Modification of Polyhedral Oligomeric Silsesquioxanes (POSS) Molecules by Ruthenium Catalyzed Cross Metathesis
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. General Procedure for the Cross Metathesis of 1 with Different Partners
3.3. 4-(POSS)but-2-en-1-yl acetate (4)
3.4. tert-butyl 4-(POSS)but-2-enoate (5)
3.5. (10R,13S)-10,13-dimethyl-1,2,3,4,7,8,9,10,11,12,13,14,15,16-tetradecahydrospiro[cyclopenta[a] phenanthrene-17,2’-[1,3]dioxolan]-3-yl 6-(POSS)hex-4-enoate (7)
3.6. (13S)-13-methyl-17-oxo-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a] phenanthren-3-yl-6-(POSS)hex-4-enoate (9)
3.7. ((13S,17R)-13-methyl-17-(4-(POSS)but-2-en-1-yl)-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol (11)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hartmann-Thompson, C. Applications of polyhedral oligomeric silsesquioxanes; Springer: Dordrecht, The Netherlands, 2011; p. 420. [Google Scholar]
- Markovic, E.; Constantopolous, K.; Matisons, J.G. Polyhedral oligomeric silsesquioxanes: From early and strategic development through to materials application. Appl. Polyhedr. Oligomeric Silsesquioxanes 2011, 3, 1–46. [Google Scholar]
- Pielichowski, K.; Njuguna, J.; Janowski, B.; Pielichowski, J. Polyhedral oligomeric silsesquioxanes (POSS)-containing nanohybrid polymers. In Supramolecular polymers polymeric betains oligomers; Donnio, B., Guillon, D., Harada, A., Hashidzume, A., Jaeger, W., Janowski, B., Kudaibergenov, S., Laschewsky, A., Njuguna, J., Pielichowski, J., et al., Eds.; Springer: Berlin, Germany, 2006; Volume 201, pp. 225–296. [Google Scholar]
- Ghanbari, H.; Cousins, B.G.; Seifalian, A.M. A nanocage for nanomedicine: Polyhedral oligomeric silsesquioxane (POSS). Macromol. Rapid Commun. 2011, 32, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- John, Ł.; Malik, M.; Janeta, M.; Szafert, S. First step towards a model system of the drug delivery network based on amide-POSS nanocarriers. RSC Adv. 2017, 7, 8394–8401. [Google Scholar] [CrossRef] [Green Version]
- John, L.; Janeta, M.; Szafert, S. Synthesis of cubic spherosilicates for self-assembled organic-inorganic biohybrids based on functionalized methacrylates. New J. Chem. 2018, 42, 39–47. [Google Scholar] [CrossRef]
- Janeta, M.; John, L.; Ejfler, J.; Lis, T.; Szafert, S. Multifunctional imine-POSS as uncommon 3D nanobuilding blocks for supramolecular hybrid materials: Synthesis, structural characterization, and properties. Dalton Trans. 2016, 45, 12312–12321. [Google Scholar] [CrossRef] [PubMed]
- Janeta, M.; John, L.; Ejfler, J.; Szafert, S. High-yield synthesis of amido-functionalized polyoctahedral oligomeric silsesquioxanes by using acyl chlorides. Chemistry-a Eur. J. 2014, 20, 15966–15974. [Google Scholar] [CrossRef] [PubMed]
- John, Ł. Selected developments and medical applications of organic–inorganic hybrid biomaterials based on functionalized spherosilicates. Mater. Sci. Eng. C 2018, 88, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Seifalian, A.M. Polyhedral oligomeric silsesquioxane nanocomposites: The next generation material for biomedical applications. Account. Chem. Res. 2005, 38, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mather, P.T. POSS polymers: Physical properties and biomaterials applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Blackwell, H.E.; O’Leary, D.J.; Chatterjee, A.K.; Washenfelder, R.A.; Bussmann, D.A.; Grubbs, R.H. New Approaches to Olefin Cross-Metathesis. J. Am. Chem. Soc. 2000, 122, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Vernall, A.J.; Abell, A.D. Cross metathesis of nitrogen-containing systems. Aldrichimica Acta 2003, 36, 93–105. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Choi, T.-L.; Sanders, D.P.; Grubbs, R.H. A general model for selectivity in olefin cross metathesis. J. Am. Chem. Soc. 2003, 125, 11360–11370. [Google Scholar] [CrossRef] [PubMed]
- Zukowska, K.; Grela, K. Cross metathesis. In Olefin Metathesis: Theory and Practice; Grela, K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 39–83, (and citation therein). [Google Scholar]
- Czaban, J.; Schertzer, B.M.; Grela, K. Low catalyst loadings in self-metathesis of 1-dodecene. Adv. Synth. Catal. 2013, 355, 1997–2006. [Google Scholar] [CrossRef]
- Kajetanowicz, A.; Sytniczuk, A.; Grela, K. Metathesis of renewable raw materials-influence of ligands in the indenylidene type catalysts on self-metathesis of methyl oleate and cross-metathesis of methyl oleate with (Z)-2-butene1,4-diol diacetate. Green Chem. 2014, 16, 1579–1585. [Google Scholar] [CrossRef]
- Olszewski, T.K.; Figlus, M.; Bieniek, M. Olefin metathesis: A versatile synthetic tool for use in preparation of active pharmaceutical ingredients. Chimica Oggi-Chemistry Today 2014, 32, 22–29. [Google Scholar] [CrossRef]
- Pederson, R.L.; Fellows, I.M.; Ung, T.A.; Ishihara, H.; Hajela, S.P. Applications of olefin cross metathesis to commercial products. Adv. Synth. Catal. 2002, 344, 728–735. [Google Scholar] [CrossRef]
- Kirschning, A.; Harmrolfs, K.; Mennecke, K.; Messinger, J.; Schön, U.; Grela, K. Homo- and heterogeneous Ru-based metathesis catalysts in cross-metathesis of 15-allylestrone—Towards 17β-hydroxysteroid dehydrogenase type 1 inhibitors. Tetrahedron Lett. 2008, 49, 3019–3022. [Google Scholar] [CrossRef]
- Bieniek, M.; Kołoda, D.; Grela, K. A highly selective synthesis of dialkenyl sulfones via cross-metathesis of divinyl sulfone. Org. Lett. 2006, 8, 5689–5692. [Google Scholar] [CrossRef] [PubMed]
- Feher, F.J.; Soulivong, D.; Eklund, A.G.; Wyndham, K.D. Cross-metathesis of alkenes with vinyl-substituted silsesquioxanes and spherosilicates: A new method for synthesizing highly-functionalized Si/O frameworks. Chem. Commun. 1997, 1185–1186. [Google Scholar] [CrossRef]
- Itami, Y.; Marciniec, B.; Kubicki, M. Functionalization of octavinylsilsesquioxane by ruthenium-catalyzed silylative coupling versus cross-metathesis. Chemistry – A Eur. J. 2004, 10, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, S.; Pietraszuk, C.; Marciniec, B. Synthesis of siloxy-modified second generation Hoveyda-Grubbs catalysts and their catalytic activity. J. Organomet. Chem. 2009, 694, 3918–3922. [Google Scholar] [CrossRef]
- Żak, P.; Pietraszuk, C.; Marciniec, B.; Spólnik, G.; Danikiewicz, W. Efficient functionalisation of cubic monovinylsilsesquioxanes via cross-metathesis and silylative coupling with olefins in the presence of ruthenium complexes. Adv. Synth. Catal. 2009, 351, 2675–2682. [Google Scholar] [CrossRef]
- Vautravers, N.R.; Andre, P.; Slawin, A.M.Z.; Cole-Hamilton, D.J. Synthesis and characterization of photoluminescent vinylbiphenyl decorated polyhedral oligomeric silsesquioxanes. Org. Biomol. Chem. 2009, 7, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Vautravers, N.R.; Andre, P.; Cole-Hamilton, D.J. Fluorescence activation of a polyhedral oligomeric silsesquioxane in the presence of reducing agents. J. Mater. Chem. 2009, 19, 4545–4550. [Google Scholar] [CrossRef]
- Bieniek, M.; Michrowska, A.; Usanov, D.L.; Grela, K. In an attempt to provide a user’s guide to the galaxy of benzylidene, alkoxybenzylidene, and indenylidene ruthenium olefin metathesis catalysts. Chem. Eur. J. 2008, 14, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Dinger, M.B.; Mol, J.C. High turnover numbers with ruthenium-based metathesis catalysts. Adv. Synth. Catal. 2002, 344, 671–677. [Google Scholar] [CrossRef]
- Michael, G.; Soley, K.; Wilson, T.; Christina, O.; Joachim, R. Development of commercially viable thermomorphic catalysts for controlled free radical polymerization. In Catalysis of organic reactions, 1st ed.; Prunier, M.L., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 319–328. [Google Scholar]
- Borré, E.; Caijo, F.; Crévisy, C.; Mauduit, M. New library of aminosulfonyl-tagged Hoveyda–Grubbs type complexes: Synthesis, kinetic studies and activity in olefin metathesis transformations. Beilstein J. Org. Chem. 2010, 6, 1159–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieniek, M.; Bujok, R.; Cabaj, M.; Lugan, N.; Lavigne, G.; Arlt, D.; Grela, K. Advanced fine-tuning of Grubbs/Hoveyda olefin metathesis catalysts: A further step toward an optimum balance between antinomic properties. J. Am. Chem. Soc. 2006, 128, 13652–13653. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, M.; Samojłowicz, C.; Sashuk, V.; Bujok, R.; Śledź, P.; Lugan, N.l.; Lavigne, G.; Arlt, D.; Grela, K. Rational design and evaluation of upgraded Grubbs/Hoveyda olefin metathesis catalysts: Polyfunctional benzylidene ethers on the test bench. Organometallics 2011, 30, 4144–4158. [Google Scholar] [CrossRef]
- Luan, X.J.; Mariz, R.; Gatti, M.; Costabile, C.; Poater, A.; Cavallo, L.; Linden, A.; Dorta, R. Identification and characterization of a new family of catalytically highly active imidazolin-2-ylidenes. J. Am. Chem. Soc. 2008, 130, 6848–6858. [Google Scholar] [CrossRef] [PubMed]
- Vieille-Petit, L.; Luan, X.; Gatti, M.; Blumentritt, S.; Linden, A.; Clavier, H.; Nolan, S.P.; Dorta, R. Improving grubbs’ II type ruthenium catalysts by appropriately modifying the N-heterocyclic carbene ligand. Chem. Commun. 2009, 3783–3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatti, M.; Vieille-Petit, L.; Luan, X.; Mariz, R.; Drinkel, E.; Linden, A.; Dorta, R. Impact of NHC ligand conformation and solvent concentration on the ruthenium-catalyzed ring-closing metathesis reaction. J. Am. Chem. Soc. 2009, 131, 9498–9499. [Google Scholar] [CrossRef] [PubMed]
- Vieille-Petit, L.; Clavier, H.; Linden, A.; Blumentritt, S.; Nolan, S.P.; Dorta, R. Ruthenium olefin metathesis catalysts with N-heterocyclic carbene ligands bearing n-naphthyl side chains. Organometallics 2010, 29, 775–788. [Google Scholar] [CrossRef]
- Samojlowicz, C.; Bieniek, M.; Zarecki, A.; Kadyrov, R.; Grela, K. The doping effect of fluorinated aromatic hydrocarbon solvents on the performance of common olefin metathesis catalysts: Application in the preparation of biologically active compounds. Chem. Commun. 2008, 6282–6284. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
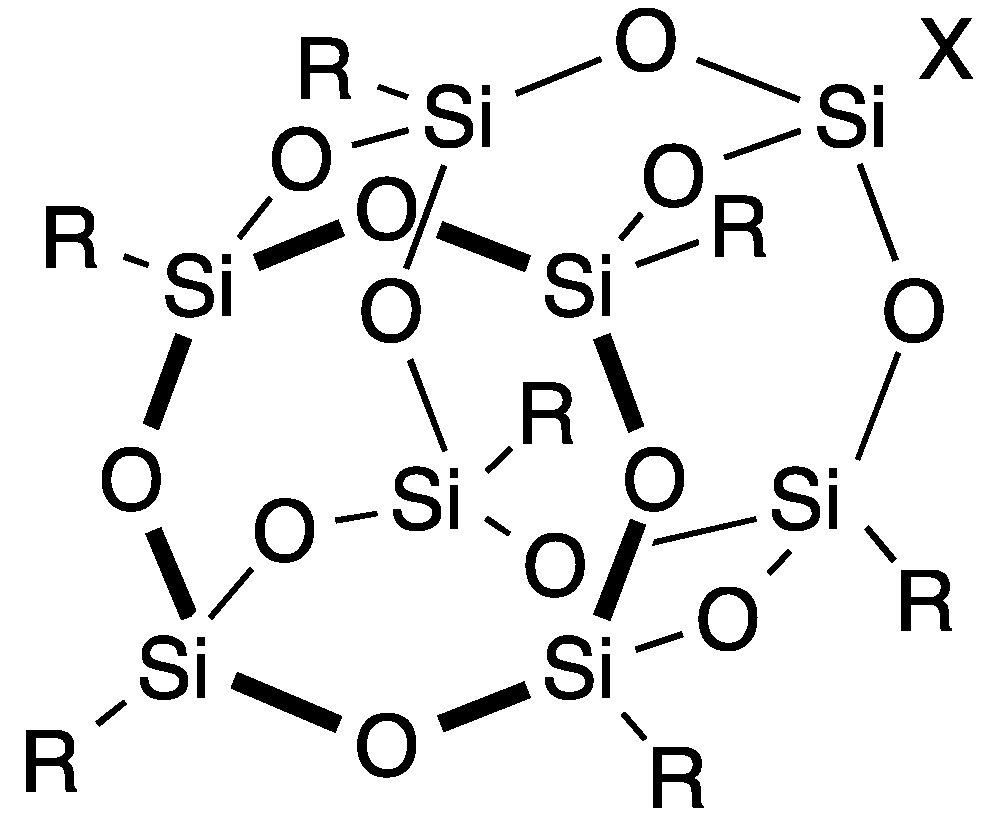
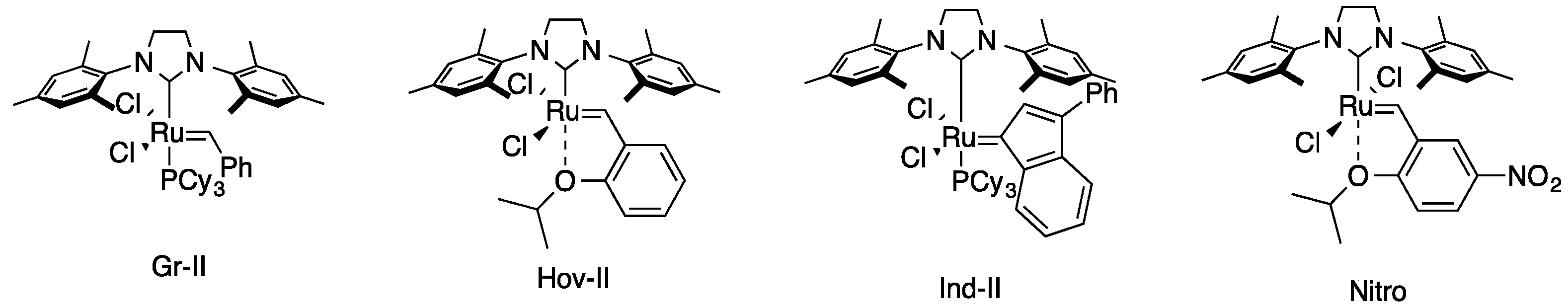
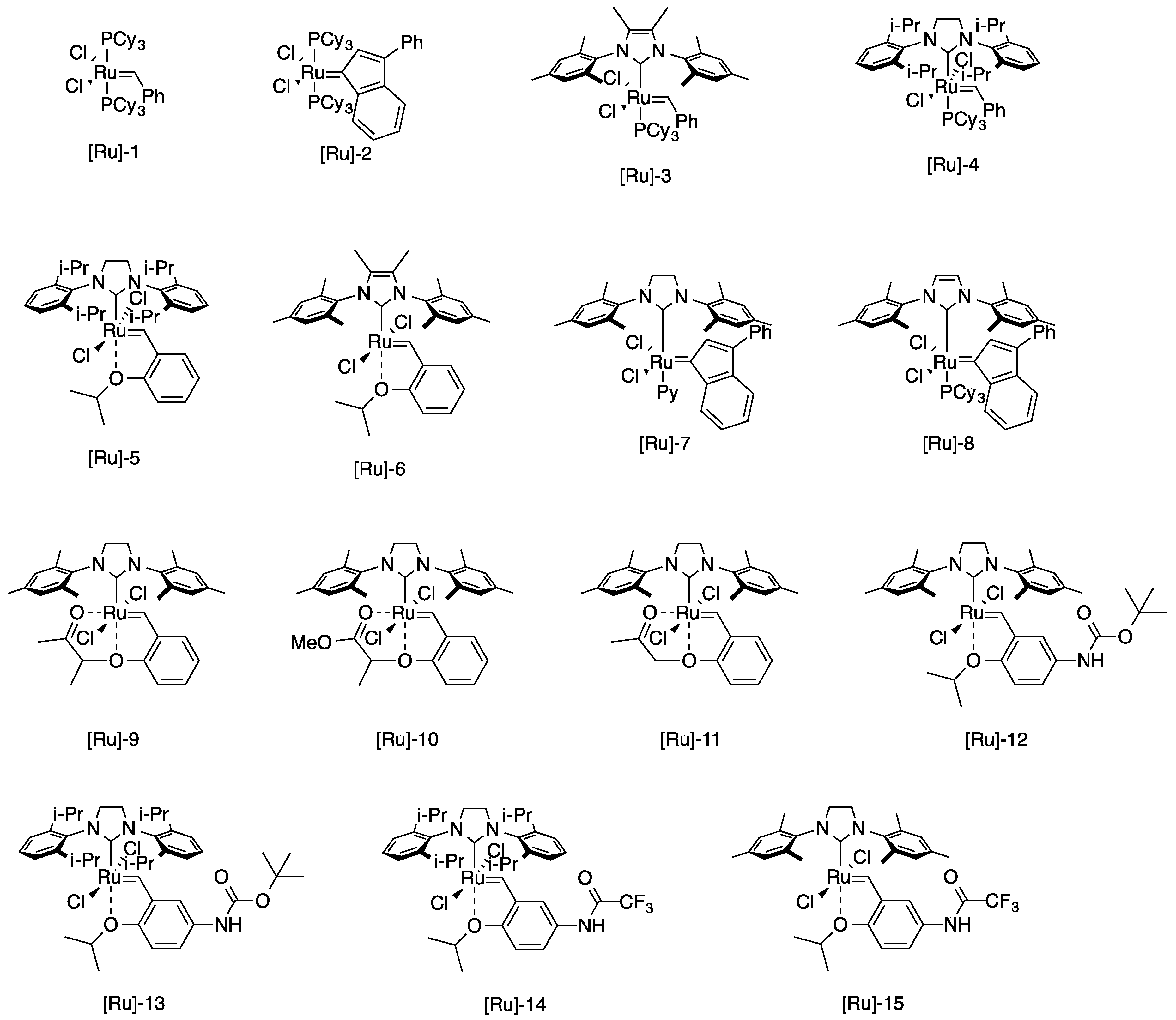

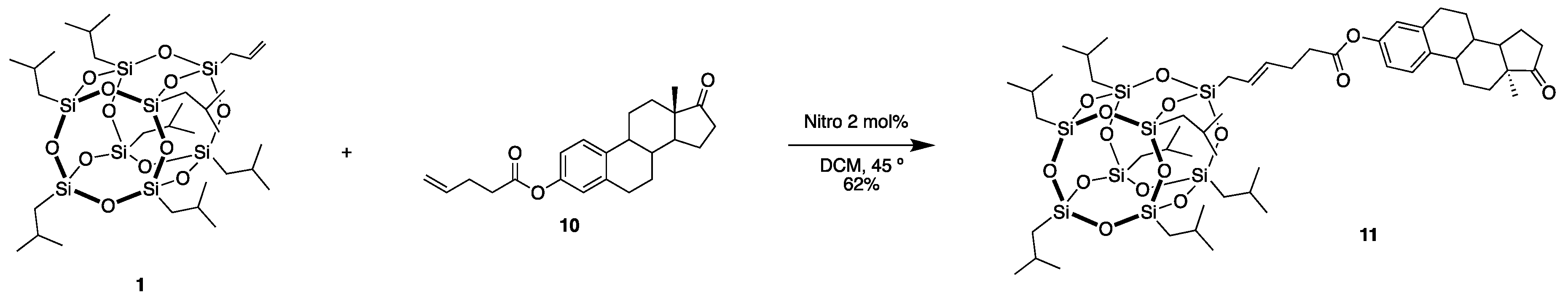

| Entry | Catalyst | Time (h) | Yield of 4 (%) | Yield of 5 (%) |
|---|---|---|---|---|
| 1 | [Ru]-1 | 5 | 6 | 33 |
| 2 | [Ru]-2 | 5 | 5 | 40 |
| 3 | Gr-II | 5 | 71 | 80 |
| 4 | [Ru]-3 | 5 | 28 | 31 |
| 5 | [Ru]-4 | 5 | 79 | 83 |
| 6 | Hov-II | 5 | 99 | 98 |
| 7 | Hov-II | 2 | 80 | 73 |
| 8 | [Ru]-5 | 5 | 99 | 69 |
| 9 | [Ru]-6 | 5 | 40 | 57 |
| 10 | Ind-II | 5 | 0 | 0 |
| 11 | [Ru]-7 | 5 | 50 | 52 |
| 12 | [Ru]-8 | 5 | 0 | 0 |
| 13 | Nitro | 24 | 90 | 64 |
| 14 | Nitro | 5 | 87 | 76 |
| 15 | [Ru]-9 | 5 | 94 | 89 |
| 16 | [Ru]-9 | 2 | 81 | 69 |
| 17 | [Ru]-10 | 5 | 99 | 66 |
| 18 | [Ru]-10 | 2 | 72 | 26 |
| 19 | [Ru]-11 | 5 | 56 | 77 |
| 20 | [Ru]-12 | 5 | 91 | 82 |
| 21 | [Ru]-12 | 2 | 93 | 90 |
| 22 | [Ru]-13 | 5 | 91 | 82 |
| 23 | [Ru]-14 | 5 | 91 | 95 |
| 24 | [Ru]-14 | 2 | 87 | 80 |
| 25 | [Ru]-15 | 5 | 85 | 83 |
| 26 | [Ru]-16 | 5 | 81 | 86 |
| 27 | [Ru]-17 | 5 | 96 | 82 |
| 28 | [Ru]-18 | 5 | 90 | 82 |
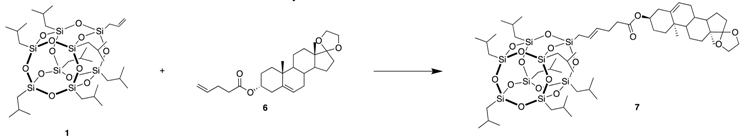
| Catalyst (mol%) | Solvent | Temperature (°C) | Time (h) | Yield of 7 (%) |
|---|---|---|---|---|
| Hov-II (0.5) | DCM | RT | 5 | 3 |
| Hov-II (2) | DCM | 45 | 5 | 36 |
| Hov-II (2) | Toluene | 100 | 5 | 69 |
| Nitro (2) | Toluene | 100 | 5 | 72 |
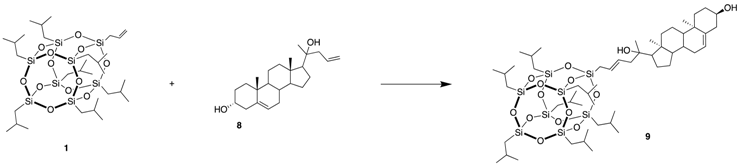
| Catalyst (mol%) | Solvent | Temperature (°C) | Time (h) | Yield of 9 (%) |
|---|---|---|---|---|
| Hov-II (0.5) | DCM | RT | 5 | 29 |
| Hov-II (2) | Toluene | 100 | 5 | 36 |
| Nitro (2) | Toluene | 100 | 10 | 46 |
| Nitro (2) | DCM | 45 | 24 | 69 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaban-Jóźwiak, J.; Woźniak, Ł.; Ulikowski, A.; Kwiecińska, K.; Rajkiewicz, A.A.; Grela, K. Modification of Polyhedral Oligomeric Silsesquioxanes (POSS) Molecules by Ruthenium Catalyzed Cross Metathesis. Molecules 2018, 23, 1722. https://doi.org/10.3390/molecules23071722
Czaban-Jóźwiak J, Woźniak Ł, Ulikowski A, Kwiecińska K, Rajkiewicz AA, Grela K. Modification of Polyhedral Oligomeric Silsesquioxanes (POSS) Molecules by Ruthenium Catalyzed Cross Metathesis. Molecules. 2018; 23(7):1722. https://doi.org/10.3390/molecules23071722
Chicago/Turabian StyleCzaban-Jóźwiak, Justyna, Łukasz Woźniak, Artur Ulikowski, Katarzyna Kwiecińska, Adam A. Rajkiewicz, and Karol Grela. 2018. "Modification of Polyhedral Oligomeric Silsesquioxanes (POSS) Molecules by Ruthenium Catalyzed Cross Metathesis" Molecules 23, no. 7: 1722. https://doi.org/10.3390/molecules23071722
APA StyleCzaban-Jóźwiak, J., Woźniak, Ł., Ulikowski, A., Kwiecińska, K., Rajkiewicz, A. A., & Grela, K. (2018). Modification of Polyhedral Oligomeric Silsesquioxanes (POSS) Molecules by Ruthenium Catalyzed Cross Metathesis. Molecules, 23(7), 1722. https://doi.org/10.3390/molecules23071722





