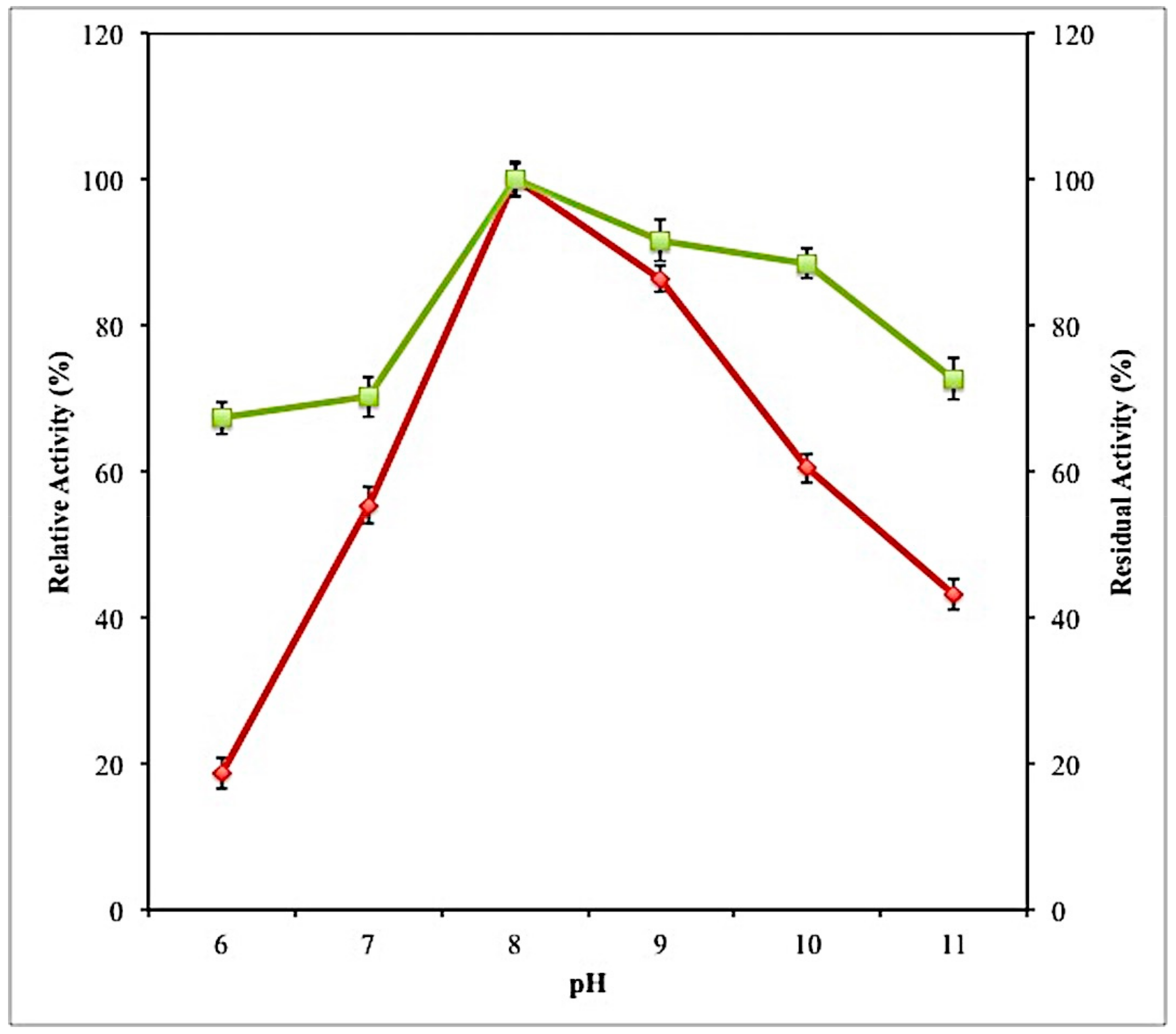
2.7.1. Effect of PH on Activity and Stability of Lipase
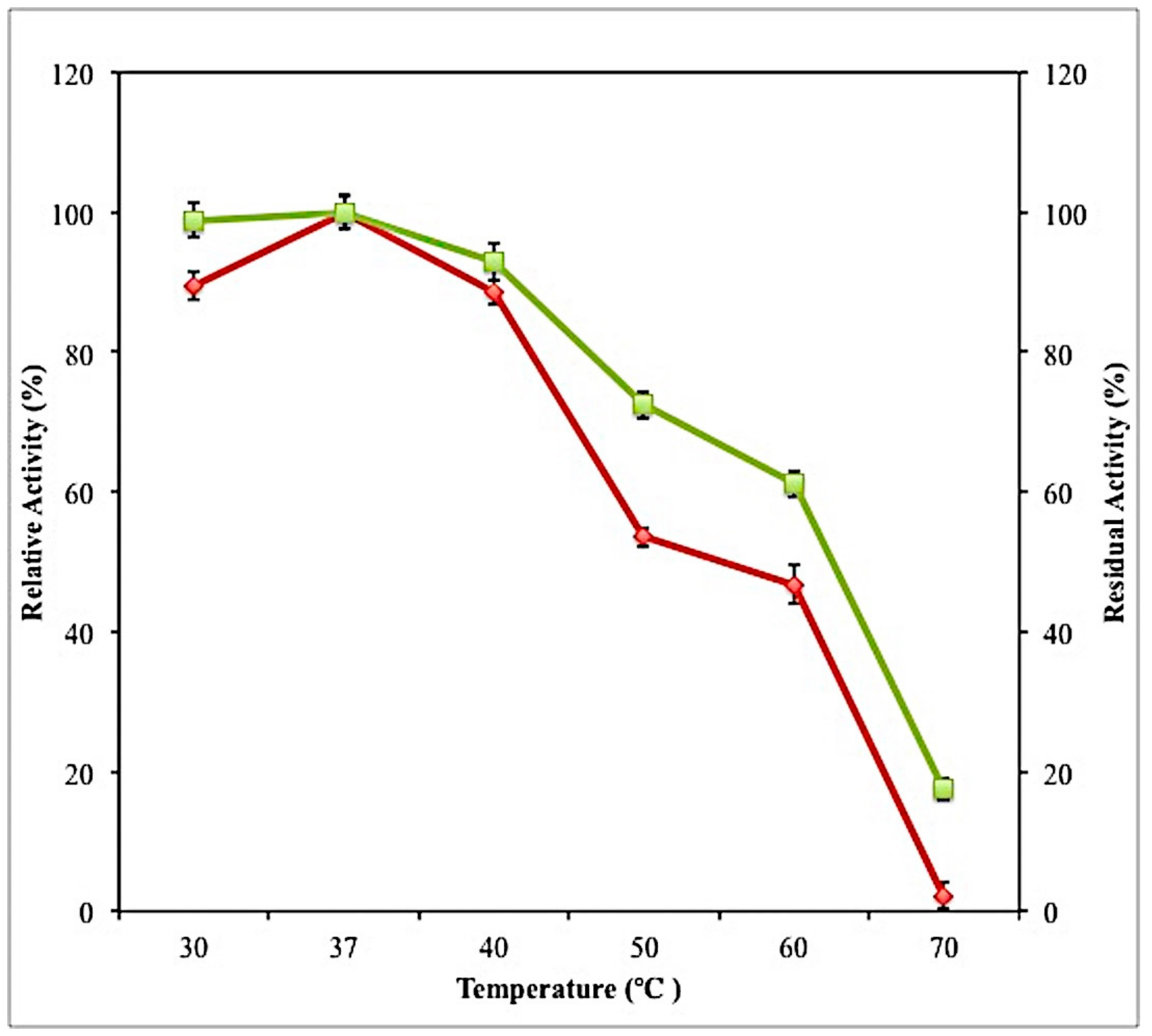
2.7.2. Effect of Temperature on Activity and Stability of Crude Lipase
2.7.3. Effect of Surfactant Agent on Lipase Activity
2.7.4. Effect of Metal Ions on Lipase Activity
3. Materials and Methods
3.1. Materials
3.2. Production of Extracellular Lipase
3.3. Binodal Curve and Tie-Lines
3.4. Lipase Partitioning Experiments
3.5. Determination of Lipase Activity and Protein Concentration
3.6. Evaluation of Lipase Partitioning
3.7. Sodium Dodecylsulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
3.8. Characterization of Purified Lipase
3.8.1. Effect of Temperature on Lipase Activity and Stability
3.8.2. Effect of PH on Lipase Activity and Stability
3.8.3. Effect of Metal Ions and Surfactants on Lipase Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raja, S.; Murty, V.R.; Thivaharan, V.; Rajasekar, V.; Ramesh, V. Aqueous two-phase systems for the recovery of biomolecules—A review. Sci. Technol. 2012, 1, 7–16. [Google Scholar] [CrossRef]
- Benavides, J.; Rito-Palomares, M.; Asenjo, J.A. Aqueous two-phase systems. In Comprehensive Biotechnology; Elsevier: New York, NY, USA, 2011; Volume 2, pp. 697–713. [Google Scholar]
- Schindler, J.; Nothwang, H.G. Aqueous polymer two-phase systems: Effective tools for plasma membrane proteomics. Proteomics 2006, 6, 5409–5417. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Khoiroh, I.; Ling, T.C.; Show, P.L. Enhanced recovery of lipase derived from Burkholderia cepacia from fermentation broth using recyclable ionic liquid/polymer-based aqueous two-phase systems. Sep. Purif. Technol. 2017, 179, 152–160. [Google Scholar] [CrossRef]
- Amid, M.; Manap, M.Y.; Hussin, M.; Mustafa, S. A novel aqueous two phase system composed of surfactant and xylitol for the purification of lipase from pumpkin (Cucurbita moschata) seeds and recycling of phase components. Molecules 2015, 20, 11184–11201. [Google Scholar] [CrossRef] [PubMed]
- De Brito Cardoso, G.; Souza, I.N.; Mourão, T.; Freire, M.G.; Soares, C.M.F.; Lima, Á.S. Novel aqueous two-phase systems composed of acetonitrile and polyols: Phase diagrams and extractive performance. Sep. Purif. Technol. 2014, 124, 54–60. [Google Scholar] [CrossRef]
- Amid, M.; Murshid, F.S.; Manap, M.Y.; Hussin, M. A novel aqueous micellar two-phase system composed of surfactant and sorbitol for purification of pectinase enzyme from Psidium guajava and recycling phase components. Biomed. Res. Int. 2015, 8, 815413. [Google Scholar]
- Emtenani, S.; Asoodeh, A.; Emtenani, S. Molecular cloning of a thermo-alkaliphilic lipase from Bacillus subtilis DR8806: Expression and biochemical characterization. Process Biochem. 2013, 48, 1679–1685. [Google Scholar] [CrossRef]
- Papagora, C.; Roukas, T.; Kotzekidou, P. Optimization of extracellular lipase production by Debaryomyces hansenii isolates from dry-salted olives using response surface methodology. Food Bioprod. Process. 2013, 91, 413–420. [Google Scholar] [CrossRef]
- Lopes, M.F.S.; Leitão, A.L.; Regalla, M.; Marques, J.J.F.; Carrondo, M.J.T.; Crespo, M.T.B. Characterization of a highly thermostable extracellular lipase from Lactobacillus plantarum. Int. J. Food. Microbiol. 2002, 76, 107–115. [Google Scholar] [CrossRef]
- Rashmi, B.S.; Gayathri, D. Partial purification, characterization of Lactobacillus sp. G5 lipase and their probiotic potential. Int. Food Res. J. 2014, 2, 1737–1743. [Google Scholar]
- Padmapriya, B.; Rajeswari, T.; Noushida, E.; Sethupalan, D.G.; Venil, C.K. Production of lipase enzyme from Lactobacillus spp. and its application in the degradation of meat. World Appl. Sci. J. 2011, 12, 1798–1802. [Google Scholar]
- Merchuk, J.C.; Andrews, B.A.; Asenjo, J.A. Aqueous two-phase systems for protein separation: Studies on phase inversion. J. Chromatogr. B 1998, 711, 285–293. [Google Scholar] [CrossRef]
- Show, P.L.; Tan, C.P.; Anuar, M.S.; Ariff, A.; Yusof, Y.A.; Chen, S.K.; Ling, T.C. Primary recovery of lipase derived from Burkholderia cenocepacia strain ST8 and recycling of phase components in an aqueous two-phase system. Biochem. Eng. J. 2012, 60, 74–80. [Google Scholar] [CrossRef]
- Dreyer, S.E. Aqueous Two-Phase Extraction Of Proteins and Enzymes Using Tetraalkylammonium-Based Ionic Liquids. Ph.D. Thesis, University of Rostock, Rostock, Germany, 2008. [Google Scholar]
- Carvalho, T.; Finotelli, P.; Bonomo, R.C.F.; Franco, M.; Amaral, P.F.F. Evaluating aqueous two-phase systems for Yarrowia lipolytica extracellular lipase purification. Process Biochem. 2017, 53, 259–266. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Goveas, L.C.; Suralikerimath, N.; Jampani, C.; Halami, P.M.; Narayan, B. Extraction and purification od lipase from Enterococcus faecium MTCC5695 by PEG/phosphate aqueous-two phase system (ATPS) and its biochemical characterization. Biocatal. Agric. Biotechnol. 2016, 6, 19–27. [Google Scholar]
- Ooi, C.W.; Tey, B.T.; Hii, S.L.; Ariff, A.; Wu, H.S.; Lan, J.C.W.; Juang, R.S.; Kamal, S.M.M.; Ling, T.C. Direct purification of Burkholderia Pseudomallei lipase from fermentation broth using aqueous two-phase systems. Biotechnol. Bioprocess Eng. 2010, 14, 811–818. [Google Scholar] [CrossRef]
- Enger, E.D.; Ross, F.C.; Bailey, D.B. Concepts in Biology, 14th ed.; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- Gobbetti, M.; Fox, P.F.; Stepaniak, L. Isolation and characterization of a tributyrin esterase from Lactobacillus plantarum 2739. J. Dairy Sci. 1997, 80, 3099–3106. [Google Scholar] [CrossRef]
- Toole, G.; Toole, S. Essential AS Biology for OCR; Nelson Thornes: Oxford, UK, 2004. [Google Scholar]
- Esakkiraj, P.; Antonyraj, C.B.; Meleppat, B.; Ankaiah, D.; Ayyanna, R.; Ahamed, S.I.B.; Arul, V. Molecular characterization and application of lipase from Bacillus sp. PU1 and investigation of structural changes based on pH and temperature using MD simulation. Int. J. Biol. Macromol. 2017, 103, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Keharia, H. Production, characterization and applications of organic solvent tolerant lipase by Pseudomonas aeruginosa AAU2. Biocatal. Agric. Biotechnol. 2013, 2, 255–266. [Google Scholar] [CrossRef]
- De Almeida, A.F.; Tauk-Tornisielo, S.M.; Carmona, E.C. Acid lipase from Candida viswanathii: Production, biochemical properties, and potential application. Biomed. Res. Int. 2013, 435818, 1–10. [Google Scholar]
- Ma, J.; Zhang, Z.; Wang, B.; Kong, X.; Wang, Y.; Cao, S.; Feng, Y. Overexpression and characterization of a lipase from Bacillus subtilis. Protein Expr. Purif. 2006, 45, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Paulraj, R. Purification and biochemical characterization of an extracellular lipase from Pseudomonas fluorescens MTCC 2421. J. Agric. Food Chem. 2009, 57, 3859–3866. [Google Scholar] [CrossRef] [PubMed]
- Ramani, K.; Chockalingam, E.; Sekaran, G. Production of a novel extracellular acidic lipase from Pseudomonas gessardii using slaughterhouse waste as a substrate. J. Ind. Microbiol. Biotechnol. 2010, 37, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.N.Z.A.; Baharum, S.N.; Salleh, A.B.; Basri, M. S5 lipase: An organic solvent tolerant enzyme. J. Microbiol. 2006, 44, 583–590. [Google Scholar] [PubMed]
- Kambourova, M.; Kirilova, N.; Mandeva, R.; Derekova, A. Purification and properties of thermostable lipase from a thermophilic Bacillus stearothermophilus MC 7. J. Mol. Catal. B Enzym. 2003, 22, 307–313. [Google Scholar] [CrossRef]
- Kaul, A. The phase diagram. In Aqueous Two-Phase Systems; Hatti-Kaul, R., Ed.; Humana Press: Clifton, NJ, USA, 2000; pp. 11–22. [Google Scholar]
- Silva, W.O.B.; Mitidieri, S.; Schrank, A.; Vainstein, M.H. Production and extraction of extracellular lipase from the entomopathogenic fungus Metarhizium anisopliae. Process Biochem. 2005, 40, 321–326. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
| Phase Component | Concentrations (%, w/w) | Relative Activity |
|---|---|---|
| Triton X-100 | 10 | 107.6 ± 0.31 a |
| 20 | 114.9 ± 0.19 b | |
| 30 | 116.2 ± 0.27 b | |
| 40 | 122.5 ± 0.32 c | |
| 50 | 101.8 ± 0.15 d | |
| 60 | 93.1 ± 0.20 e | |
| Maltitol | 5 | 128.9 ± 0.64 a |
| 10 | 120.3 ± 0.35 b | |
| 20 | 113.8 ± 0.52 c | |
| 30 | 105.2 ± 0.22 d | |
| 40 | 99.1 ± 0.27 e |
| Polyol | Weight Fraction, % (w/w) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| [Tri X-100]M | [Maltitol]M | [Tri X-100]T | [Maltitol]T | [Tri X-100]B | [Maltitol]B | TLL | Selectivity | PF | |
| Maltitol | 29.91 0.04 | 10.05 0.03 | 34.47 0.02 | 7.49 0.04 | 15.67 0.01 | 17.62 0.05 | 21.4 | 4.76 | 2.68 |
| 34.79 0.07 | 10.26 0.06 | 43.97 0.04 | 5.44 0.09 | 13.05 0.04 | 21.83 0.70 | 35.0 | 6.11 | 4.19 | |
| 30.06 0.03 | 15.12 0.05 | 44.96 0.07 | 5.27 0.02 | 12.15 0.11 | 24.31 0.07 | 37.9 | 7.43 | 4.32 | |
| 35.21 0.14 | 9.83 0.08 | 51.78 0.08 | 4.26 0.05 | 11.57 0.61 | 27.33 0.22 | 46.4 | 10.25 | 7.41 | |
| Phase-Forming Components | Bacterial Sources | Yield (%) | PF | Reference |
|---|---|---|---|---|
| PEG 8000/Na2HPO4 | Enterococcus faecium MTCC5695 | 82.09 | 5.99 | [17] |
| PPG 400/[Ch][BES] | Burkholderia cepacia ST8 | 99.3 | 17.96 | [4] |
| PEG6000 + K2HPO4/KH2PO4 + NaCl | Burkholderia pseudomallei | 93.0 | 12.4 | [18] |
| EOPO 3900/KH2PO4 | Burkholderia cenocepacia ST8 | 99.0 | 14.0 | [14] |
| Triton X-100/Maltitol | L.mesenteroides subsp. mesenteroides ATCC 8293 | 94.7 | 17.28 | This work |
| Relative Activity (%) | ||
|---|---|---|
| Control | 100 0.00 a | |
| Surfactants | Triton X-100 | 106.92 0.05 b |
| Tween 20 | 139.43 0.04 c | |
| Tween 80 | 100.21 0.09 a | |
| SDS | 21.36 0.03 d | |
| Metal ions | Mg2+ | 153.99 0.05 e |
| K+ | 141.72 0.04 f | |
| Ca2+ | 121.71 0.05 g | |
| Na+ | 105.22 0.07 h | |
| Zn2+ | 54.43 0.04 i | |
| Cu2+ | 53.16 0.06 i |
Sample Availability: Samples of the lipase are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eko Sukohidayat, N.H.; Zarei, M.; Baharin, B.S.; Manap, M.Y. Purification and Characterization of Lipase Produced by Leuconostoc mesenteroides Subsp. mesenteroides ATCC 8293 Using an Aqueous Two-Phase System (ATPS) Composed of Triton X-100 and Maltitol. Molecules 2018, 23, 1800. https://doi.org/10.3390/molecules23071800
Eko Sukohidayat NH, Zarei M, Baharin BS, Manap MY. Purification and Characterization of Lipase Produced by Leuconostoc mesenteroides Subsp. mesenteroides ATCC 8293 Using an Aqueous Two-Phase System (ATPS) Composed of Triton X-100 and Maltitol. Molecules. 2018; 23(7):1800. https://doi.org/10.3390/molecules23071800
Chicago/Turabian StyleEko Sukohidayat, Nurfadhilah Hidayah, Mohammad Zarei, Badlishah Sham Baharin, and Mohd Yazid Manap. 2018. "Purification and Characterization of Lipase Produced by Leuconostoc mesenteroides Subsp. mesenteroides ATCC 8293 Using an Aqueous Two-Phase System (ATPS) Composed of Triton X-100 and Maltitol" Molecules 23, no. 7: 1800. https://doi.org/10.3390/molecules23071800
APA StyleEko Sukohidayat, N. H., Zarei, M., Baharin, B. S., & Manap, M. Y. (2018). Purification and Characterization of Lipase Produced by Leuconostoc mesenteroides Subsp. mesenteroides ATCC 8293 Using an Aqueous Two-Phase System (ATPS) Composed of Triton X-100 and Maltitol. Molecules, 23(7), 1800. https://doi.org/10.3390/molecules23071800






