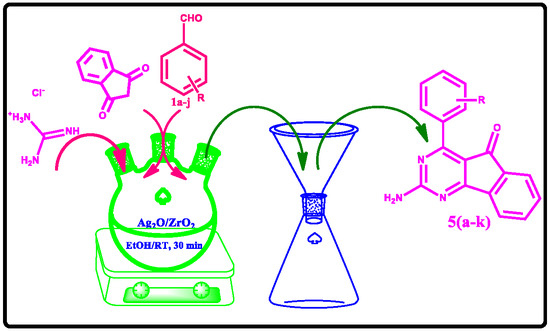
Ag2O on ZrO2 as a Recyclable Catalyst for Multicomponent Synthesis of Indenopyrimidine Derivatives
Abstract
1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
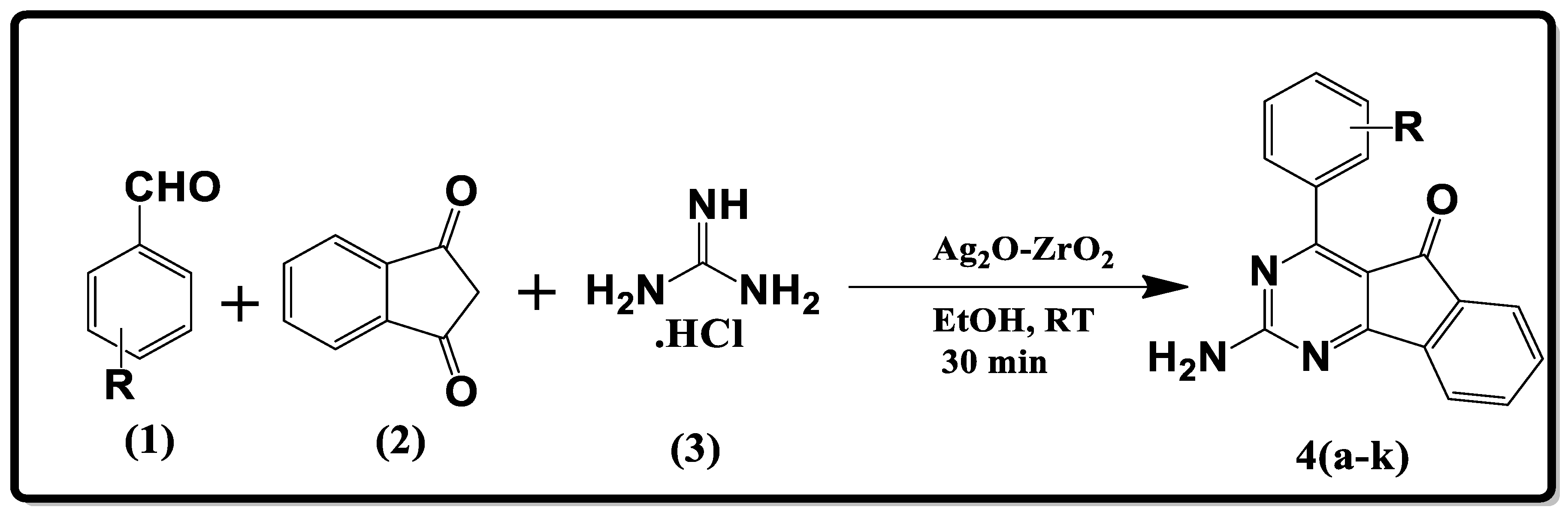
2.2. General Procedure for the Synthesis of Indenopyrimidine Derivatives
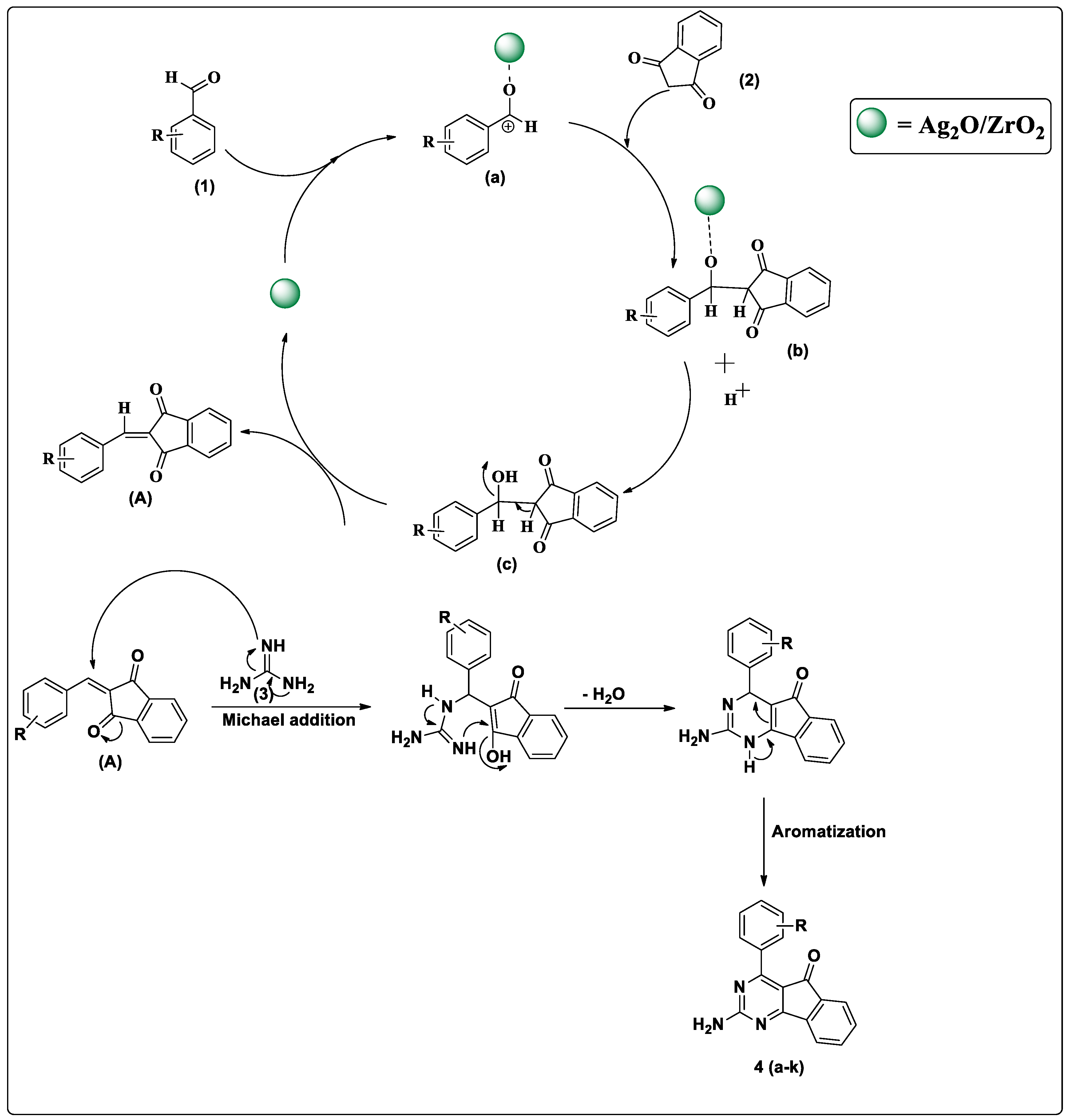
3. Results and Discussion
3.1. Crystallinity by Powder XRD (PXRD) Studies
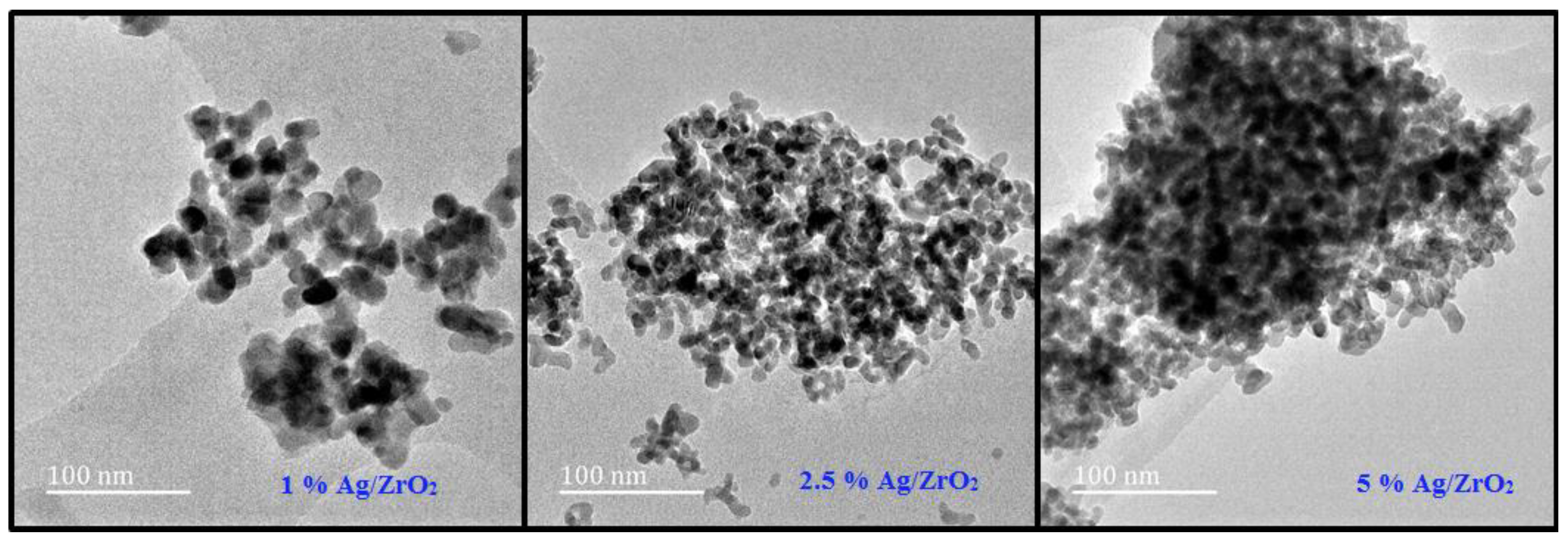
3.2. TEM Analysis
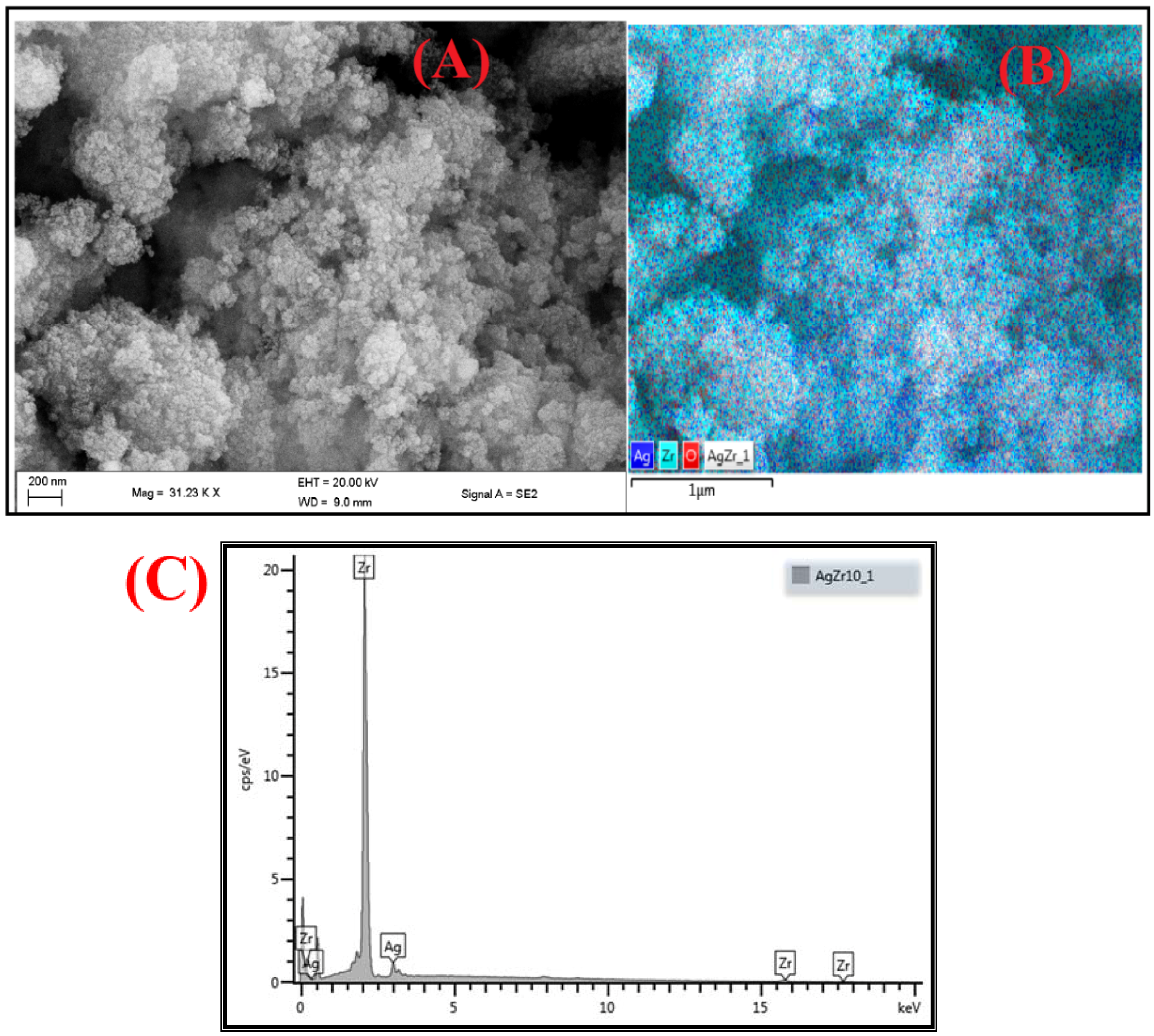
3.3. SEM Analysis
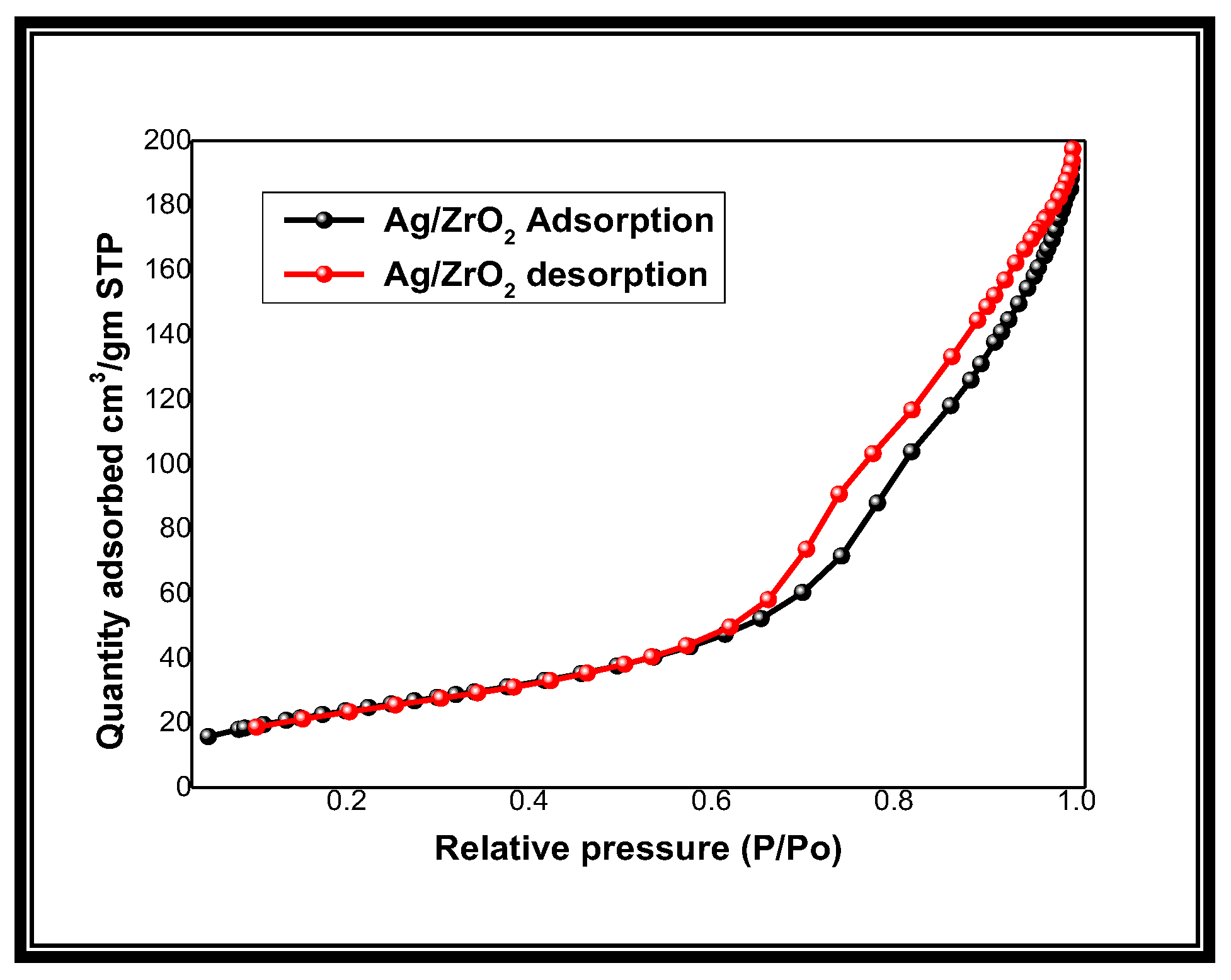
3.4. BET Surface Area Analysis
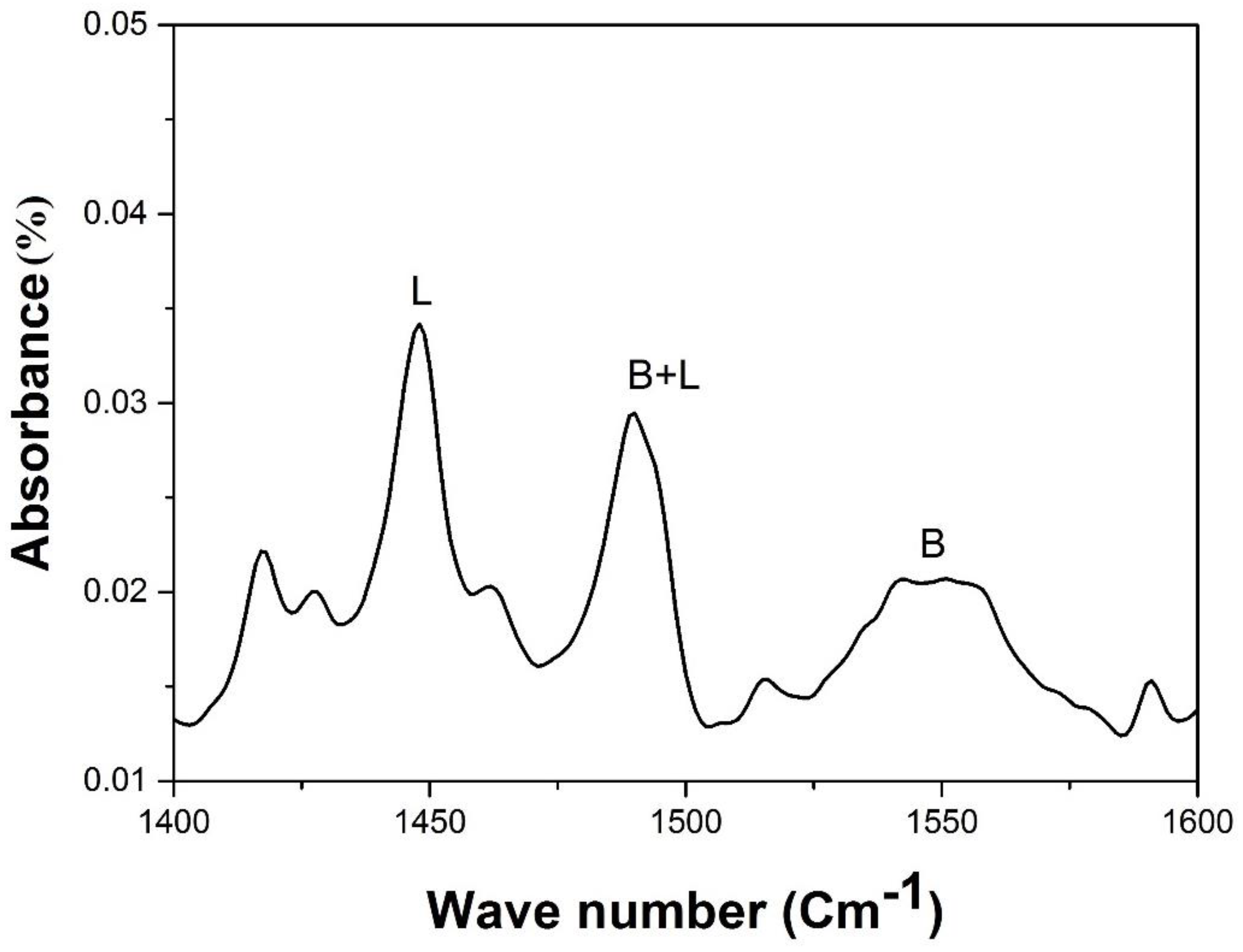
3.5. Pyridine Adsorbed FT-IR Spectroscopy
4. Reaction Optimization
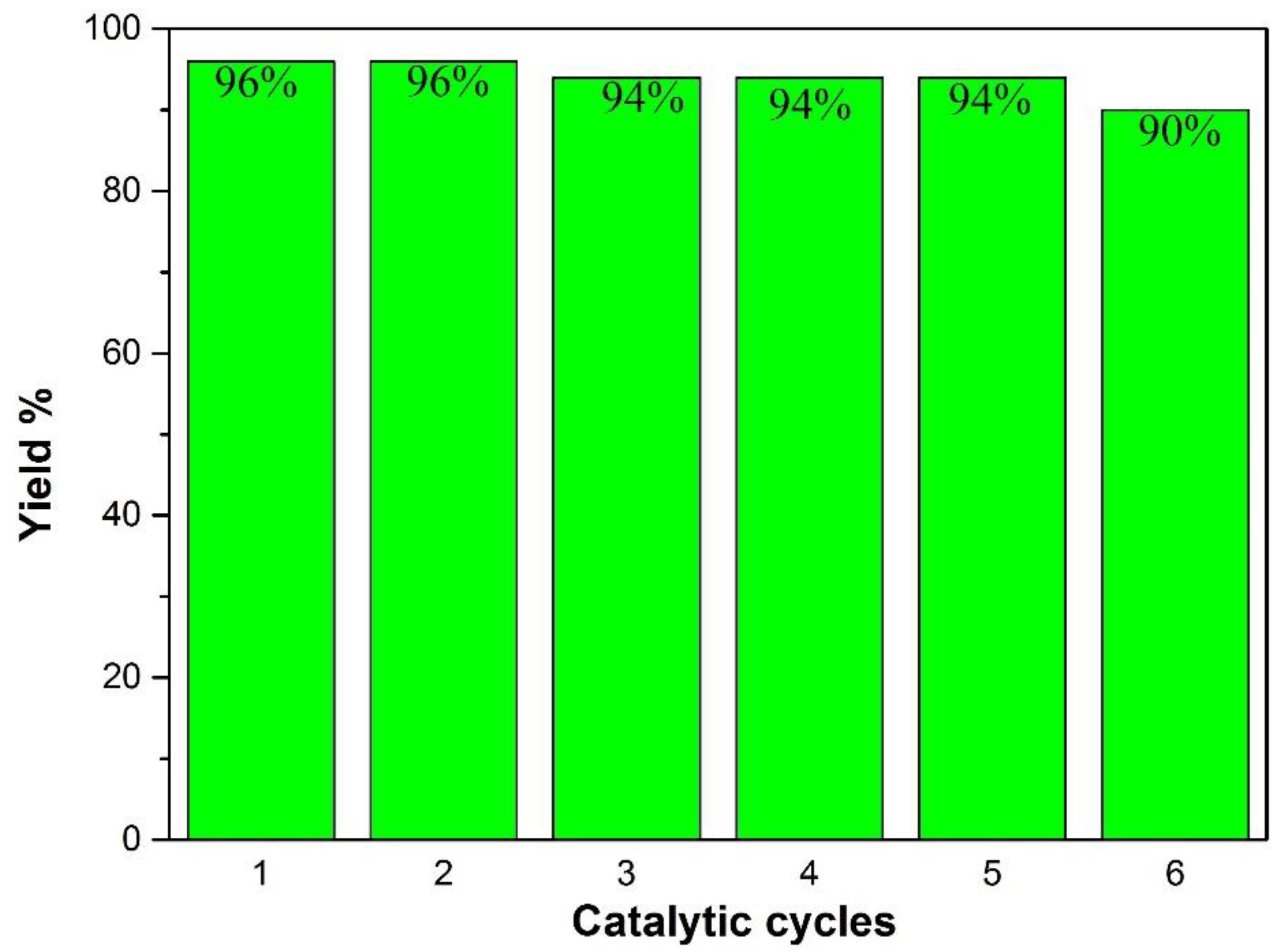
5. Reusability of Catalyst
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rotstein, B.H.; Zaretsky, S.; Rai, V.; Yudin, A.K. Small heterocycles in multicomponent reactions. Chem. Rev. 2014, 114, 8323–8359. [Google Scholar] [CrossRef] [PubMed]
- Domling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Gangu, K.K.; Maddila, S.; Maddila, S.N.; Jonnalagadda, S.B. Novel iron doped calcium oxalates as promising heterogeneous catalysts for one-pot multi-component synthesis of pyranopyrazoles. RSC Adv. 2017, 7, 423–432. [Google Scholar] [CrossRef]
- Bhaskaruni, S.V.H.S.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on multi-component green synthesis of N-containing heterocycles using mixed oxides as heterogeneous catalysts. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Bhaskaruni, S.V.H.S.; Maddila, S.; van Zyl, W.E.; Jonnalagadda, S.B. RuO2/ZrO2 as an efficient reusable catalyst for the facile, green, one-pot synthesis of novel functionalized halopyridine derivatives. Catal. Commun. 2017, 100, 24–28. [Google Scholar] [CrossRef]
- Pagadala, R.; Maddila, S.; Moodley, V.; van Zyl, W.E.; Jonnalagadda, S.B. An efficient method for the multicomponent synthesis of multisubstituted pyridines, a rapid procedure using Au/MgO as the catalyst. Tetrahedron Lett. 2014, 55, 4006–4010. [Google Scholar] [CrossRef]
- Bhaskaruni, S.V.H.S.; Maddila, S.; van Zyl, W.E.; Jonnalagadda, S.B. V2O5/ZrO2 as an efficient reusable catalyst for the facile, green, one-pot synthesis of novel functionalized 1,4-dihydropyridine derivatives. Catal. Today 2017. [Google Scholar] [CrossRef]
- Maddila, S.N.; Maddila, S.; van Zyl, W.E.; Jonnalagadda, S.B. Mn doped ZrO2 as a green, efficient and reusable heterogeneous catalyst for the multicomponent synthesis of pyrano[2,3-d]-pyrimidine derivatives. RSC Adv. 2015, 5, 37360–37366. [Google Scholar] [CrossRef]
- Wen, C.; Yin, A.; Dai, W.-L. Recent advances in silver-based heterogeneous catalysts for green chemistry processes. Appl. Catal. B Environ. 2014, 160–161, 730–741. [Google Scholar] [CrossRef]
- Maddila, S.; Jonnalagadda, S.B.; Gangu, K.K.; Maddila, S.N. Recent Advances in the Synthesis of Pyrazole Derivatives Using Multicomponent Reactions. Curr. Org. Synth. 2017, 14, 634–653. [Google Scholar] [CrossRef]
- Gore, R.P.; Rajput, A.P. A review on recent progress in multicomponent reactions of pyrimidine synthesis. Drug Invent. Today 2013, 5, 148–152. [Google Scholar] [CrossRef]
- Yalagala, K.; Maddila, S.; Rana, S.; Maddila, S.N.; Kalva, S.; Skelton, A.A.; Jonnalagadda, S.B. Synthesis, antimicrobial activity and molecular docking studies of pyrano[2,3-d]pyrimidine formimidate derivatives. Res. Chem. Intermed. 2016, 42, 3763–3774. [Google Scholar] [CrossRef]
- Maddila, S.; Gorle, S.; Seshadri, N.; Lavanya, P.; Jonnalagadda, S.B. Synthesis, antibacterial and antifungal activity of novel benzothiazole pyrimidine derivatives. Arab. J. Chem. 2016, 9, 681–687. [Google Scholar] [CrossRef]
- Raić-Malić, S.; Svedružić, D.; Gazivoda, T.; Marunović, A.; Hergold-Brundić, A.; Nagl, A.; Balzarini, J.; De Clercq, E.; Mintas, M. Synthesis and Antitumor Activities of Novel Pyrimidine Derivatives of 2,3-O,O-Dibenzyl-6-deoxy-l-ascorbic Acid and 4,5-Didehydro-5,6-dideoxy-l-ascorbic Acid. J. Med. Chem. 2000, 43, 4806–4811. [Google Scholar] [CrossRef] [PubMed]
- Miazga, A.; Ziemkowski, P.; Siwecka, M.A.; Lipniacki, A.; Piasek, A.; Kulikowski, T. Synthesis, biological properties and anti-HIV-1 activity of new pyrimidine P1,P2-dinucleotides. Nucleosides Nucleotides Nucleic Acids 2010, 29, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Yadlapalli, R.K.; Chourasia, O.P.; Vemuri, K.; Sritharan, M.; Perali, R.S. Synthesis and in vitro anticancer and antitubercular activity of diarylpyrazole ligated dihydropyrimidines possessing lipophilic carbamoyl group. Bioorg. Med. Chem. Lett. 2012, 22, 2708–2711. [Google Scholar] [CrossRef] [PubMed]
- De Assis, S.P.O.; da Silva, M.T.; de Oliveira, R.N.; de Lima, V.L.M. Synthesis and Anti-Inflammatory Activity of New Alkyl-Substituted Phthalimide 1H-1,2,3-Triazole Derivatives. Sci. World J. 2012, 2012, 925925. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Kaur, T. Pyrimidine-based antimalarials: Design strategies and antiplasmodial effects. Medchemcomm 2016, 7, 749–768. [Google Scholar] [CrossRef]
- Lim, H.-K.; Chen, J.; Sensenhauser, C.; Cook, K.; Preston, R.; Thomas, T.; Shook, B.; Jackson, P.F.; Rassnick, S.; Rhodes, K.; et al. Overcoming the Genotoxicity of a Pyrrolidine Substituted Arylindenopyrimidine As a Potent Dual Adenosine A2A/A1 Antagonist by Minimizing Bioactivation to an Iminium Ion Reactive Intermediate. Chem. Res. Toxicol. 2011, 24, 1012–1030. [Google Scholar] [CrossRef] [PubMed]
- Patravale, A.A.; Gore, A.H.; Patil, D.R.; Kolekar, G.B.; Deshmukh, M.B.; Anbhule, P.V. Trouble-Free Multicomponent Method for Combinatorial Synthesis of 2-Amino-4-phenyl-5-H-indeno[1,2-d]pyrimidine-5-one and Their Screening against Cancer Cell Lines. Ind. Eng. Chem. Res. 2014, 53, 16568–16578. [Google Scholar] [CrossRef]
- Undare, S.S.; Valekar, N.J.; Patravale, A.A.; Jamale, D.K.; Vibhute, S.S.; Walekar, L.S.; Kolekar, G.B.; Deshmukh, M.B.; Anbhule, P.V. Synthesis, anti-inflammatory, ulcerogenic and cyclooxygenase activities of indenopyrimidine derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Yin, S.; Tao, S.; Shi, Y.; Rong, L.; Wei, X.; Zong, Z. An efficient and facile synthesis of novel substituted pyrimidine derivatives: 4-amino-5-carbonitrile-2-nitroaminopyrimidine. Res. Chem. Intermed. 2012, 38, 2435–2442. [Google Scholar] [CrossRef]
- Jagadale, S.D.; Sawant, A.D.; Deshmukh, M.B. Synthesis and Antimicrobial Evaluation of Novel Dibenzo-18-Crown-6-Ether Functionalized Pyrimidines. J. Heterocycl. Chem. 2017, 54, 2307–2312. [Google Scholar] [CrossRef]
- Gogoi, P.; Dutta, A.K.; Saikia, S.; Borah, R. Heterogenized hybrid catalyst of 1-sulfonic acid-3-methyl imidazolium ferric chloride over NaY zeolite for one-pot synthesis of 2-amino-4-arylpyrimidine derivatives: A viable approach. Appl. Catal. A Gen. 2016, 523, 321–331. [Google Scholar] [CrossRef]
- Aryan, R.; Beyzaei, H.; Nojavan, M.; Dianatipour, T. Secondary amines immobilized inside magnetic mesoporous materials as a recyclable basic and oxidative heterogeneous nanocatalyst for the synthesis of trisubstituted pyrimidine derivatives. Res. Chem. Intermed. 2016, 42, 4417–4431. [Google Scholar] [CrossRef]
- Kamali, M.; Shockravi, A.; Doost, M.S.; Hooshmand, S.E. One-pot, solvent-free synthesis via Biginelli reaction: Catalyst-free and new recyclable catalysts. Cogent Chem. 2015, 24, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Wang, S.; Huang, T.; Hao, Y.; Qi, Y. Multi-mode photocatalytic degradation and photocatalytic hydrogen evolution of honeycomb-like three-dimensionally ordered macroporous composite Ag/ZrO2. RSC Adv. 2016, 6, 13991–14001. [Google Scholar] [CrossRef]
- Lee, C.; Shul, Y.-G.; Einaga, H. Silver and manganese oxide catalysts supported on mesoporous ZrO2 nanofiber mats for catalytic removal of benzene and diesel soot. Catal. Today 2017, 281, 460–466. [Google Scholar] [CrossRef]
- Shabalala, N.G.; Maddila, S.; Jonnalagadda, S.B. Facile one-pot green synthesis of tetrahydrobiphenylene-1,3-dicarbonitriles in aqueous media under ultrasound irradiation. Res. Chem. Intermed. 2016, 42, 8097–8108. [Google Scholar] [CrossRef]
- Shabalala, N.; Maddila, S.; Jonnalagadda, S.B. Catalyst-free, one-pot, four-component green synthesis of functionalized 1-(2-fluorophenyl)-1,4-dihydropyridines under ultrasound irradiation. New J. Chem. 2016, 40, 5107–5112. [Google Scholar] [CrossRef]
- Shabalala, S.; Maddila, S.; van Zyl, W.E.; Jonnalagadda, S.B. Sustainable CeO2/ZrO2 Mixed Oxide Catalyst For the Green Synthesis of Highly Functionalized 1,4-Dihydropyridine-2,3-dicarboxylate Derivatives. Curr. Org. Synth. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Shabalala, S.; Maddila, S.; van Zyl, W.E.; Jonnalagadda, S.B. Innovative Efficient Method for the Synthesis of 1,4-Dihydropyridines Using Y2O3 Loaded on ZrO2 as Catalyst. Ind. Eng. Chem. Res. 2017, 56, 11372–11379. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Maddila, S.N.; Jonnalagadda, S.B. Nanostructured samarium doped fluorapatites and their catalytic activity towards synthesis of 1,2,4-triazoles. Molecules 2016, 21, 1281. [Google Scholar] [CrossRef] [PubMed]
- Maddila, S.; Lavanya, P.; Jonnalagadda, S.B. Cesium loaded on silica as an efficient and recyclable catalyst for the novel synthesis of selenophenes. Arab. J. Chem. 2016, 9, 891–897. [Google Scholar] [CrossRef]
- Maddila, S.; Valand, J.; Bandaru, H.; Yalagala, K.; Lavanya, P. Ag Loaded on SiO2 as an Efficient and Recyclable Heterogeneous Catalyst for the Synthesis of Chloro-8-substituted-9H-purines. J. Heterocycl. Chem. 2016, 53, 319–324. [Google Scholar] [CrossRef]
- Maddila, S.; Maddila, S.N.; Jonnalagadda, S.B.; Lavanya, P. Reusable Ce-V Loaded Alumina Catalyst for Multicomponent Synthesis of Substituted Pyridines in Green Media. J. Heterocycl. Chem. 2016, 53, 658–664. [Google Scholar] [CrossRef]
- Maddila, S.; Gorle, S.; Shabalala, S.; Oyetade, O.; Maddila, S.N.; Lavanya, P.; Jonnalagadda, S.B. Ultrasound mediated green synthesis of pyrano[2,3-c]pyrazoles by using Mn doped ZrO2. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- Maddila, S.; Dasireddy, V.D.B.C.; Jonnalagadda, S.B. Ce-V loaded metal oxides as catalysts for dechlorination of chloronitrophenol by ozone. Appl. Catal. B Environ. 2014, 150–151, 305–314. [Google Scholar] [CrossRef]
- Maddila, S.; Dasireddy, V.D.B.C.; Jonnalagadda, S.B. Dechlorination of tetrachloro-o-benzoquinone by ozonation catalyzed by cesium loaded metal oxides. Appl. Catal. B Environ. 2013, 138–139, 149–160. [Google Scholar] [CrossRef]
- Balaga, V.; Pedada, J.; Friedrich, H.B.; Singh, S. Tuning surface composition of Cs exchanged phosphomolybdic acid catalysts in CH bond activation of toluene to benzaldehyde at room temperature. J. Mol. Catal. A Chem. 2016, 425, 116–123. [Google Scholar] [CrossRef]
- Védrine, J.C. Acid-base characterization of heterogeneous catalysts: An up-to-date overview. Res. Chem. Intermed. 2015, 41, 9387–9423. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Yu, Y.; Chen, Y.; Dai, L. Tailoring a magnetically separable NiFe2O4 nanoparticle catalyst for Knoevenagel condensation. Tetrahedron 2016, 72, 8358–8363. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Entry | Catalyst | Solvent | Condition | Time (h) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | -- | -- | RT | 10 | -- |
| 2 | -- | -- | Reflux | 10 | -- |
| 3 | TEA | EtOH | RT | 8.0 | 9 |
| 4 | Pyridine | EtOH | RT | 8.5 | 13 |
| 5 | NaOH | EtOH | RT | 7.5 | 25 |
| 6 | K2CO3 | EtOH | RT | 7.0 | 19 |
| 7 | (Bmim)BF4 | EtOH | RT | 10 | 23 |
| 8 | L-proline | EtOH | RT | 10 | 27 |
| 9 | AcOH | EtOH | RT | 5.0 | 43 |
| 10 | FeCl3 | EtOH | RT | 4.5 | 50 |
| 11 | PTSA | EtOH | RT | 5.0 | 45 |
| 12 | SiO2 | EtOH | RT | 2.5 | 62 |
| 13 | ZrO2 | EtOH | RT | 2.0 | 79 |
| 14 | Al2O3 | EtOH | RT | 3.0 | 59 |
| 15 | 2.5% CuO/ZrO2 | EtOH | RT | 1.5 | 82 |
| 16 | 2.5% MnO2/ZrO2 | EtOH | RT | 1.0 | 87 |
| 17 | 2.5% Ag2O/ZrO2 | EtOH | RT | 0.20 | 96 |
| Entry | Solvent | Time (min) | Yield (%) |
|---|---|---|---|
| 1 | No solvent | 120 | -- |
| 2 | n-hexane | 120 | -- |
| 3 | toluene | 90 | -- |
| 4 | THF | 75 | 10 |
| 5 | DMF | 65 | 18 |
| 6 | MeCN | 60 | 25 |
| 7 | MeOH | 45 | 81 |
| 8 | EtOH | 30 | 96 |
| Entry | Catalyst (mg) | Time (min) | Yield (%) |
|---|---|---|---|
| 1 | 20 | 100 | 56 |
| 2 | 40 | 50 | 79 |
| 3 | 60 | 30 | 96 |
| 4 | 80 | 30 | 96 |
| 5 | 100 | 30 | 96 |
| 6 | 120 | 40 | 96 |
| Entry | R | Product | Yield * (%) | m.p. (°C) | Lit. m.p. (°C) |
|---|---|---|---|---|---|
| 1 | 2-OMe | 4a | 94 | 119–121 | - |
| 2 | 4-OMe | 4b | 93 | 136–137 | 100 [20] |
| 3 | 2,3-(OMe)2 | 4c | 94 | 183–184 | - |
| 4 | 2,5-(OMe)2 | 4d | 92 | 200–201 | - |
| 5 | 2-Br | 4e | 94 | 197–198 | - |
| 6 | 2-F | 4f | 92 | 239–241 | - |
| 7 | 3,4-(OMe)2 | 4g | 96 | 176–178 | 178 [20] |
| 8 | 3-OH | 4h | 95 | 246–248 | - |
| 9 | 4-Br | 4i | 94 | 221–223 | 210 [20] |
| 10 | 4-Cl | 4j | 90 | 239–241 | 244 [20] |
| 11 | 4-Et | 4k | 94 | 204–206 | - |
| Catalyst | Solvent | Reaction Condition | Yield (%) [Ref] |
|---|---|---|---|
| NaOH | EtOH | Reflux, 6–10 h | 81–94 [20] |
| NaOH | EtOH | Reflux, 7–8.4 h | 75–86 [21] |
| NaOH | EtOH | Reflux, 0.5–1 h | 85–94 [22] |
| NaOMe | EtOH | Reflux, 10–14 h | 60–70 [23] |
| α-Fe2O3-MCM-41-P | Solvent free | 80 °C, 1 h | 82–95 [25] |
| Uranyl acetate/succinimide sulfonic acid | Solvent free | 90 °C, 4 h | 75–96 [26] |
| 2.5% Ag2O–ZrO2 | EtOH | RT, 30 min | 90–96 [Present Work] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhaskaruni, S.V.H.S.; Maddila, S.; Van Zyl, W.E.; Jonnalagadda, S.B. Ag2O on ZrO2 as a Recyclable Catalyst for Multicomponent Synthesis of Indenopyrimidine Derivatives. Molecules 2018, 23, 1648. https://doi.org/10.3390/molecules23071648
Bhaskaruni SVHS, Maddila S, Van Zyl WE, Jonnalagadda SB. Ag2O on ZrO2 as a Recyclable Catalyst for Multicomponent Synthesis of Indenopyrimidine Derivatives. Molecules. 2018; 23(7):1648. https://doi.org/10.3390/molecules23071648
Chicago/Turabian StyleBhaskaruni, Sandeep V. H. S., Suresh Maddila, Werner E. Van Zyl, and Sreekantha B. Jonnalagadda. 2018. "Ag2O on ZrO2 as a Recyclable Catalyst for Multicomponent Synthesis of Indenopyrimidine Derivatives" Molecules 23, no. 7: 1648. https://doi.org/10.3390/molecules23071648
APA StyleBhaskaruni, S. V. H. S., Maddila, S., Van Zyl, W. E., & Jonnalagadda, S. B. (2018). Ag2O on ZrO2 as a Recyclable Catalyst for Multicomponent Synthesis of Indenopyrimidine Derivatives. Molecules, 23(7), 1648. https://doi.org/10.3390/molecules23071648