Modification of Polyhedral Oligomeric Silsesquioxanes (POSS) Molecules by Ruthenium Catalyzed Cross Metathesis
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. General Procedure for the Cross Metathesis of 1 with Different Partners
3.3. 4-(POSS)but-2-en-1-yl acetate (4)
3.4. tert-butyl 4-(POSS)but-2-enoate (5)
3.5. (10R,13S)-10,13-dimethyl-1,2,3,4,7,8,9,10,11,12,13,14,15,16-tetradecahydrospiro[cyclopenta[a] phenanthrene-17,2’-[1,3]dioxolan]-3-yl 6-(POSS)hex-4-enoate (7)
3.6. (13S)-13-methyl-17-oxo-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a] phenanthren-3-yl-6-(POSS)hex-4-enoate (9)
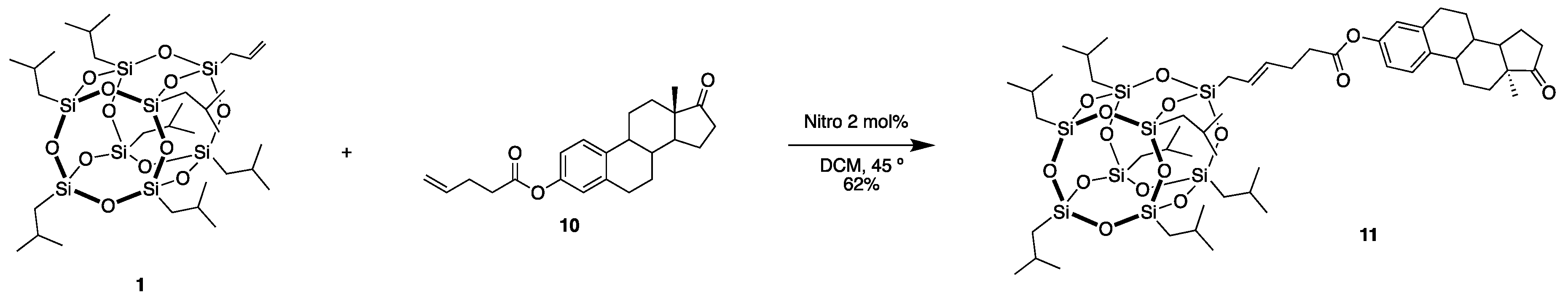
3.7. ((13S,17R)-13-methyl-17-(4-(POSS)but-2-en-1-yl)-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol (11)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hartmann-Thompson, C. Applications of polyhedral oligomeric silsesquioxanes; Springer: Dordrecht, The Netherlands, 2011; p. 420. [Google Scholar]
- Markovic, E.; Constantopolous, K.; Matisons, J.G. Polyhedral oligomeric silsesquioxanes: From early and strategic development through to materials application. Appl. Polyhedr. Oligomeric Silsesquioxanes 2011, 3, 1–46. [Google Scholar]
- Pielichowski, K.; Njuguna, J.; Janowski, B.; Pielichowski, J. Polyhedral oligomeric silsesquioxanes (POSS)-containing nanohybrid polymers. In Supramolecular polymers polymeric betains oligomers; Donnio, B., Guillon, D., Harada, A., Hashidzume, A., Jaeger, W., Janowski, B., Kudaibergenov, S., Laschewsky, A., Njuguna, J., Pielichowski, J., et al., Eds.; Springer: Berlin, Germany, 2006; Volume 201, pp. 225–296. [Google Scholar]
- Ghanbari, H.; Cousins, B.G.; Seifalian, A.M. A nanocage for nanomedicine: Polyhedral oligomeric silsesquioxane (POSS). Macromol. Rapid Commun. 2011, 32, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- John, Ł.; Malik, M.; Janeta, M.; Szafert, S. First step towards a model system of the drug delivery network based on amide-POSS nanocarriers. RSC Adv. 2017, 7, 8394–8401. [Google Scholar] [CrossRef]
- John, L.; Janeta, M.; Szafert, S. Synthesis of cubic spherosilicates for self-assembled organic-inorganic biohybrids based on functionalized methacrylates. New J. Chem. 2018, 42, 39–47. [Google Scholar] [CrossRef]
- Janeta, M.; John, L.; Ejfler, J.; Lis, T.; Szafert, S. Multifunctional imine-POSS as uncommon 3D nanobuilding blocks for supramolecular hybrid materials: Synthesis, structural characterization, and properties. Dalton Trans. 2016, 45, 12312–12321. [Google Scholar] [CrossRef] [PubMed]
- Janeta, M.; John, L.; Ejfler, J.; Szafert, S. High-yield synthesis of amido-functionalized polyoctahedral oligomeric silsesquioxanes by using acyl chlorides. Chemistry-a Eur. J. 2014, 20, 15966–15974. [Google Scholar] [CrossRef] [PubMed]
- John, Ł. Selected developments and medical applications of organic–inorganic hybrid biomaterials based on functionalized spherosilicates. Mater. Sci. Eng. C 2018, 88, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Seifalian, A.M. Polyhedral oligomeric silsesquioxane nanocomposites: The next generation material for biomedical applications. Account. Chem. Res. 2005, 38, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mather, P.T. POSS polymers: Physical properties and biomaterials applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Blackwell, H.E.; O’Leary, D.J.; Chatterjee, A.K.; Washenfelder, R.A.; Bussmann, D.A.; Grubbs, R.H. New Approaches to Olefin Cross-Metathesis. J. Am. Chem. Soc. 2000, 122, 58–71. [Google Scholar] [CrossRef]
- Vernall, A.J.; Abell, A.D. Cross metathesis of nitrogen-containing systems. Aldrichimica Acta 2003, 36, 93–105. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Choi, T.-L.; Sanders, D.P.; Grubbs, R.H. A general model for selectivity in olefin cross metathesis. J. Am. Chem. Soc. 2003, 125, 11360–11370. [Google Scholar] [CrossRef] [PubMed]
- Zukowska, K.; Grela, K. Cross metathesis. In Olefin Metathesis: Theory and Practice; Grela, K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 39–83, (and citation therein). [Google Scholar]
- Czaban, J.; Schertzer, B.M.; Grela, K. Low catalyst loadings in self-metathesis of 1-dodecene. Adv. Synth. Catal. 2013, 355, 1997–2006. [Google Scholar] [CrossRef]
- Kajetanowicz, A.; Sytniczuk, A.; Grela, K. Metathesis of renewable raw materials-influence of ligands in the indenylidene type catalysts on self-metathesis of methyl oleate and cross-metathesis of methyl oleate with (Z)-2-butene1,4-diol diacetate. Green Chem. 2014, 16, 1579–1585. [Google Scholar] [CrossRef]
- Olszewski, T.K.; Figlus, M.; Bieniek, M. Olefin metathesis: A versatile synthetic tool for use in preparation of active pharmaceutical ingredients. Chimica Oggi-Chemistry Today 2014, 32, 22–29. [Google Scholar] [CrossRef]
- Pederson, R.L.; Fellows, I.M.; Ung, T.A.; Ishihara, H.; Hajela, S.P. Applications of olefin cross metathesis to commercial products. Adv. Synth. Catal. 2002, 344, 728–735. [Google Scholar] [CrossRef]
- Kirschning, A.; Harmrolfs, K.; Mennecke, K.; Messinger, J.; Schön, U.; Grela, K. Homo- and heterogeneous Ru-based metathesis catalysts in cross-metathesis of 15-allylestrone—Towards 17β-hydroxysteroid dehydrogenase type 1 inhibitors. Tetrahedron Lett. 2008, 49, 3019–3022. [Google Scholar] [CrossRef]
- Bieniek, M.; Kołoda, D.; Grela, K. A highly selective synthesis of dialkenyl sulfones via cross-metathesis of divinyl sulfone. Org. Lett. 2006, 8, 5689–5692. [Google Scholar] [CrossRef] [PubMed]
- Feher, F.J.; Soulivong, D.; Eklund, A.G.; Wyndham, K.D. Cross-metathesis of alkenes with vinyl-substituted silsesquioxanes and spherosilicates: A new method for synthesizing highly-functionalized Si/O frameworks. Chem. Commun. 1997, 1185–1186. [Google Scholar] [CrossRef]
- Itami, Y.; Marciniec, B.; Kubicki, M. Functionalization of octavinylsilsesquioxane by ruthenium-catalyzed silylative coupling versus cross-metathesis. Chemistry – A Eur. J. 2004, 10, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, S.; Pietraszuk, C.; Marciniec, B. Synthesis of siloxy-modified second generation Hoveyda-Grubbs catalysts and their catalytic activity. J. Organomet. Chem. 2009, 694, 3918–3922. [Google Scholar] [CrossRef]
- Żak, P.; Pietraszuk, C.; Marciniec, B.; Spólnik, G.; Danikiewicz, W. Efficient functionalisation of cubic monovinylsilsesquioxanes via cross-metathesis and silylative coupling with olefins in the presence of ruthenium complexes. Adv. Synth. Catal. 2009, 351, 2675–2682. [Google Scholar] [CrossRef]
- Vautravers, N.R.; Andre, P.; Slawin, A.M.Z.; Cole-Hamilton, D.J. Synthesis and characterization of photoluminescent vinylbiphenyl decorated polyhedral oligomeric silsesquioxanes. Org. Biomol. Chem. 2009, 7, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Vautravers, N.R.; Andre, P.; Cole-Hamilton, D.J. Fluorescence activation of a polyhedral oligomeric silsesquioxane in the presence of reducing agents. J. Mater. Chem. 2009, 19, 4545–4550. [Google Scholar] [CrossRef]
- Bieniek, M.; Michrowska, A.; Usanov, D.L.; Grela, K. In an attempt to provide a user’s guide to the galaxy of benzylidene, alkoxybenzylidene, and indenylidene ruthenium olefin metathesis catalysts. Chem. Eur. J. 2008, 14, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Dinger, M.B.; Mol, J.C. High turnover numbers with ruthenium-based metathesis catalysts. Adv. Synth. Catal. 2002, 344, 671–677. [Google Scholar] [CrossRef]
- Michael, G.; Soley, K.; Wilson, T.; Christina, O.; Joachim, R. Development of commercially viable thermomorphic catalysts for controlled free radical polymerization. In Catalysis of organic reactions, 1st ed.; Prunier, M.L., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 319–328. [Google Scholar]
- Borré, E.; Caijo, F.; Crévisy, C.; Mauduit, M. New library of aminosulfonyl-tagged Hoveyda–Grubbs type complexes: Synthesis, kinetic studies and activity in olefin metathesis transformations. Beilstein J. Org. Chem. 2010, 6, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, M.; Bujok, R.; Cabaj, M.; Lugan, N.; Lavigne, G.; Arlt, D.; Grela, K. Advanced fine-tuning of Grubbs/Hoveyda olefin metathesis catalysts: A further step toward an optimum balance between antinomic properties. J. Am. Chem. Soc. 2006, 128, 13652–13653. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, M.; Samojłowicz, C.; Sashuk, V.; Bujok, R.; Śledź, P.; Lugan, N.l.; Lavigne, G.; Arlt, D.; Grela, K. Rational design and evaluation of upgraded Grubbs/Hoveyda olefin metathesis catalysts: Polyfunctional benzylidene ethers on the test bench. Organometallics 2011, 30, 4144–4158. [Google Scholar] [CrossRef]
- Luan, X.J.; Mariz, R.; Gatti, M.; Costabile, C.; Poater, A.; Cavallo, L.; Linden, A.; Dorta, R. Identification and characterization of a new family of catalytically highly active imidazolin-2-ylidenes. J. Am. Chem. Soc. 2008, 130, 6848–6858. [Google Scholar] [CrossRef] [PubMed]
- Vieille-Petit, L.; Luan, X.; Gatti, M.; Blumentritt, S.; Linden, A.; Clavier, H.; Nolan, S.P.; Dorta, R. Improving grubbs’ II type ruthenium catalysts by appropriately modifying the N-heterocyclic carbene ligand. Chem. Commun. 2009, 3783–3785. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Vieille-Petit, L.; Luan, X.; Mariz, R.; Drinkel, E.; Linden, A.; Dorta, R. Impact of NHC ligand conformation and solvent concentration on the ruthenium-catalyzed ring-closing metathesis reaction. J. Am. Chem. Soc. 2009, 131, 9498–9499. [Google Scholar] [CrossRef] [PubMed]
- Vieille-Petit, L.; Clavier, H.; Linden, A.; Blumentritt, S.; Nolan, S.P.; Dorta, R. Ruthenium olefin metathesis catalysts with N-heterocyclic carbene ligands bearing n-naphthyl side chains. Organometallics 2010, 29, 775–788. [Google Scholar] [CrossRef]
- Samojlowicz, C.; Bieniek, M.; Zarecki, A.; Kadyrov, R.; Grela, K. The doping effect of fluorinated aromatic hydrocarbon solvents on the performance of common olefin metathesis catalysts: Application in the preparation of biologically active compounds. Chem. Commun. 2008, 6282–6284. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
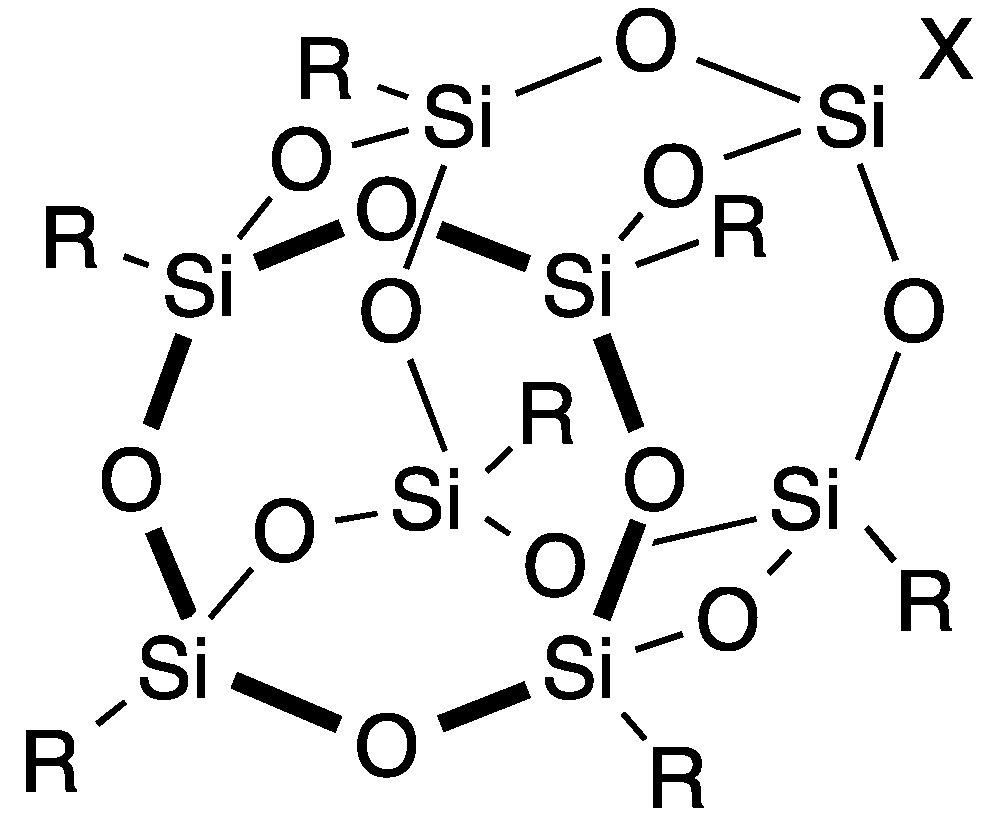
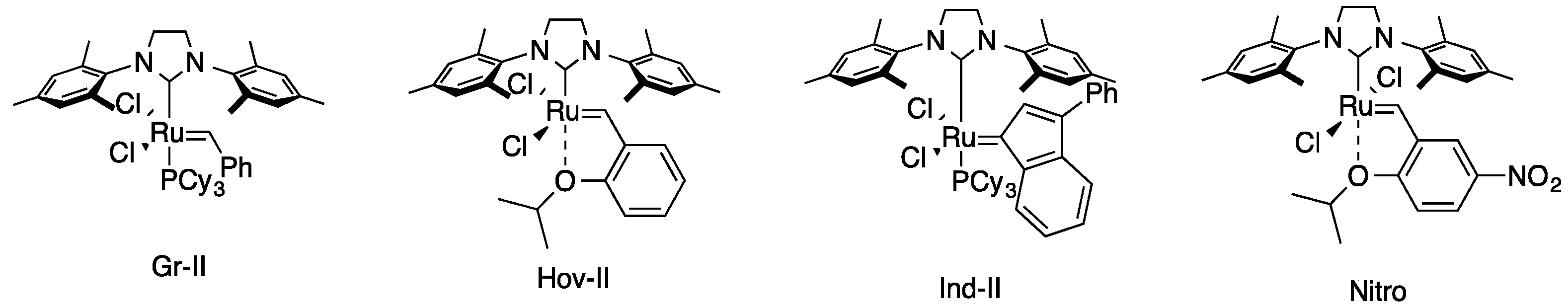
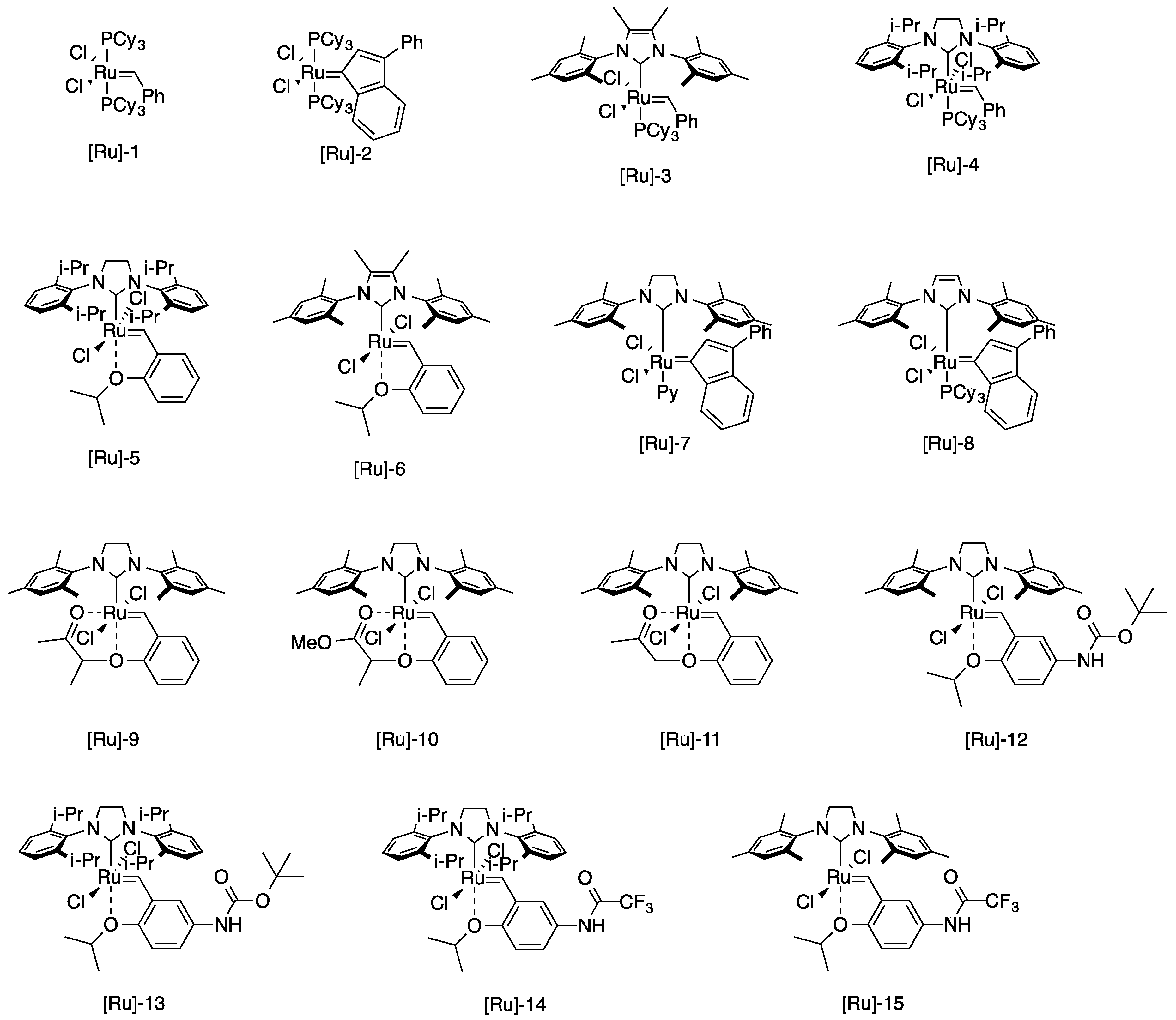


| Entry | Catalyst | Time (h) | Yield of 4 (%) | Yield of 5 (%) |
|---|---|---|---|---|
| 1 | [Ru]-1 | 5 | 6 | 33 |
| 2 | [Ru]-2 | 5 | 5 | 40 |
| 3 | Gr-II | 5 | 71 | 80 |
| 4 | [Ru]-3 | 5 | 28 | 31 |
| 5 | [Ru]-4 | 5 | 79 | 83 |
| 6 | Hov-II | 5 | 99 | 98 |
| 7 | Hov-II | 2 | 80 | 73 |
| 8 | [Ru]-5 | 5 | 99 | 69 |
| 9 | [Ru]-6 | 5 | 40 | 57 |
| 10 | Ind-II | 5 | 0 | 0 |
| 11 | [Ru]-7 | 5 | 50 | 52 |
| 12 | [Ru]-8 | 5 | 0 | 0 |
| 13 | Nitro | 24 | 90 | 64 |
| 14 | Nitro | 5 | 87 | 76 |
| 15 | [Ru]-9 | 5 | 94 | 89 |
| 16 | [Ru]-9 | 2 | 81 | 69 |
| 17 | [Ru]-10 | 5 | 99 | 66 |
| 18 | [Ru]-10 | 2 | 72 | 26 |
| 19 | [Ru]-11 | 5 | 56 | 77 |
| 20 | [Ru]-12 | 5 | 91 | 82 |
| 21 | [Ru]-12 | 2 | 93 | 90 |
| 22 | [Ru]-13 | 5 | 91 | 82 |
| 23 | [Ru]-14 | 5 | 91 | 95 |
| 24 | [Ru]-14 | 2 | 87 | 80 |
| 25 | [Ru]-15 | 5 | 85 | 83 |
| 26 | [Ru]-16 | 5 | 81 | 86 |
| 27 | [Ru]-17 | 5 | 96 | 82 |
| 28 | [Ru]-18 | 5 | 90 | 82 |
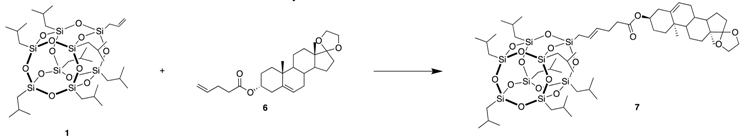
| Catalyst (mol%) | Solvent | Temperature (°C) | Time (h) | Yield of 7 (%) |
|---|---|---|---|---|
| Hov-II (0.5) | DCM | RT | 5 | 3 |
| Hov-II (2) | DCM | 45 | 5 | 36 |
| Hov-II (2) | Toluene | 100 | 5 | 69 |
| Nitro (2) | Toluene | 100 | 5 | 72 |
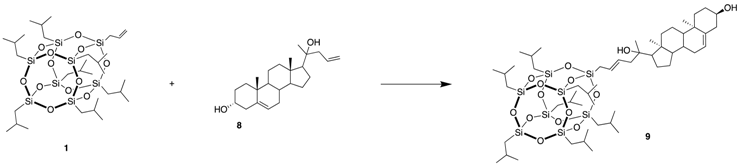
| Catalyst (mol%) | Solvent | Temperature (°C) | Time (h) | Yield of 9 (%) |
|---|---|---|---|---|
| Hov-II (0.5) | DCM | RT | 5 | 29 |
| Hov-II (2) | Toluene | 100 | 5 | 36 |
| Nitro (2) | Toluene | 100 | 10 | 46 |
| Nitro (2) | DCM | 45 | 24 | 69 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaban-Jóźwiak, J.; Woźniak, Ł.; Ulikowski, A.; Kwiecińska, K.; Rajkiewicz, A.A.; Grela, K. Modification of Polyhedral Oligomeric Silsesquioxanes (POSS) Molecules by Ruthenium Catalyzed Cross Metathesis. Molecules 2018, 23, 1722. https://doi.org/10.3390/molecules23071722
Czaban-Jóźwiak J, Woźniak Ł, Ulikowski A, Kwiecińska K, Rajkiewicz AA, Grela K. Modification of Polyhedral Oligomeric Silsesquioxanes (POSS) Molecules by Ruthenium Catalyzed Cross Metathesis. Molecules. 2018; 23(7):1722. https://doi.org/10.3390/molecules23071722
Chicago/Turabian StyleCzaban-Jóźwiak, Justyna, Łukasz Woźniak, Artur Ulikowski, Katarzyna Kwiecińska, Adam A. Rajkiewicz, and Karol Grela. 2018. "Modification of Polyhedral Oligomeric Silsesquioxanes (POSS) Molecules by Ruthenium Catalyzed Cross Metathesis" Molecules 23, no. 7: 1722. https://doi.org/10.3390/molecules23071722
APA StyleCzaban-Jóźwiak, J., Woźniak, Ł., Ulikowski, A., Kwiecińska, K., Rajkiewicz, A. A., & Grela, K. (2018). Modification of Polyhedral Oligomeric Silsesquioxanes (POSS) Molecules by Ruthenium Catalyzed Cross Metathesis. Molecules, 23(7), 1722. https://doi.org/10.3390/molecules23071722





