Garcinol Enhances TRAIL-Induced Apoptotic Cell Death through Up-Regulation of DR5 and Down-Regulation of c-FLIP Expression
Abstract
1. Introduction
2. Results
2.1. Effect of Garcinol on TRAIL Sensitization
2.2. Garcinol Induces TRAIL Sensitization through Down-Regulation of c-FLIP Expression
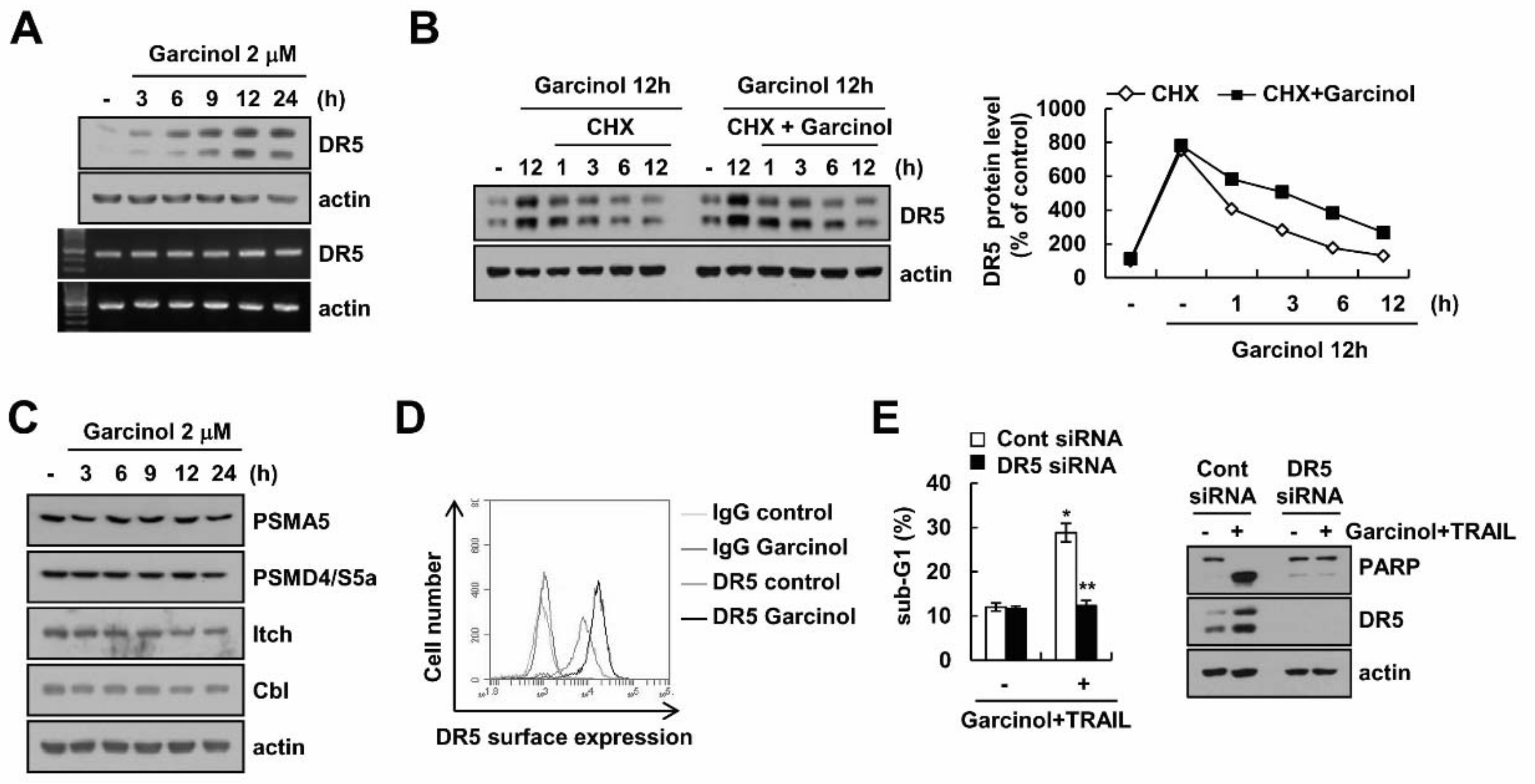
2.3. Combined Treatment Garcinol and TRAIL Induces Up-Regulation of DR5 Expression
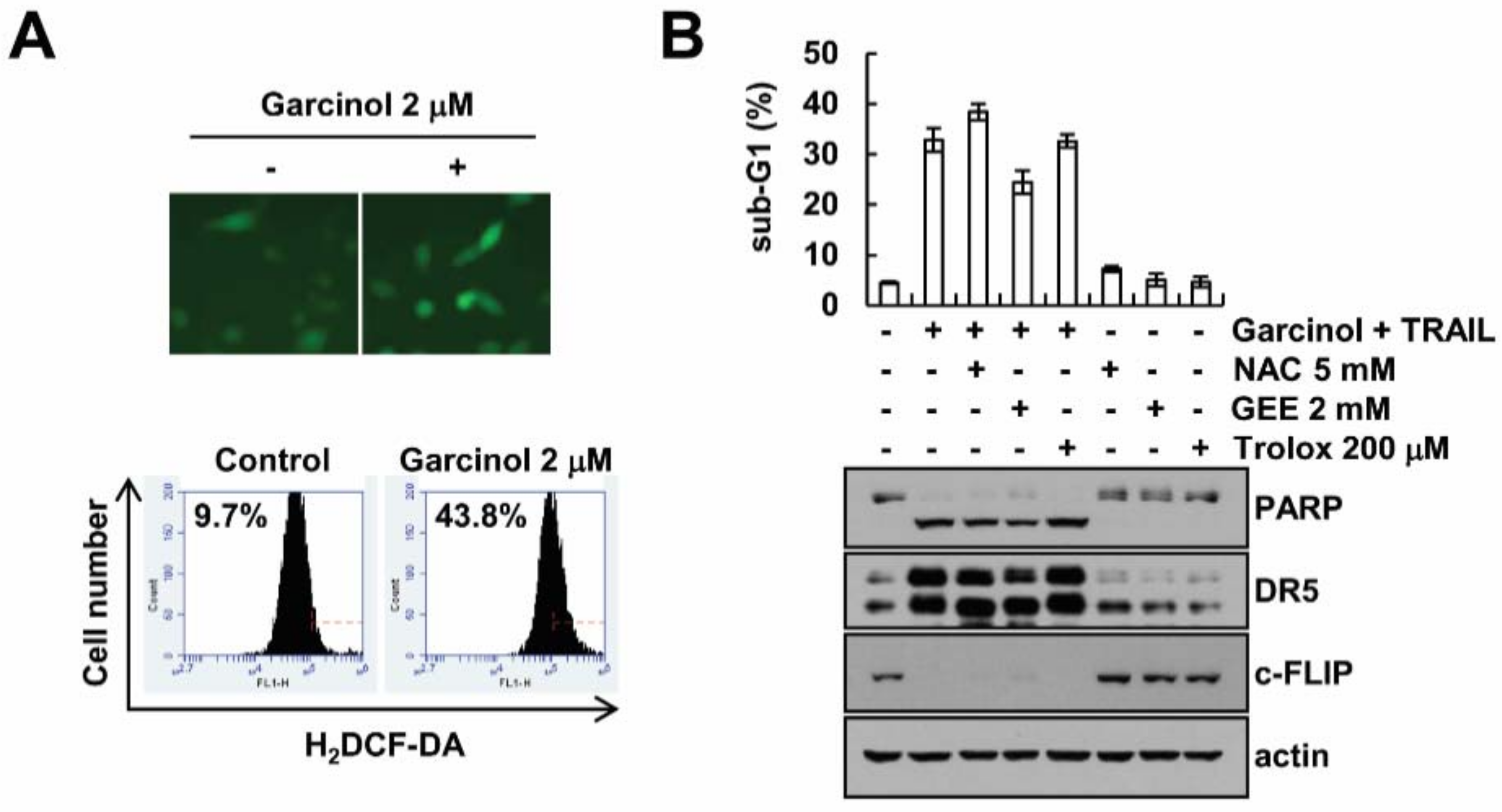
2.4. Garcinol-Mediated TRAIL Sensitization Is Not Associated with Reactive Oxygen Species (ROS) Signaling Pathway
2.5. Garcinol Induces TRAIL Sensitization in Other Cancer Cells, but Garcinol Plus TRAIL Had No Effect on Apoptosis in Normal Cells
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Materials
4.2. Flow Cytometry and Western Blot Analysis
4.3. 4′,6′-Diamidino-2-Phenylindole Staining (DAPI) Staining and DNA Fragmentation Assay
4.4. Detection of Caspase-3 Activity
4.5. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.6. Detection of DR5 on Cell Surface
4.7. Detection of Reactive Oxygen Species (ROS) Production
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| STAT | Signal transducer and activator of transcription |
| NF-κB | Nuclear factor-κB |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| ROS | Reactive oxygen species |
| DR | Death receptor |
| cFLIP | Cellular FADD-like IL-1β-converting enzyme-inhibitory protein |
| CHX | cycloheximide |
| PSMA5 | 20S proteasome subunit type 5 |
| PSMD4/S5a | 26S proteasome non-ATPase regulatory 4 |
References
- Yamaguchi, F.; Ariga, T.; Yoshimura, Y.; Nakazawa, H. Antioxidative and anti-glycation activity of garcinol from Garcinia indica fruit rind. J. Agric. Food Chem. 2000, 48, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Yasmin, T.; Bagchi, D.; Stohs, S.J. The bactericidal effects of lactobacillus acidophilus, garcinol and protykin compared to clarithromycin, on helicobacter pylori. Mol. Cell. Biochem. 2003, 243, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Sang, S.; Liang, Y.C.; Ho, C.T.; Lin, J.K. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 in downregulating nuclear factor-kappa b pathway by garcinol. Mol. Carcinog. 2004, 41, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Chatterjee, S.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Wong, K.F.; Kumar, A.P.; Senapati, P.; Behera, A.K.; Hui, K.M.; et al. Inhibition of stat3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Mol. Cancer 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Sang, S.; Ho, C.T.; Lin, J.K. Garcinol modulates tyrosine phosphorylation of FAK and subsequently induces apoptosis through down-regulation of Src, ERK, and Akt survival signaling in human colon cancer cells. J. Cell. Biochem. 2005, 96, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Wang, Z.; Ali, R.; Maitah, M.Y.; Kong, D.; Banerjee, S.; Padhye, S.; Sarkar, F.H. Apoptosis-inducing effect of garcinol is mediated by NF-κB signaling in breast cancer cells. J. Cell. Biochem. 2010, 109, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Das, S.N. Garcinol inhibits tumour cell proliferation, angiogenesis, cell cycle progression and induces apoptosis via NF-κB inhibition in oral cancer. Tumor Biol. 2016, 37, 7175–7184. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shanmugam, M.K.; Siveen, K.S.; Wang, F.; Ong, T.H.; Loo, S.Y.; Swamy, M.M.; Mandal, S.; Kumar, A.P.; Goh, B.C.; et al. Garcinol sensitizes human head and neck carcinoma to cisplatin in a xenograft mouse model despite downregulation of proliferative biomarkers. Oncotarget 2015, 6, 5147–5163. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Ravindran, J.; Sung, B.; Pandey, M.K.; Aggarwal, B.B. Garcinol potentiates trail-induced apoptosis through modulation of death receptors and antiapoptotic proteins. Mol. Cancer Ther. 2010, 9, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A.; et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef]
- Pitti, R.M.; Marsters, S.A.; Ruppert, S.; Donahue, C.J.; Moore, A.; Ashkenazi, A. Induction of apoptosis by APO-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 1996, 271, 12687–12690. [Google Scholar] [CrossRef] [PubMed]
- Eggert, A.; Grotzer, M.A.; Zuzak, T.J.; Wiewrodt, B.R.; Ho, R.; Ikegaki, N.; Brodeur, G.M. Resistance to tumor necrosis factor-related apoptosis-inducing ligand (trail)-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression. Cancer Res. 2001, 61, 1314–1319. [Google Scholar] [PubMed]
- Limami, Y.; Pinon, A.; Riaz, A.; Simon, A. Trail and targeting cancer cells: Between promises and obstacles. Cell. Mol. Biol. 2015, 61, 33–38. [Google Scholar] [PubMed]
- O’Leary, L.; van der Sloot, A.M.; Reis, C.R.; Deegan, S.; Ryan, A.E.; Dhami, S.P.; Murillo, L.S.; Cool, R.H.; Correa de Sampaio, P.; Thompson, K.; et al. Decoy receptors block trail sensitivity at a supracellular level: The role of stromal cells in controlling tumour trail sensitivity. Oncogene 2016, 35, 1261–1270. [Google Scholar]
- Han, M.A.; Lee, D.H.; Woo, S.M.; Seo, B.R.; Min, K.J.; Kim, S.; Park, J.W.; Kim, S.H.; Choi, Y.H.; Kwon, T.K. Galangin sensitizes trail-induced apoptosis through down-regulation of anti-apoptotic proteins in renal carcinoma CAKI cells. Sci. Rep. 2016, 6, 18642. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Seo, B.R.; Bae, Y.C.; Yoo, Y.H.; Kwon, T.K. Antipsychotic agent thioridazine sensitizes renal carcinoma Caki cells to TRAIL-induced apoptosis through reactive oxygen species-mediated inhibition of AKT signaling and downregulation of Mcl-1 and c-FLIP(L). Cell Death Dis. 2014, 5, e1063. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Mishra, D.P. Trailing trail resistance: Novel targets for trail sensitization in cancer cells. Front. Oncol. 2015, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Wilkie-Grantham, R.P.; Matsuzawa, S.; Reed, J.C. Novel phosphorylation and ubiquitination sites regulate reactive oxygen species-dependent degradation of anti-apoptotic c-flip protein. J. Biol. Chem. 2013, 288, 12777–12790. [Google Scholar] [CrossRef] [PubMed]
- Chanvorachote, P.; Nimmannit, U.; Wang, L.; Stehlik, C.; Lu, B.; Azad, N.; Rojanasakul, Y. Nitric oxide negatively regulates Fas CD95-induced apoptosis through inhibition of ubiquitin-proteasome-mediated degradation of FLICE inhibitory protein. J. Biol. Chem. 2005, 280, 42044–42050. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.R.; Min, K.J.; Woo, S.M.; Choe, M.; Choi, K.S.; Lee, Y.K.; Yoon, G.; Kwon, T.K. Inhibition of cathepsin S induces mitochondrial ROS that sensitizes TRAIL-mediated apoptosis through p53-mediated downregulation of Bcl-2 and c-FLIP. Antioxid. Redox Signal. 2017, 27, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.T.; Qian, G.; Deng, J.; Sun, S.Y. Monocyte chemotactic protein-induced protein-1 enhances DR5 degradation and negatively regulates dr5 activation-induced apoptosis through its deubiquitinase function. Oncogene 2018, 37, 3415–3425. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Kwon, Y.J.; Chun, Y.J. CYP1B1 activates Wnt/β-catenin signaling through suppression of HERC5-mediated ISGylation for protein degradation on β-catenin in HeLa cells. Toxicol. Res. 2017, 33, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Shin, D.Y. Repression of the F-box protein Skp2 is essential for actin damage-induced tetraploid G1 arrest. BMB Rep. 2017, 50, 379–383. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Seo, S.U.; Min, K.-J.; Woo, S.M.; Nam, J.-O.; Kubatka, P.; Kim, S.; Park, J.-W.; Kwon, T.K. Garcinol Enhances TRAIL-Induced Apoptotic Cell Death through Up-Regulation of DR5 and Down-Regulation of c-FLIP Expression. Molecules 2018, 23, 1614. https://doi.org/10.3390/molecules23071614
Kim S, Seo SU, Min K-J, Woo SM, Nam J-O, Kubatka P, Kim S, Park J-W, Kwon TK. Garcinol Enhances TRAIL-Induced Apoptotic Cell Death through Up-Regulation of DR5 and Down-Regulation of c-FLIP Expression. Molecules. 2018; 23(7):1614. https://doi.org/10.3390/molecules23071614
Chicago/Turabian StyleKim, Seok, Seung Un Seo, Kyoung-Jin Min, Seon Min Woo, Ju-Ock Nam, Peter Kubatka, Shin Kim, Jong-Wook Park, and Taeg Kyu Kwon. 2018. "Garcinol Enhances TRAIL-Induced Apoptotic Cell Death through Up-Regulation of DR5 and Down-Regulation of c-FLIP Expression" Molecules 23, no. 7: 1614. https://doi.org/10.3390/molecules23071614
APA StyleKim, S., Seo, S. U., Min, K.-J., Woo, S. M., Nam, J.-O., Kubatka, P., Kim, S., Park, J.-W., & Kwon, T. K. (2018). Garcinol Enhances TRAIL-Induced Apoptotic Cell Death through Up-Regulation of DR5 and Down-Regulation of c-FLIP Expression. Molecules, 23(7), 1614. https://doi.org/10.3390/molecules23071614







