Protein Hydrolysates’ Absorption Characteristics in the Dynamic Small Intestine In Vivo
Abstract
1. Introduction
2. Results
2.1. Food Running Speed
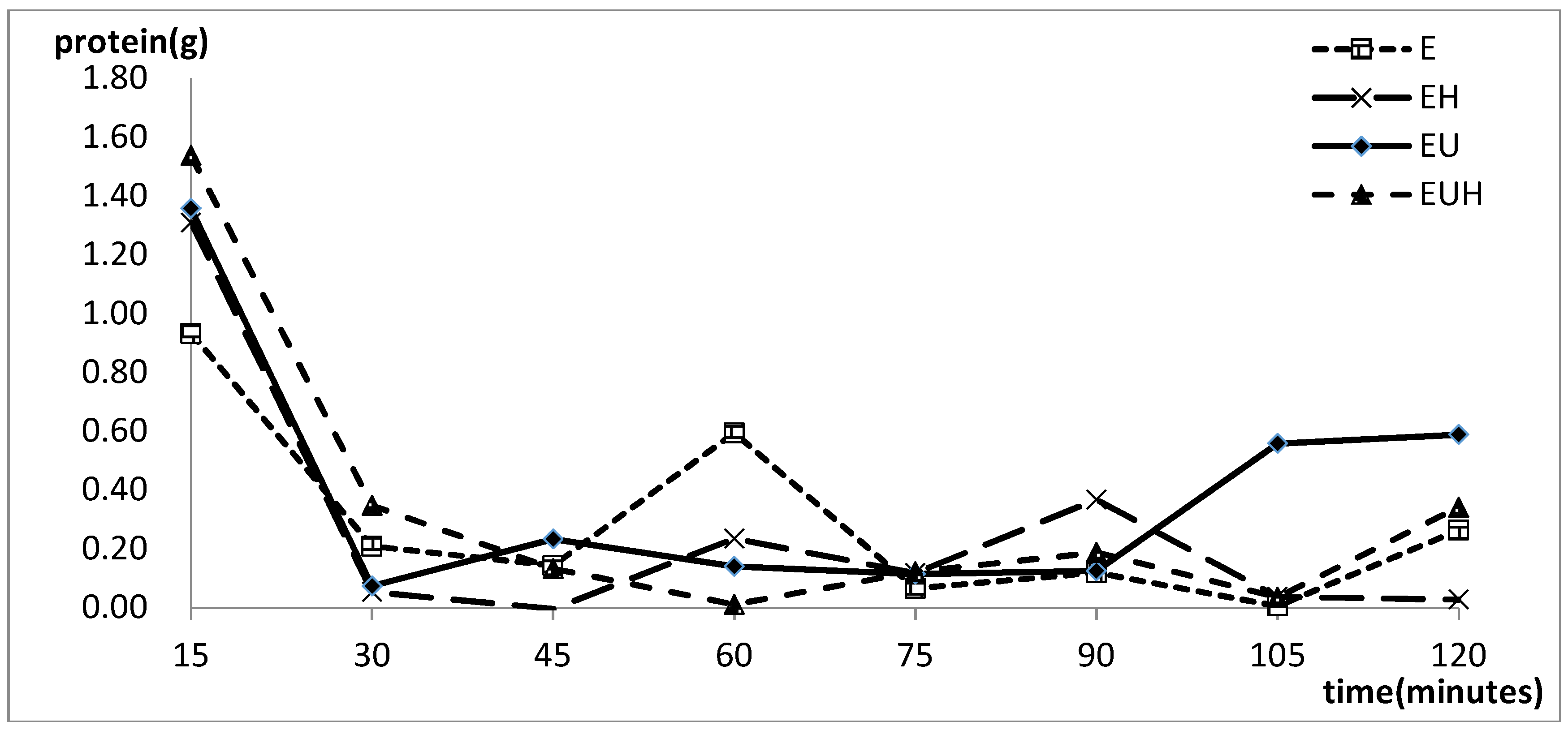
2.2. Protein Absorption Change with the Time
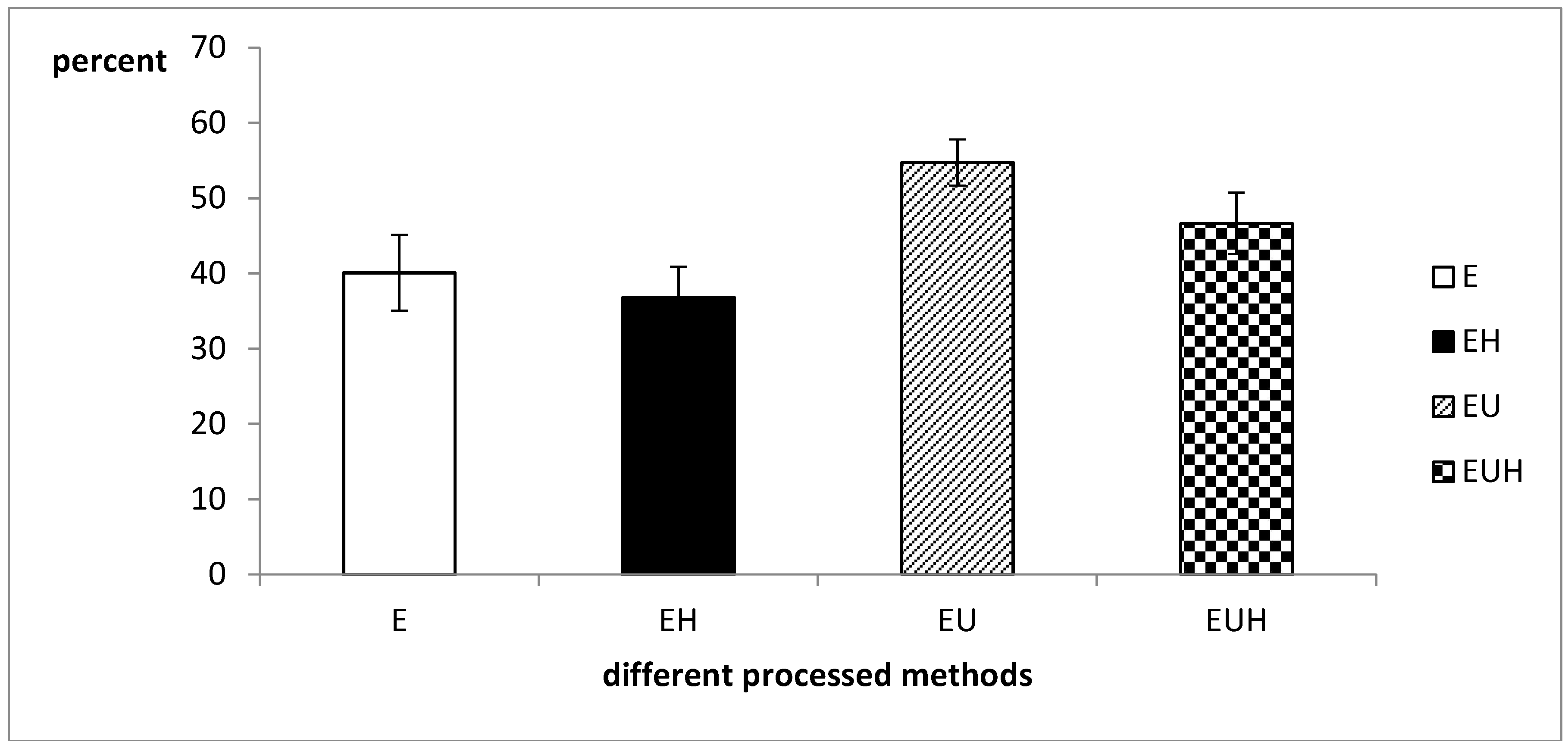
2.3. Total Protein Absorption Difference
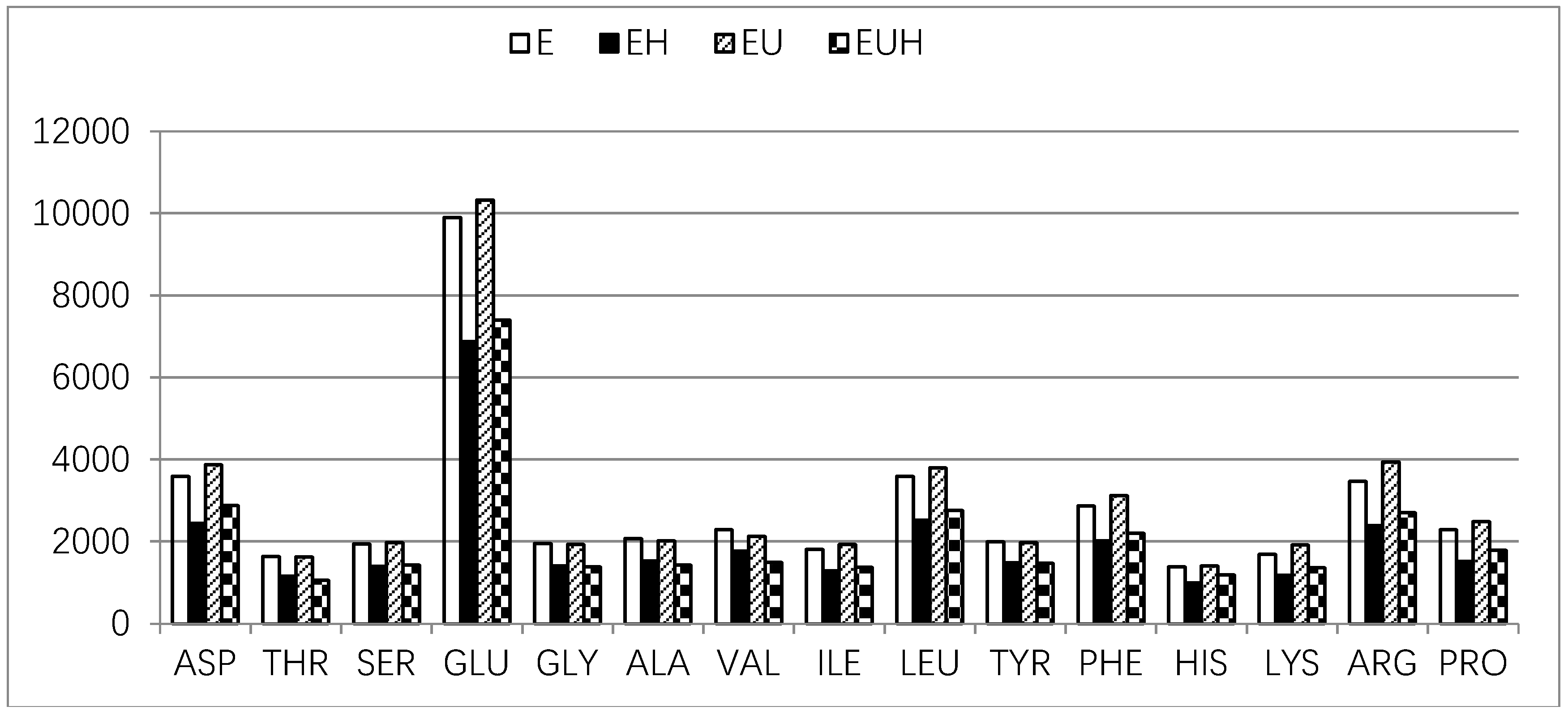
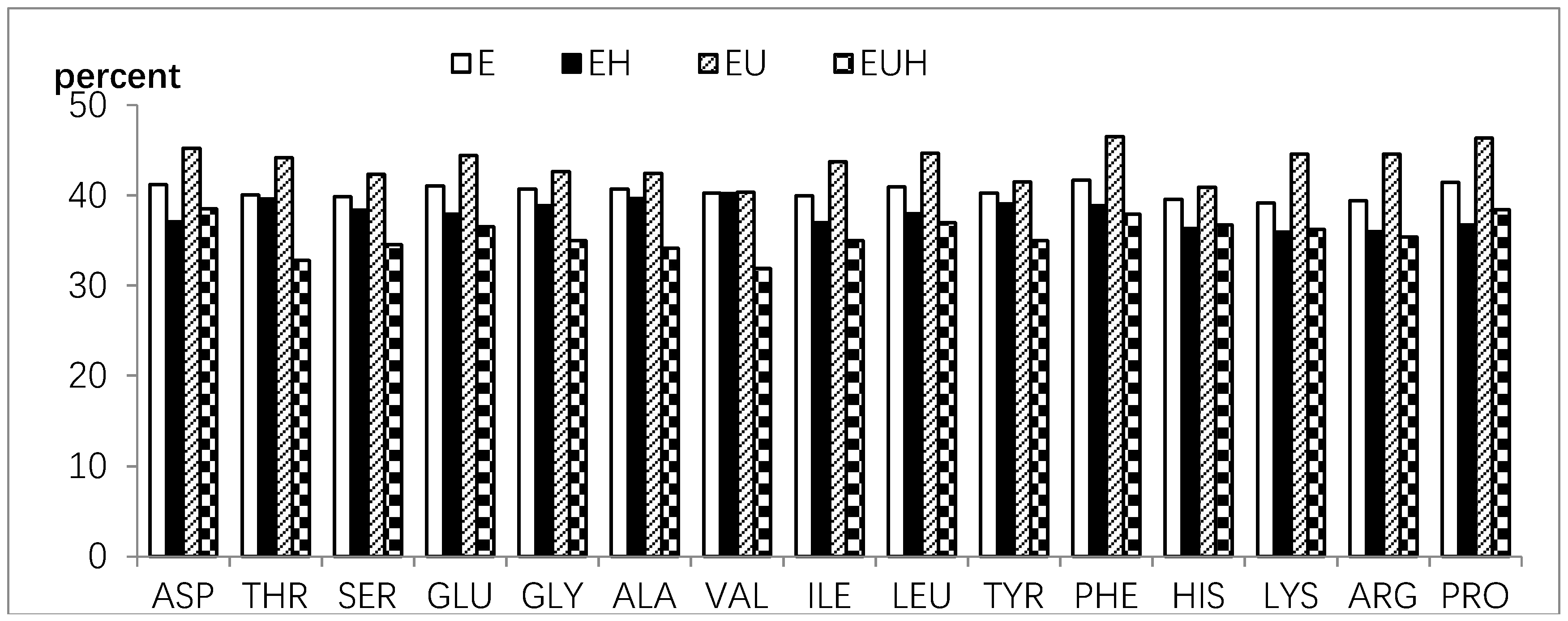
2.4. Amino Acid Absorption Difference
3. Discussion
4. Materials and Methods
4.1. Protein Preparation
4.2. Preparation of Protein Hydrolysates
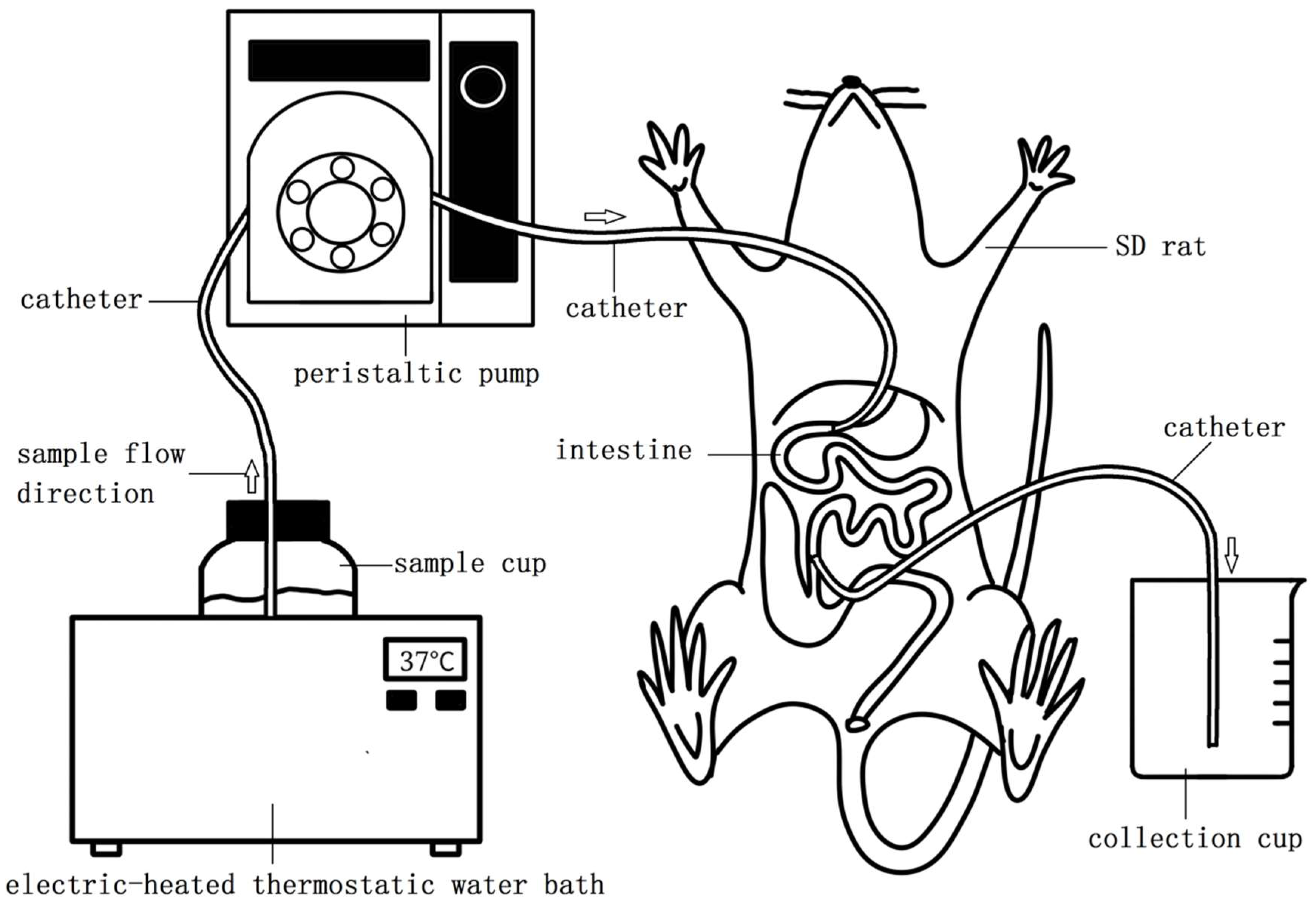
4.3. Small Intestinal Model (SIM)
4.4. In Vivo Intestinal Food Running Speed Measurement
4.5. Protein Absorption Change with the Time
4.6. Total Protein and Amino Acid Absorption
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manikkam, V.; Vasiljevic, T.; Donkor, O.N.; Mathai, M.L. A Review of Potential Marine-derived Hypotensive and Anti-obesity Peptides. Crit. Rev. Food Sci. Nutr. 2016, 56, 92–112. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rivera, L.; Martinez-Maqueda, D.; Cruz-Huerta, E.; Miralles, B.; Recio, I. Peptidomics for discovery, bioavailability and monitoring of dairy bioactive peptides. Food Res. Int. 2014, 63, 170–181. [Google Scholar] [CrossRef]
- Jin, J.; Ma, H.L.; Zhou, C.S.; Luo, M.; Liu, W.; Qu, W.J.; He, R.H.; Luo, L.; Yagoub, A.A. Effect of degree of hydrolysis on the bioavailability of corn gluten meal hydrolysates. J. Sci. Food Agric. 2015, 95, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015, 52, 5377–5392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; McClements, D.J. Control of protein digestion under simulated gastrointestinal conditions using biopolymer microgels. Food Res. Int. 2017, 100, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Tharakan, A.; Norton, I.T.; Fryer, P.J.; Bakalis, S. Mass transfer and nutrient absorption in a simulated model of small intestine. J. Food Sci. 2010, 75, E339–E346. [Google Scholar] [CrossRef] [PubMed]
- Jaime-Fonseca, M.R.; Gouseti, O.; Fryer, P.J.; Wickham, M.S.; Bakalis, S. Digestion of starch in a dynamic small intestinal model. Eur. J. Nutr. 2016, 55, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Boland, M. Human digestion—A processing perspective. J. Sci. Food Agric. 2016, 96, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Macierzanka, A.; Bottger, F.; Rigby, N.M.; Lille, M.; Poutanen, K.; Mills, E.N.C.; Mackie, A.R. Enzymatically Structured Emulsions in Simulated Gastrointestinal Environment: Impact on Interfacial Proteolysis and Diffusion in Intestinal Mucus. Langmuir 2012, 28, 17349–17362. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Venema, K.; Havenaar, R.; Minekus, M. Improving in vitro simulation of the stomach and intestines. In Designing Functional Foods: Measuring and Controlling Food Structure Breakdown and Nutrient Absorption; McClements, D.J., Decker, E.A., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 314–339. [Google Scholar]
- Verwei, M.; Minekus, M.; Zeijdner, E.; Schilderink, R.; Havenaar, R. Evaluation of two dynamic in vitro models simulating fasted and fed state conditions in the upper gastrointestinal tract (TIM-1 and tiny-TIM) for investigating the bioaccessibility of pharmaceutical compounds from oral dosage forms. Int. J. Pharm. 2016, 498, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Singh, R.P. A human gastric simulator (HGS) to study food digestion in human stomach. J. Food Sci. 2010, 75, E627–E635. [Google Scholar] [CrossRef] [PubMed]
- Wickham, M.J.S.; Faulks, R.M.; Mann, J.; Mandalari, G. The Design, Operation, and Application of a Dynamic Gastric Model. Dissolut. Technol. 2012, 19, 15–22. [Google Scholar] [CrossRef]
- Kozu, H.; Nakata, Y.; Nakajima, M.; Neves, M.A.; Uemura, K.; Sato, S.; Kobayashi, I.; Ichikawa, S. Development of a Human Gastric Digestion Simulator Equipped with Peristalsis Function for the Direct Observation and Analysis of the Food Digestion Process. Food Sci. Technol. Res. 2014, 20, 225–233. [Google Scholar] [CrossRef]
- Barros, L.; Retamal, C.; Torres, H.; Zúñiga, R.N.; Troncoso, E. Development of an in vitro mechanical gastric system (IMGS) with realistic peristalsis to assess lipid digestibility. Food Res. Int. 2016, 90, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Havenaar, R.; de Jong, A.; Koenen, M.E.; van Bilsen, J.; Janssen, A.M.; Labij, E.; Westerbeek, H.J.M. Digestibility of Transglutaminase Cross-Linked Caseinate versus Native Caseinate in an In Vitro Multicompartmental Model Simulating Young Child and Adult Gastrointestinal Conditions. J. Agric. Food Chem. 2013, 61, 7636–7644. [Google Scholar] [CrossRef] [PubMed]
- Argyri, K.; Birba, A.; Miller, D.D.; Komaitis, M.; Kapsokefalou, M. Predicting relative concentrations of bioavailable iron in foods using in vitro digestion: New developments. Food Chem. 2009, 113, 602–607. [Google Scholar] [CrossRef]
- Menezes, E.A.; Oliveira, A.F.; Franca, C.J.; Souza, G.B.; Nogueira, A.R.A. Bioaccessibility of Ca, Cu, Fe, Mg, Zn, and crude protein in beef, pork and chicken after thermal processing. Food Chem. 2018, 240, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Shiowatana, J.; Kitthikhun, W.; Sottimai, U.; Promchan, J.; Kunajiraporn, K. Dynamic continuous-flow dialysis method to simulate intestinal digestion for in vitro estimation of mineral bioavailability of food. Talanta 2006, 68, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Awati, A.; Rutherfurd, S.M.; Plugge, W.; Reynolds, G.W.; Marrant, H.; Kies, A.K.; Moughan, P.J. Ussing chamber results for amino acid absorption of protein hydrolysates in porcine jejunum must be corrected for endogenous protein. J. Sci. Food Agric. 2009, 89, 1857–1861. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Yuan-Qing, H.; Chao-Yue, M.; Ye, P.; Li-Jing, Y.; Jie, Z.; Yuqing, D.; Haihui, Z.; Haile, M. Bioavailability of corn gluten meal hydrolysates and their effects on the immune system. Czech J. Food Sci. 2018, 36, 1–7. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- Saadi, S.; Saari, N.; Anwar, F.; Hamid, A.A.; Mohd Ghazali, H. Recent advances in food biopeptides: Production, biological functionalities and therapeutic applications. Biotechnol. Adv. 2015, 33, 80–116. [Google Scholar] [CrossRef] [PubMed]
- Mourad, F.H.; Saade, N.E. Neural regulation of intestinal nutrient absorption. Prog. Neurobiol. 2011, 95, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e2. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Burmann, J.; Fassbender, K.; Schwiertz, A.; Schafer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
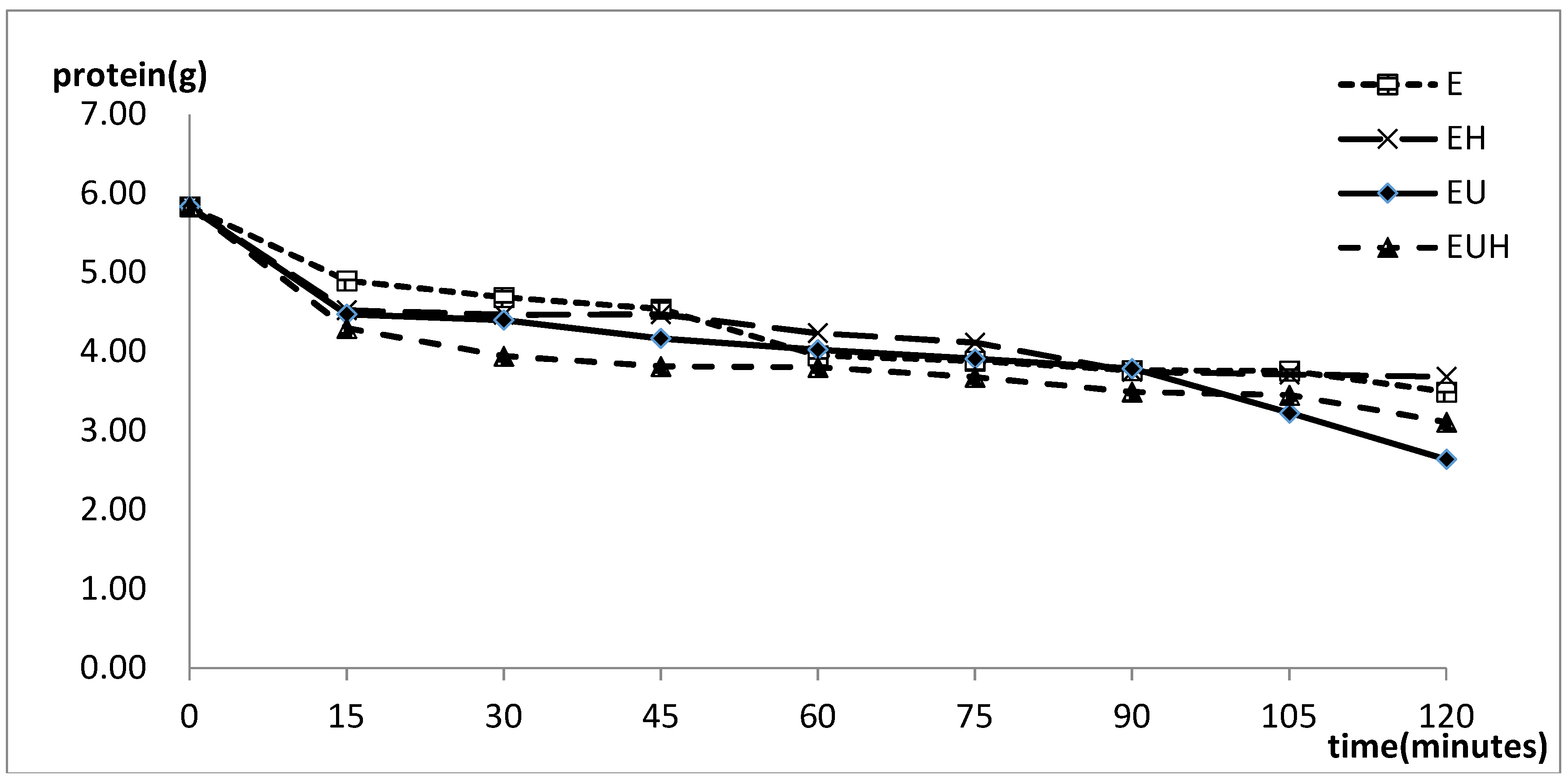
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Shen, L.; Ma, C.; Chen, M.; Pan, Y.; Yin, L.; Zhou, J.; Lei, X.; Ren, Q.; Duan, Y.; et al. Protein Hydrolysates’ Absorption Characteristics in the Dynamic Small Intestine In Vivo. Molecules 2018, 23, 1591. https://doi.org/10.3390/molecules23071591
He Y, Shen L, Ma C, Chen M, Pan Y, Yin L, Zhou J, Lei X, Ren Q, Duan Y, et al. Protein Hydrolysates’ Absorption Characteristics in the Dynamic Small Intestine In Vivo. Molecules. 2018; 23(7):1591. https://doi.org/10.3390/molecules23071591
Chicago/Turabian StyleHe, Yuanqing, Lingling Shen, Chaoyue Ma, Min Chen, Ye Pan, Lijing Yin, Jie Zhou, Xiaochun Lei, Qian Ren, Yuqing Duan, and et al. 2018. "Protein Hydrolysates’ Absorption Characteristics in the Dynamic Small Intestine In Vivo" Molecules 23, no. 7: 1591. https://doi.org/10.3390/molecules23071591
APA StyleHe, Y., Shen, L., Ma, C., Chen, M., Pan, Y., Yin, L., Zhou, J., Lei, X., Ren, Q., Duan, Y., Zhang, H., & Ma, H. (2018). Protein Hydrolysates’ Absorption Characteristics in the Dynamic Small Intestine In Vivo. Molecules, 23(7), 1591. https://doi.org/10.3390/molecules23071591





