Tetrel Bonding Interactions in Perchlorinated Cyclopenta- and Cyclohexatetrelanes: A Combined DFT and CSD Study
Abstract
1. Introduction
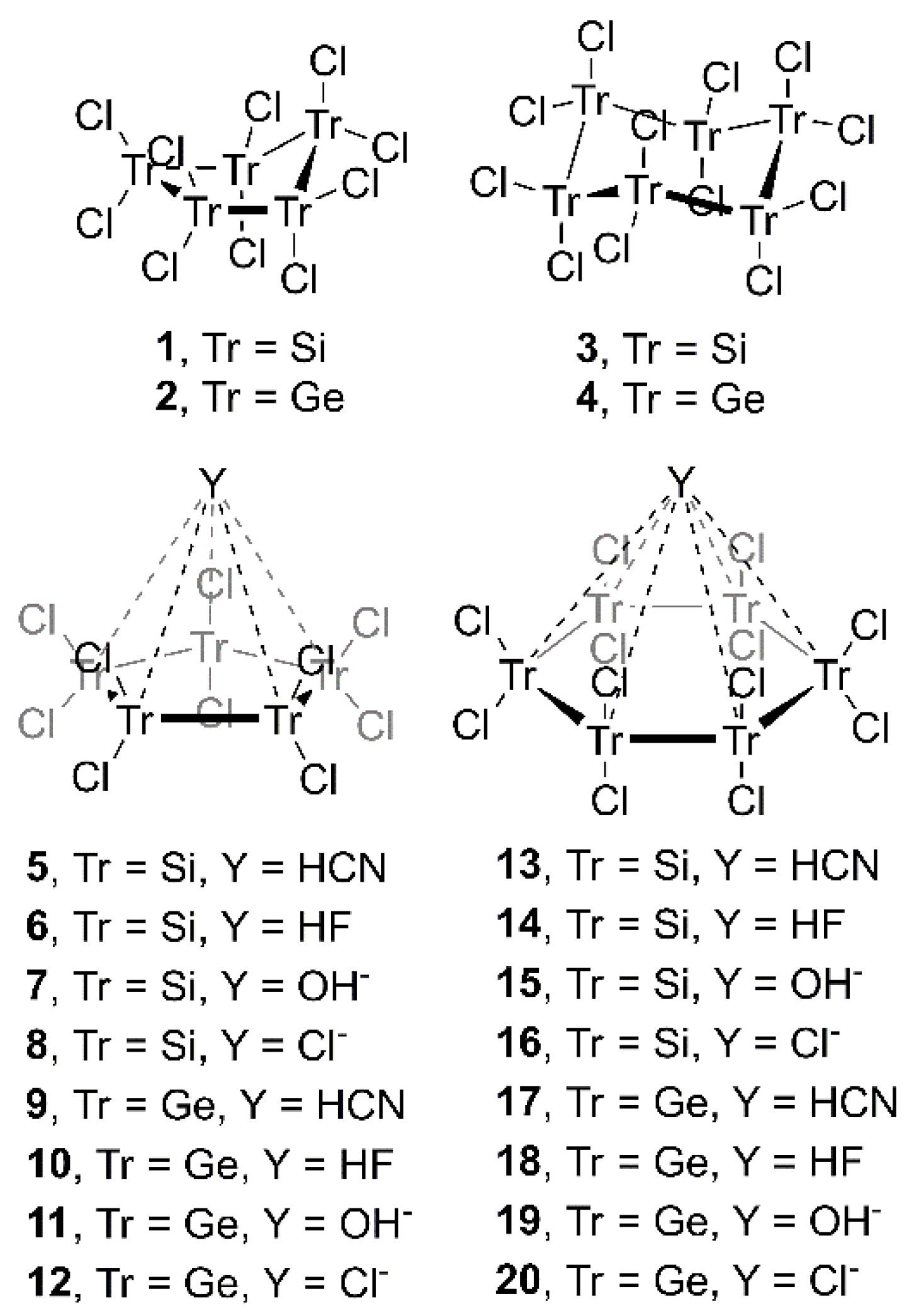
2. Results and Discussion
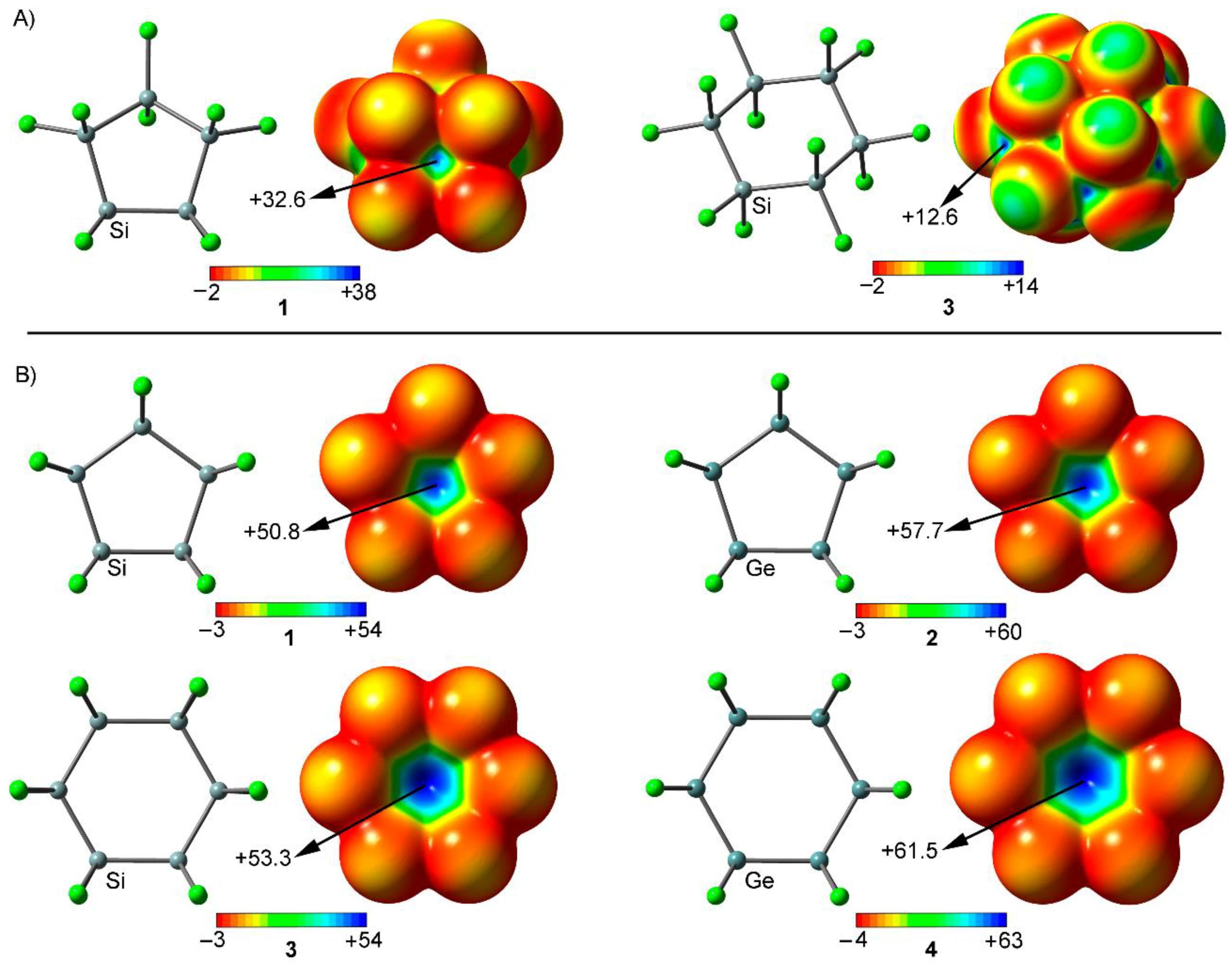
2.1. Preliminary MEP Analysis
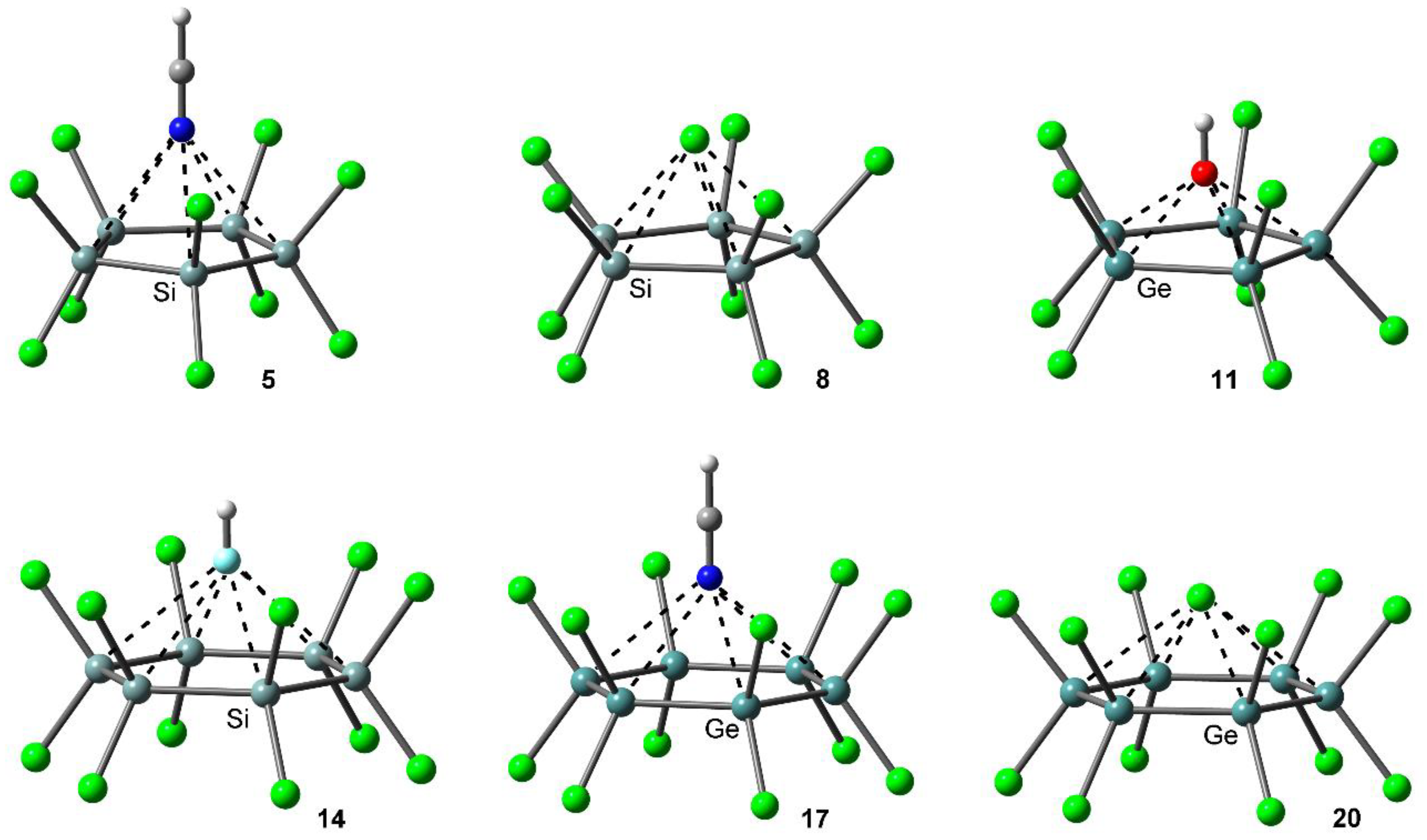
2.2. Energetic and Geometric Results
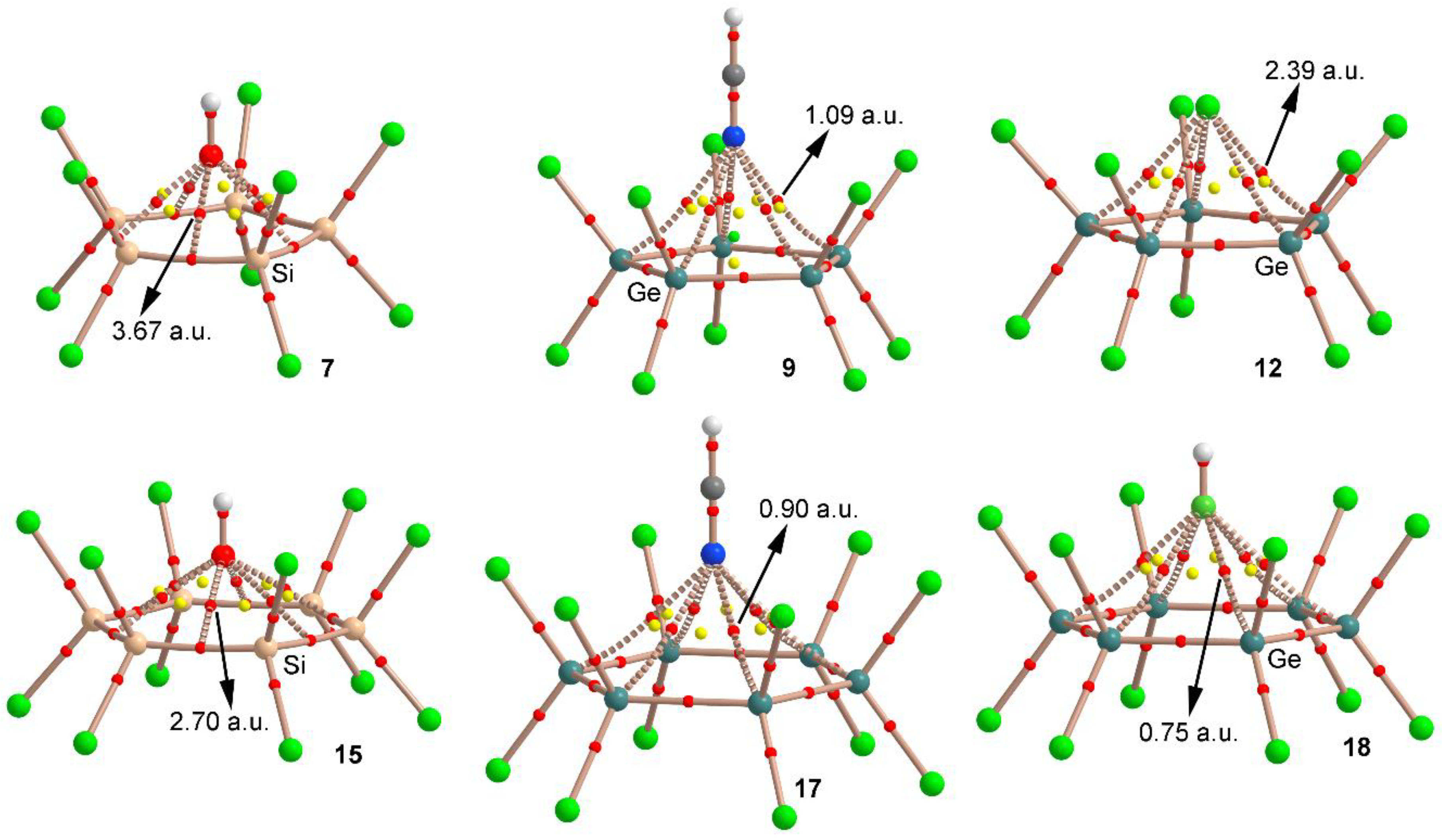
2.3. AIM and NCI Analyses
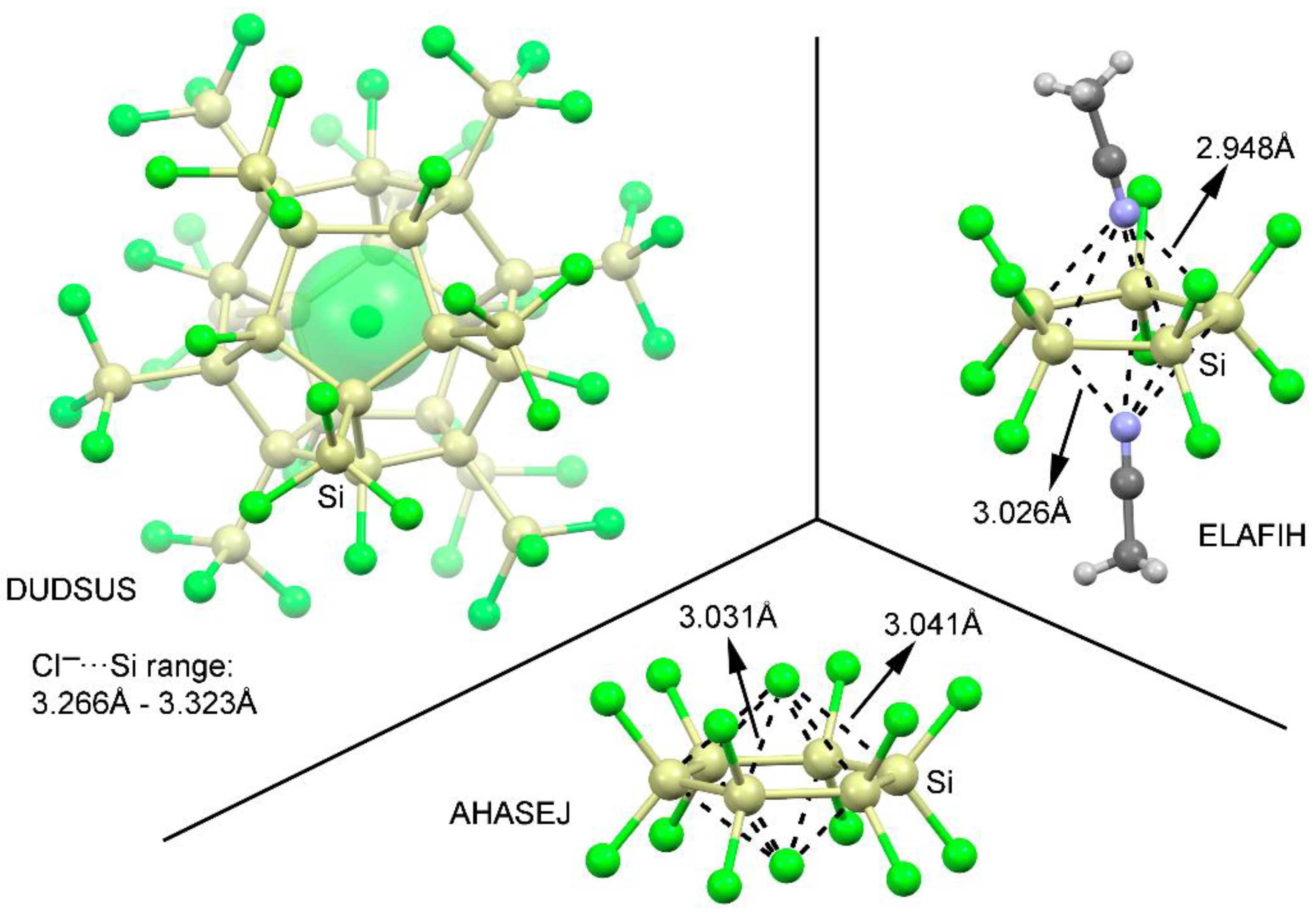
2.4. CSD Search
3. Theoretical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIM | Atoms in molecules |
| MEP | Molecular electrostatic potential |
| BSSE | Basis Set Superposition Error |
| CSD | Cambridge Structural Database |
| CP | Critical point |
| NCIplot | Non Covalent Interactions plot |
| MINECO | Ministerio de Economía y Competitividad |
| AEI | Agencia Española de Investigación |
References
- Schneider, H.J. Binding mechanisms in supramolecular complexes. Angew. Chem. Int. Ed. 2009, 48, 3924–3977. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.J.; Yatsimirski, A. Principles and Methods in Supramolecular Chemistry; John Wiley: Chichester, UK, 2000. [Google Scholar]
- Lehn, J.M. Supramolecular Chemistry Concepts and Perspectives; Wiley–VCH: Weinheim, Germany, 1995. [Google Scholar]
- Vögtle, F. Supramolecular Chemistry: An Introduction; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Beer, P.D.; Gale, P.A.; Smith, D.K. Supramolecular Chemistry; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; Wiley: Chichester, UK, 2000. [Google Scholar]
- Grabowski, S.J. What is the covalency of hydrogen bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Resnati, G. Halogen bonding: A paradigm in supramolecular chemistry. Chem. Eur. J. 2001, 7, 2511–2519. [Google Scholar] [CrossRef]
- Murrayrust, P.; Motherwell, W.D.S. Computer retrieval and analysis of molecular geometry. 4. Intermolecular interactions. J. Am. Chem. Soc. 1979, 101, 4374–4376. [Google Scholar] [CrossRef]
- Bauzá, A.; Quiñonero, D.; Deyà, P.M.; Frontera, A. Halogen bonding versus chalcogen and pnicogen bonding: A combined Cambridge structural database and theoretical study. CrystEngComm 2013, 15, 3137–3144. [Google Scholar] [CrossRef]
- Brown, A.; Beer, P.D. Halogen bonding anion recognition. Chem. Commun. 2016, 52, 8645–8658. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. Halogen bonding: An interim discussion. ChemPhysChem 2013, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. Supramolecular nanotubes based on halogen bonding interactions: Cooperativity and interaction with small guests. Phys. Chem. Chem. Phys. 2017, 19, 12936–12941. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P. Σ-holes, π-holes and electrostatically-driven interactions. J. Mol. Model. 2012, 18, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. The Bright Future of Unconventional σ/π-Hole Interactions. ChemPhysChem 2015, 16, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. Aerogen Bonding Interaction: A New Supramolecular Force? Angew. Chem. Int. Ed. 2015, 54, 7340–7343. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Triel Bonds, π-Hole-π-Electrons Interactions in Complexes of Boron and Aluminium Trihalides and Trihydrides with Acetylene and Ethylene. Molecules 2015, 20, 11297–11316. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Directionality of π-holes in nitro compounds. Chem. Commun. 2015, 51, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Triel bonds-complexes of boron and aluminum trihalides and trihydrides with benzene. Struct. Chem. 2017, 28, 1163–1171. [Google Scholar] [CrossRef]
- Adriaenssens, L.; Gil-Ramírez, G.; Frontera, A.; Quiñonero, D.; Escudero-Adán, E.C.; Ballester, P. Thermodynamic characterization of halide–π interactions in solution using “two-wall” aryl extended calix[4]pyrroles as model system. J. Am. Chem. Soc. 2014, 136, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. The Pnicogen Bond: Its Relation to Hydrogen, Halogen, and Other Noncovalent Bonds. Acc. Chem. Res. 2013, 46, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Marín-Luna, M.; Alkorta, I.; Elguero, J. Cooperativity in Tetrel Bonds. J. Phys. Chem. A 2016, 120, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ji, B.; Zhang, Y. Chalcogen Bond: A Sister Noncovalent Bond to Halogen Bond. J. Phys. Chem. A 2009, 113, 8132–8135. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. Theoretical study on the dual behavior of XeO3 and XeF4 toward aromatic rings: Lone pair–π versus aerogen–π interactions. ChemPhysChem 2015, 16, 3625–3630. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. π-Hole aerogen bonding interactions Phys. Chem. Chem. Phys. 2015, 17, 24748–24753. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.; Frontera, A. Tetrel-bonding interaction: Rediscovered supramolecular force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321. [Google Scholar] [CrossRef] [PubMed]
- Southern, S.A.; Bryce, D.L. NMR investigations of noncovalent carbon tetrel bonds. computational assessment and initial experimental observation. J. Phys. Chem. A 2015, 119, 11891–11899. [Google Scholar] [CrossRef] [PubMed]
- Southern, S.A.; Errulat, D.; Frost, J.M.; Gabidullin, B.; Bryce, D.L. Prospects for (207)Pb solid-state NMR studies of lead tetrel bonds. Faraday Discuss. 2017, 203, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Systematic elucidation of factors that influence the strength of tetrel bonds. J. Phys. Chem. A 2017, 121, 5561–5568. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Chopra, D. Characterization of the short O=C···O=C π-hole tetrel bond in the solid state. CrystEngComm 2018, 20, 3308–3312. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A. RCH3···O interactions in biological systems: Are they trifurcated H-bonds or noncovalent carbon bonds? Crystals 2016, 8, 26. [Google Scholar] [CrossRef]
- Grabowski, S.J. Tetrel bond-σ-hole bond as a preliminary stage of the SN2 reaction. Phys. Chem. Chem. Phys. 2014, 16, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Kim, B.K.; Boudjouk, P.; Grier, D.G. Amine-promoted disproportionation and redistribution of trichlorosilane: formation of tetradecachlorocyclohexasilane dianion. J. Am. Chem. Soc. 2001, 123, 8117–8118. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Schulz, D.L.; Braun, C.W.; Ugrinov, A.; Boudjouk, P. “Inverse Sandwich” complexes of perhalogenated cyclohexasilane. Organometallics 2010, 29, 2203–2205. [Google Scholar] [CrossRef]
- Teichmann, J.; Köstler, B.; Tillmann, J.; Moxter, M.; Kupec, R.; Bolte, M.; Lerner, H.-W.; Wagner, M. Halide-ion diadducts of perhalogenated cyclopenta- and cyclohexasilanes. Z. Anorg. Allg. Chem. 2018. [Google Scholar] [CrossRef]
- Robertazzi, A.; Platts, J.A.; Gamez, P. Anion···Si interactions in an inverse sandwich complex: A computational study. ChemPhysChem 2014, 15, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Pokhodnya, K.; Anderson, K.; Kilina, S.; Boudjouk, P. Toward the mechanism of perchlorinated cyclopentasilane (Si5Cl10) ring flattening in the [Si5Cl10·2Cl]2− dianion. J. Phys. Chem. A 2017, 121, 3494–3500. [Google Scholar] [CrossRef] [PubMed]
- Pokhodnya, K.; Anderson, K.; Kilina, S.; Naveen, D.; Boudjouk, P. Mechanism of charged, neutral, mono-, and polyatomic donor ligand coordination to perchlorinated cyclohexasilane (Si6Cl12). J. Phys. Chem. A 2018, 122, 4067–4075. [Google Scholar] [CrossRef] [PubMed]
- Vedha, S.A.; Solomon, R.V.; Venuvanalingam, P. On the nature of hypercoordination in dihalogenated perhalocyclohexasilanes. J. Phys. Chem. A 2013, 117, 3529–3538. [Google Scholar] [CrossRef] [PubMed]
- Geboes, Y.; de Proft, F.; Herrebout, W.A. Lone pair···π interactions involving an aromatic π-system: Complexes of hexafluorobenzene with dimethyl ether and trimethylamine. Chem. Phys. Lett. 2016, 647, 26–30. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A program for plotting noncovalent interaction regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, J.; Wender, J.H.; Bahr, U.; Bolte, M.; Lerner, H.-W.; Holthausen, M.C.; Wagner, M. One-step synthesis of a [20] silafullerane with an endohedral chloride ion. Angew. Chem. Int. Ed. 2015, 54, 5429–5433. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Anderson, K.J.; Schulz, D.L.; Boudjouk, P. Coordination chemistry of Si5Cl10 with organocyanides. Dalton Trans. 2010, 39, 11188–11192. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, J.; Lerner, H.-W.; Bats, J.W. CCDC 1414760: Experimental Crystal Structure Determination. CSD Commun. 2015. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Bär, M.; Hacer, M.; Horn, H.; Kömel, C. Electronic structure calculations on workstation computers: The program system Turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Boys, S.B.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll (Version 13.05.06); TK Gristmill Software: Overland Park, KS, USA, 2013. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
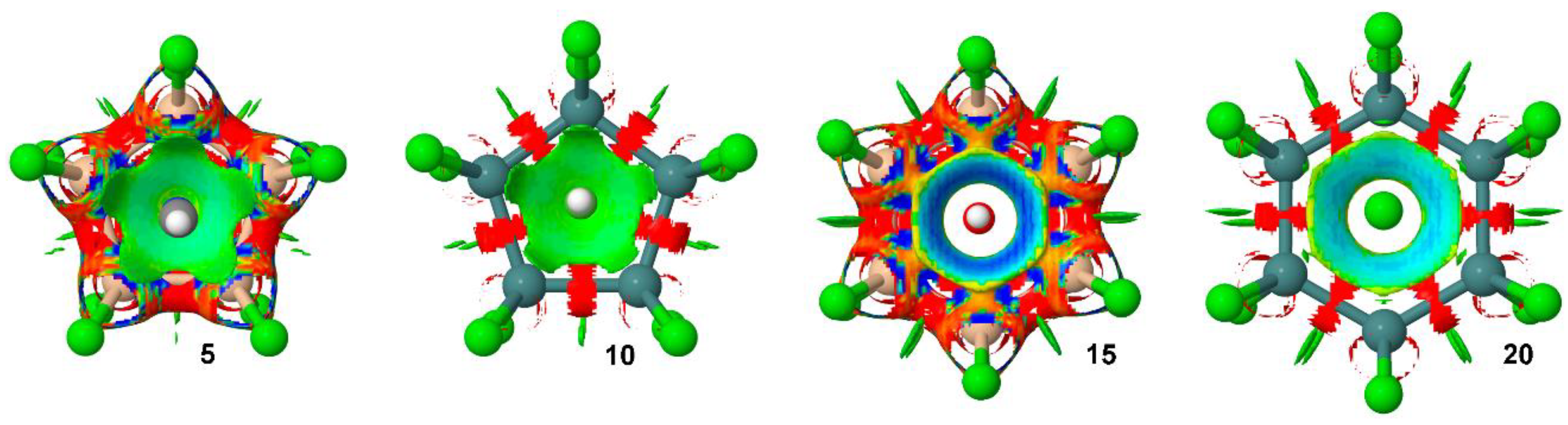
| Complex | ΔE a | ΔEBSSE | R b | 102 × ρ |
|---|---|---|---|---|
| 5 | −7.8 | −7.1 | 2.350 | 1.17 |
| 6 | −5.0 | −3.7 | 2.271 | 0.94 |
| 7 | −116.3 | −102.4 | 1.401 | 3.67 |
| 8 | −67.3 | −61.4 | 2.067 | 2.72 |
| 9 | −12.3 | −11.4 | 2.340 | 0.97 |
| 10 | −9.1 | −7.6 | 2.237 | 0.81 |
| 11 | −121.1 | −107.5 | 1.430 | 3.36 |
| 12 | −73.8 | −68.0 | 2.121 | 2.39 |
| 13 | −1.2 | −0.5 | 2.145 | 1.09 |
| 13Aa | −14.1 | −12.6 | 2.157 | - |
| 14 | +1.9 | +3.2 | 2.051 | 0.91 |
| 14Aa | −8.3 | −5.7 | 2.062 | - |
| 15 | −109.1 | −95.3 | 1.223 | 2.70 |
| 16 | −65.4 | −59.3 | 1.849 | 2.23 |
| 17 | −9.2 | −8.3 | 2.118 | 0.90 |
| 18 | −5.6 | −4.1 | 2.021 | 0.75 |
| 19 | −120.0 | −106.3 | 1.196 | 2.46 |
| 20 | −76.2 | −70.0 | 1.870 | 1.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauzá, A.; Frontera, A. Tetrel Bonding Interactions in Perchlorinated Cyclopenta- and Cyclohexatetrelanes: A Combined DFT and CSD Study. Molecules 2018, 23, 1770. https://doi.org/10.3390/molecules23071770
Bauzá A, Frontera A. Tetrel Bonding Interactions in Perchlorinated Cyclopenta- and Cyclohexatetrelanes: A Combined DFT and CSD Study. Molecules. 2018; 23(7):1770. https://doi.org/10.3390/molecules23071770
Chicago/Turabian StyleBauzá, Antonio, and Antonio Frontera. 2018. "Tetrel Bonding Interactions in Perchlorinated Cyclopenta- and Cyclohexatetrelanes: A Combined DFT and CSD Study" Molecules 23, no. 7: 1770. https://doi.org/10.3390/molecules23071770
APA StyleBauzá, A., & Frontera, A. (2018). Tetrel Bonding Interactions in Perchlorinated Cyclopenta- and Cyclohexatetrelanes: A Combined DFT and CSD Study. Molecules, 23(7), 1770. https://doi.org/10.3390/molecules23071770








