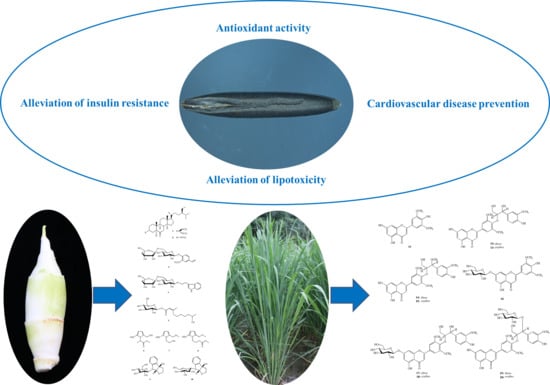
Morphological Characteristics, Nutrients, and Bioactive Compounds of Zizania latifolia, and Health Benefits of Its Seeds
Abstract
:1. Introduction
2. Methods Used in Searching Research Paper
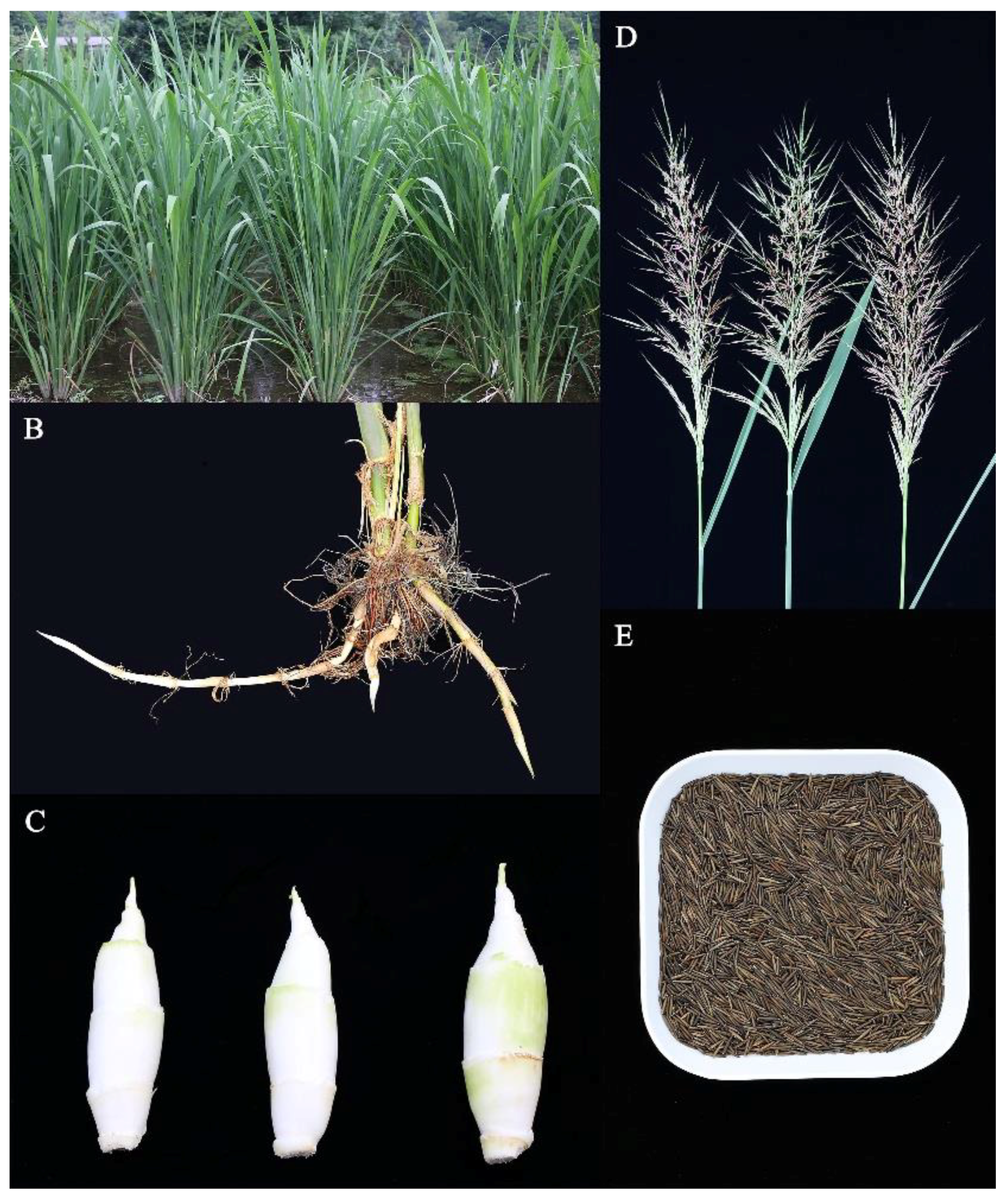
3. Morphological Characteristics of the Z. latifolia
4. History and Present Situation of the Z. latifolia Seeds (Chinese Wild Rice)
5. Nutrients and Bioactive Compounds of Chinese Wild Rice
5.1. Nutrients of Chinese Wild Rice
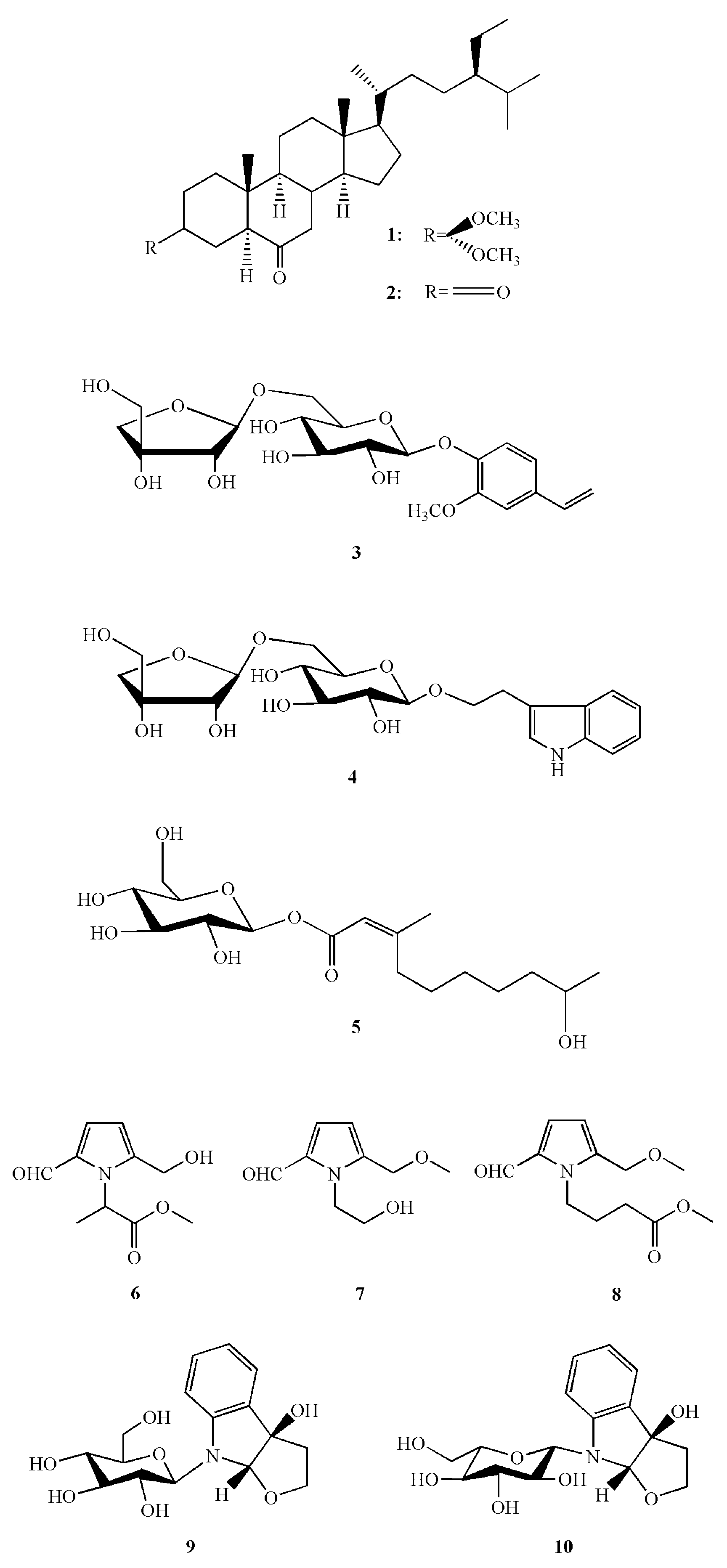
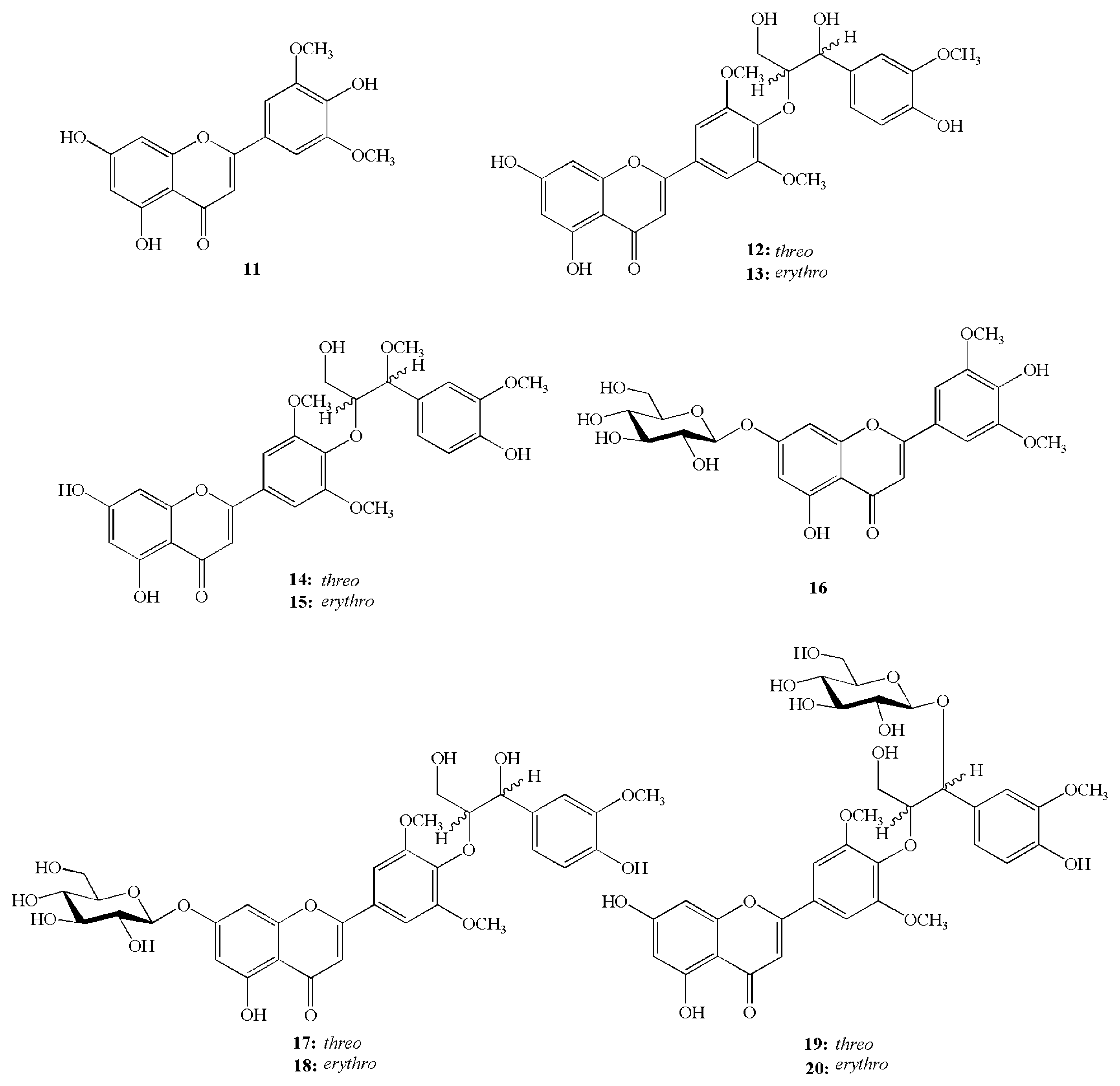
5.2. Bioactive Compounds in Chinese Wild Rice
6. Bioactivity and Health Benefits of Chinese Wild Rice
6.1. Antioxidant Activity
6.2. Alleviation of Insulin Resistance and Lipotoxicity
6.3. Cardiovascular Disease Prevention
7. Nutrients and Bioactive Compounds of the Swollen Culm of Z. latifolia
8. Nutrients and Bioactive Compounds of the Aerial Parts of Z. latifolia
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, D.; Li, K.; Gao, J.; Liu, Y.; Gao, L.Z. The complete plastid genome sequence of the wild rice Zizania latifolia and comparative chloroplast genomics of the rice Tribe Oryzeae, Poaceae. Front. Ecol. Evol. 2016, 4, 88. [Google Scholar] [CrossRef]
- Sahoo, D.; Ummalyma, S.B.; Kumar, O.A.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Surendiran, G.; Alsaif, M.; Kapourchali, F.R.; Moghadasian, M.H. Nutritional constituents and health benefits of wild rice (Zizania spp.). Nutr. Rev. 2014, 72, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Zengin, G.; Alkharfy, K.M.; Marcu, M. Wild rice (Zizania sp.): A potential source of valuable ingredients for nutraceuticals and functional foods. Riv. Ital. Sostanze Gr. 2017, 565, 81–89. [Google Scholar]
- Xu, X.; Wu, J.; Qi, M.; Lu, Q.; Lee, P.F.; Lutz, S.; Ge, S.; Wen, J. Comparative phylogeography of the wild-rice genus Zizania (Poaceae) in eastern Asia and North America. Am. J. Bot. 2015, 102, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.R.; Ren, X.R.; Liu, Y.L.; Chen, Y.Y. Genetic structure of wild rice Zizania latifolia and the implications for its management in the Sanjiang Plain, Northeast China. Biochem. Syst. Ecol. 2016, 64, 81–88. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Fan, X.; Li, W.; Liu, Y. Landscape-scale genetic structure of wild rice Zizania latifolia: The roles of rivers, mountains, and fragmentation. Front. Ecol. Evol. 2017, 5, 17. [Google Scholar] [CrossRef]
- Xu, X.; Walters, C.; Antolin, M.F.; Alexander, M.L.; Lutz, S.; Ge, S.; Wen, J. Phylogeny and biogeography of the eastern Asian-North American disjunct wild-rice genus (Zizania L., Poaceae). Mol. Phylogenet. Evol. 2010, 55, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, J.; Liu, F.; Li, W. Morphological changes and resource allocation of Zizania latifolia (Griseb.) Stapf in response to different submergence depth and duration. Flora 2014, 209, 279–284. [Google Scholar] [CrossRef]
- Asaeda, T.; Fujino, T.; Manatunge, J. Morphological adaptations of emergent plants to water flow: A case study with Typha angustifolia, Zizania latifolia, and Phragmites australis. Freshw. Biol. 2005, 50, 1991–2001. [Google Scholar] [CrossRef]
- Hung, L.Q.; Asaeda, T.; Fujino, T.; Mnaya, B.J. Inhibition of Zizania latifolia growth by Phragmites australis: An experimental study. Wetl. Ecol. Manag. 2007, 15, 105–111. [Google Scholar] [CrossRef]
- Kwon, G.J.; Lee, B.A.; Byun, C.H.; Nam, J.M.; Kim, J.G. The optimal environmental ranges for wetland plants: I. Zizania latifolia and Typha angustifolia. J. Korea Soc. Environ. Restor. Technol. 2006, 9, 72–88. [Google Scholar]
- Guo, H.; Li, S.; Peng, J.; Ke, W. Zizania latifolia Turcz. cultivated in China. Genet. Resour. Crop Evol. 2007, 54, 1211–1217. [Google Scholar] [CrossRef]
- Yan, N.; Wang, X.; Wang, Z.; Zhang, Y.; Xue, H.; Guo, D. Antioxidative system and chlorophyll fluorescence of Zizania latifolia Turcz. plants are affected by Ustilago esculenta infection. Aata Ecol. Sin. 2013, 33, 1584–1593. [Google Scholar]
- Yan, N.; Wang, X.; Xu, X.; Guo, D.; Wang, Z.; Zhang, J.; Hyde, K.D.; Liu, H. Plant growth and photosynthetic performance of Zizania latifolia are altered by endophytic Ustilago esculenta infection. Physiol. Mol. Plant Pathol. 2013, 83, 75–83. [Google Scholar] [CrossRef]
- Yan, N.; Xu, X.; Wang, Z.; Huang, J.; Guo, D. Interactive effects of temperature and light intensity on photosynthesis and antioxidant enzyme activity in Zizania latifolia Turcz. plants. Photosynthetica 2013, 51, 127–138. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Chu, F.Q.; Guo, D.P.; Hyde, K.D.; Xie, G.L. Cytology and ultrastructure of interactions between Ustilago esculenta and Zizania latifolia. Mycol. Prog. 2012, 11, 499–508. [Google Scholar] [CrossRef]
- Guo, L.; Qiu, J.; Han, Z.; Ye, Z.; Chen, C.; Liu, C.; Xin, X.; Ye, C.Y.; Wang, Y.Y.; Xie, H.; et al. A host plant genome (Zizania latifolia) after a century-long endophyte infection. Plant J. 2015, 83, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Pan, Y.; Zhang, Y.; Cui, H.; Jin, G.; McHardy, A.C.; Fan, L.; Yu, X. Comparative whole-genome analysis reveals artificial selection effects on Ustilago esculenta genome. DNA Res. 2017, 24, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.R.; Tzeng, D.D. Biosynthesis of indole-3-acetic acid by the gall-inducing fungus Ustilago esculenta. J. Biol. Sci. 2004, 4, 744–750. [Google Scholar]
- Wang, Z.D.; Yan, N.; Wang, Z.H.; Zhang, X.H.; Zhang, J.Z.; Xue, H.M.; Wang, L.X.; Zhan, Q.; Xu, Y.P.; Guo, D.P. RNA-seq analysis provides insight into reprogramming of culm development in Zizania latifolia induced by Ustilago esculenta. Plant Mol. Biol. 2017, 95, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Zhang, Y.; Xue, H.; Zhang, X.; Wang, Z.; Shi, L.; Guo, D. Changes in plant growth and photosynthetic performance of Zizania latifolia exposed to different phosphorus concentrations under hydroponic condition. Photosynthetica 2015, 53, 630–635. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Fan, L. Main agronomic traits, domestication and breeding of Gu (Zizania latifolia). J. Zhejiang Univ. (Agric. Life Sci.) 2013, 39, 629–635. [Google Scholar]
- Zhai, C.; Tang, W.; Jang, X.; Lorenz, K. Studies of the safety of Chinese wild rice. Food Chem. Toxicol. 1996, 34, 347–352. [Google Scholar] [CrossRef]
- Chen, Y.; Chu, H.; Liu, H.; Liu, Y. Abundant genetic diversity of the wild rice Zizania latifolia in central China revealed by microsatellites. Ann. Appl. Biol. 2012, 161, 192–201. [Google Scholar] [CrossRef]
- Zhai, C.; Sun, G.; Lu, C.; Jiang, Z.; Zhang, X. On Chinese Zizania L. resources and their utilization value. Resour. Sci. 2000, 22, 22–26. [Google Scholar]
- Zhang, X.; Wan, A.; Wang, H.; Zhu, L.; Yin, J.; Liu, Z.; Yu, D. The overgrowth of Zizania latifolia in a subtropical floodplain lake: Changes in its distribution and possible water level control measures. Ecol. Eng. 2016, 84, 119–120. [Google Scholar] [CrossRef]
- Jiang, M.X.; Zhai, L.J.; Yang, H.; Zhai, S.M.; Zhai, C.K. Analysis of active components and proteomics of Chinese wild rice (Zizania latifolia (Griseb) Turcz) and Indica Rice (Nagina22). J. Med. Food 2016, 19, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Zhang, X.; Sun, G.; Lu, C.; Jiang, Z. Study on nutrition composition and protein quality of a Chinese wild rice. J. Hyg. Res. 2000, 29, 375–378. [Google Scholar]
- Zhai, C.; Jiang, X.; Xu, Y.; Lorenz, K. Protein and amino acid composition of Chinese and North American wild rice. LWT Food Sci. Technol. 1994, 7, 380–383. [Google Scholar] [CrossRef]
- Zhai, C.; Lu, C.; Zhang, X.; Sun, G.; Lorenz, K. Comparative study on nutritional value of Chinese and North American wild rice. J. Food Compos. Anal. 2001, 14, 371–382. [Google Scholar] [CrossRef]
- Qian, B.; Luo, Y.; Deng, Y.; Cao, L.; Yang, H.; Shen, Y.; Ping, J. Chemical composition, angiotensin-converting enzyme-inhibitory activity and antioxidant activities of few-flower wild rice (Zizania latifolia Turcz.). J. Sci. Food Agric. 2012, 92, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, L.; Wu, J.; Zhang, Z.; Yu, G.; Zheng, W.; Yang, G.; Cai, Z. Analysis of nutritional quality and evaluation of quality and safety of Jiaobai. J. Zhejiang Agric. Sci. 2015, 56, 98–101. [Google Scholar]
- Zhou, C.; Li, C. A review of the nutrition and utilization of Jiaobai in China. J. Changjiang Veg. 2017, 20, 32–35. [Google Scholar]
- Przybylski, R.; Klensporf-Pawlik, D.; Anwar, F.; Rudzinska, M. Lipid components of North American wild rice (Zizania palustris). J. Am. Oil Chem. Soc. 2009, 86, 553–559. [Google Scholar] [CrossRef]
- Aladedunye, F.; Przybylski, R.; Rudzinska, M.; Klensporf-Pawlik, D. γ-Oryzanols of North American wild rice (Zizania palustris). J. Am. Oil Chem. Soc. 2013, 90, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Bunzel, M.; Allerdings, E.; Sinwell, V.; Ralph, J.; Steinhart, H. Cell wall hydroxycinnamates in wild rice (Zizania aquatica L.) insoluble dietary fibre. Eur. Food Res. Technol. 2002, 214, 482–488. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, Q.; Beta, T. Antioxidant activity of commercial wild rice and identification of flavonoid compounds in active fractions. J. Agric. Food Chem. 2009, 57, 7543–7551. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liu, Q.; Beta, T. Antioxidant properties of commercial wild rice and analysis of soluble and insoluble phenolic acids. Food Chem. 2010, 121, 140–147. [Google Scholar] [CrossRef]
- Deng, G.; Xu, X.; Zhang, Y.; Li, D.; Gan, R.; Li, H. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. A review on structure–activity relationship of dietary polyphenols inhibiting α-amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. Advance in dietary polyphenols as aldose reductases inhibitors: Structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2015, 55, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant properties of diverse cereal grains: A review on in vitro and in vivo studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, W.; Addis, P.B.; Epley, R.J.; Salih, A.M.; Lehrfeld, J. Antioxidant properties of wild rice. J. Agric. Food Chem. 1994, 42, 34–37. [Google Scholar] [CrossRef]
- Sumczynski, D.; Kotásková, E.; Orsavová, J.; Valášek, P. Contribution of individual phenolics to antioxidant activity and in vitro digestibility of wild rices (Zizania aquatica L.). Food Chem. 2017, 218, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, P.; Agellon, L.B.; Zhai, C. Wild rice (Zizania latifolia (Griseb) Turcz) improves the serum lipid profile and antioxidant status of rats fed with a high fat/cholesterol diet. Br. J. Nutr. 2009, 102, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, C. Effects of Chinese and North American wild rice on blood lipids, oxidative stress, and inflammation factors in hyperlipidemic rats. Cereal Chem. 2016, 93, 357–363. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Zhang, H.; Zhai, C.; Chi, J.; Tan, Q. Effect of Chinese wild rice on insulin in resistance in rats induced by high-fat/cholesterol diets. Acta Nutrimenta Sin. 2012, 34, 449–453. [Google Scholar]
- Zhang, H.; Liu, Y.; Zhao, J.; Zhai, C. Determination of the glycemic index of the wild rice and the effects of wild rice on insulin resistance in rats. J. Hyg. Res. 2015, 44, 173–178. [Google Scholar]
- Han, S.; Zhang, H.; Qin, L.; Zhai, C. Effects of dietary carbohydrate replaced with wild rice (Zizania latifolia (Griseb) Turcz) on insulin resistance in rats fed with a high-fat/cholesterol diet. Nutrients 2013, 5, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, P.; Zhai, C.; Ding, Z.; Guo, Y.; Zhang, Q. Effect of Chinese wild rice on lipid metabolism and inflammatory factors in rats fed with high cholesterol diets. Acta Nutrimenta Sin. 2009, 31, 222–225. [Google Scholar]
- Zhang, H.; Han, S.; Cao, P.; Zhai, C. Effect of Chinese wild rice on lipid metabolism and lipotoxicity in rats fed with high fat/cholesterol diet. J. Hyg. Res. 2013, 42, 190–195. [Google Scholar]
- Han, S.; Zhang, H.; Zhai, C. Protective potentials of wild rice (Zizania latifolia (Griseb) Turcz) against obesity and lipotoxicity induced by a high-fat/cholesterol diet in rats. Food Chem. Toxicol. 2012, 50, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Zhai, C.; Jin, X.; Zhang, H.; Han, S.; Tan, Q. Effect of Chinese wild rice on formation of atherosclerosis in rats. Acta Nutrimenta Sin. 2012, 34, 576–581. [Google Scholar]
- Schaffer, J.E. Lipotoxicity: Many roads to cell dysfunction and cell death: Introduction to a thematic review series. J. Lipid Res. 2016, 57, 1327–1328. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sánchez-Zapata, E.; Pérez-Álvarez, J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- Surendiran, G.; Goh, C.; Le, K.; Zhao, Z.; Askarian, F.; Othman, R.; Nicholson, T.; Moghadasian, P.; Wang, Y.J.; Aliani, M.; et al. Wild rice (Zizania palustris L.) prevents atherogenesis in LDL receptor knockout mice. Atherosclerosis 2013, 230, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Moghadasian, M.H.; Alsaif, M.; Le, K.; Gangadaran, S.; Masisi, K.; Beta, T.; Shen, G.X. Combination effects of wild rice and phytosterols on prevention of atherosclerosis in LDL receptor knockout mice. J. Nutr. Biochem. 2016, 33, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Moghadasian, M.H.; Zhao, R.; Ghazawwi, N.; Le, K.; Apea-Bah, F.B.; Beta, T.; Shen, G.X. Inhibitory effects of North American wild rice on monocyte adhesion and inflammatory modulators in low-density lipoprotein receptor-knockout mice. J. Agric. Food Chem. 2017, 65, 9054–9060. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.C.; Goyari, S.; Louis, B.; Waikhom, S.D.; Handique, P.J.; Talukdar, N.C. Investigation on the biotrophic interaction of Ustilago esculenta on Zizania latifolia found in the Indo-Burma biodiversity hotspot. Microb. Pathog. 2016, 98, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, Q.; Hu, P.; Cui, H.; Yu, X.; Ye, Z. Investigation on the differentiation of two Ustilago esculenta strains-implications of a relationship with the host phenotypes appearing in the fields. BMC Microbiol. 2017, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Singh, H.B.; Bhattacharyya, P.R. The ethnobotany and nutritional values of wild rice [Zizania latifolia (Griseb.) Turcz. ex Stapf](Poaceae) in Manipur. Indian J. Tradit. Knowl. 2012, 11, 66–69. [Google Scholar]
- Choi, J.H.; Suzuki, T.; Kawaguchi, T.; Yamashita, K.; Morita, A.; Masuda, K.; Yazawa, K.; Hirai, H.; Kawagishi, H. Makomotines A to D from Makomotake, Zizania latifolia infected with Ustilago esculenta. Tetrahedron Lett. 2014, 55, 3596–3599. [Google Scholar] [CrossRef]
- Kawagishi, H.; Hota, K.; Masuda, K.; Yamaguchi, K.; Yazawa, K.; Shibata, K.; Uzuka, N.; Matahira, Y. Osteoclast-forming suppressive compounds from Makomotake, Zizania latifolia infected with Ustilago esculenta. Biosci. Biotechol. Biochem. 2006, 70, 2800–2802. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Choi, J.H.; Kawaguchi, T.; Yamashita, K.; Morita, A.; Hirai, H.; Nagai, K.; Hirose, T.; Omura, S.; Sunazuka, T.; et al. Makomotindoline from Makomotake, Zizania latifolia infected with Ustilago esculenta. Bioorg. Med. Chem. Lett. 2012, 22, 4246–4248. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, P.; Zhao, S.; Nie, C.; Wang, N.; Du, X.; Zhou, Y. Characterization, antioxidant activity and immunomodulatory activity of polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2017, 95, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, S.; Zhu, P.; Nie, C.; Ma, S.; Wang, N.; Du, X.; Zhou, Y. Purification, characterization and immunomodulatory activity of water extractable polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2018, 107, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liang, S.; Gao, J.; Ngo, H.H.; Guo, W.; Guo, Z.; Wang, J.; Li, Y. Enhancement of Cr (VI) removal by modifying activated carbon developed from Zizania caduciflora with tartaric acid during phosphoric acid activation. Chem. Eng. J. 2014, 246, 168–174. [Google Scholar] [CrossRef]
- Kim, N.H.; Moon, P.D.; Pak, S.C.; Kim, H.M.; Jeong, H.J. Anti-fatigue effect of Zizania caudiflora (Turczaninow) Nakai. Am. J. Chin. Med. 2012, 40, 111. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Whang, E.Y.; Whang, K.; Lee, I.S.; Yang, S.A. Anti-allergic effect of Zizania latifolia Turcz extracts. Korean J. Food. Sci. Technol. 2009, 41, 717–721. [Google Scholar]
- Lee, S.S.; Baek, N.I.; Baek, Y.S.; Chung, D.K.; Song, M.C.; Bang, M.H. New flavonolignan glycosides from the aerial parts of Zizania latifolia. Molecules 2015, 20, 5616–5624. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Baek, Y.S.; Eun, C.S.; Yu, M.H.; Baek, N.I.; Chung, D.K.; Bang, M.H.; Yang, S.A. Tricin derivatives as anti-inflammatory and anti-allergic constituents from the aerial part of Zizania latifolia. Biosci. Biotechnol. Biochem. 2015, 79, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Yu, M.H.; Garcia, C.V.; Jhee, K.H.; Yang, S.A. Inhibitory effect of Zizania latifolia chloroform fraction on allergy-related mediator production in RBL-2H3 cells. Food Sci. Biotechnol. 2017, 26, 481–487. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Unit per 100 g | Seed [28,31] | Swollen Culm [32,33,34] |
|---|---|---|
| Moisture (g) | 9.51 ± 0.13 | 92.001 ± 0.90 |
| Protein (g) | 13.30 ± 1.38 | 2.818 ± 0.019 |
| Fat (g) | 1.08 ± 0.12 | 2.258 ± 0.071 |
| Total carbohydrates (g) | 73.18 ± 1.68 | 2.43 ± 0.19 |
| Total dietary fibre (g) | 7.24 ± 1.29 | 4.22 ± 0.006 |
| Total minerals (g) | 1.30 ± 0.04 | 0.531 ± 0.001 |
| Vitamin B1 (mg) | 0.59 ± 0.04 | 0.02 |
| Vitamin B2 (mg) | 0.11 ± 0.03 | – |
| Vitamin E (mg) | 0.29 ± 0.11 | 0.99 |
| Alanine (g) | 0.69 ± 0.07 | 0.06 ± 0.008 |
| Arginine (g) | 1.13 ± 0.13 | 0.04 ± 0.005 |
| Aspartic acid (g) | 1.19 ± 0.15 | 0.15 ± 0.028 |
| Cysteine (g) | 0.37 ± 0.02 | – |
| Glutamic acid (g) | 2.40 ± 0.24 | 0.11 ± 0.010 |
| Glycine (g) | 0.59 ± 0.06 | 0.04 ± 0.005 |
| Histidine (g) | 0.38 ± 0.03 | 0.05 ± 0.005 |
| Isoleucine (g) | 0.50 ± 0.05 | 0.04 ± 0.003 |
| Leucine (g) | 0.95 ± 0.10 | 0.07 ± 0.007 |
| Lysine (g) | 0.66 ± 0.07 | 0.07 ± 0.007 |
| Methionine (g) | 0.28 ± 0.01 | 0.01 ± 0.000 |
| Phenylalanine (g) | 0.65 ± 0.08 | 0.04 ± 0.005 |
| Proline (g) | 0.37 ± 0.05 | 0.04 ± 0.005 |
| Serine (g) | 0.66 ± 0.07 | 0.06 ± 0.007 |
| Threonine (g) | 0.44 ± 0.04 | 0.05 ± 0.007 |
| Tryptophan (g) | 0.21 ± 0.03 | – |
| Tyrosine (g) | 0.44 ± 0.05 | 0.03 ± 0.003 |
| Valine (g) | 0.70 ± 0.08 | 0.05 ± 0.005 |
| Calcium (mg) | 23.74 ± 0.47 | 4.00 |
| Chromium (mg) | 0.12 ± 0.03 | – |
| Copper (mg) | 0.22 ± 0.13 | – |
| Iron (mg) | 2.80 ± 0.27 | 0.40 |
| Magnesium (mg) | 114.74 ± 7.21 | 8.00 |
| Manganese (mg) | 1.34 ± 0.11 | 0.49 |
| Phosphorus (mg) | 291.20 ± 37.64 | 36.00 |
| Potassium (mg) | 218.47 ± 11.06 | 209.00 |
| Sodium (mg) | 4.48 ± 0.87 | 5.80 |
| Zinc (mg) | 2.40 ± 0.20 | 0.33 |
| Flavonoids (mg) | 352.00 ± 3.12 | 383.70 |
| Saponins (mg) | 354.11 ± 22.70 | – |
| Anthocyanins (mg) | 258.00 ± 17.31 | – |
| Chlorophyll (mg) | 108.40 ± 2.41 | – |
| Phytosterols (mg) | 71.28 ± 8.12 | – |
| Bioactivity or Health Benefit | Putative Functional Compounds | Potential Mechanism | Reference |
|---|---|---|---|
| Antioxidant activity | Vitamins, minerals, phytosterols, phenolic acid, phytic acid, flavonoids, saponins, anthocyanins, and chlorophyll | Suppressing oxidative stress by increasing antioxidant capacity and superoxide dismutase activity, and reducing malondialdehyde concentration | [47,48] |
| Alleviation of insulin resistance and lipotoxicity | Dietary fibre, resistant starch, vitamins, minerals, polyunsaturated fatty acids, phytosterols, phenolic acids, flavonoids, saponins, anthocyanins, and chlorophyll | (1) Improving glucose metabolism and insulin sensitivity by inhibiting the expression of protein tyrosine phosphatase 1B and enhancing the expression of insulin receptor substrate-2 in the liver (2) Decreasing triglyceride and free fatty acid levels in a liver homogenate; increasing adiponectin, and decreasing lipocalin-2 and visfatin concentrations in the serum (3) Increasing the expression of adiponectin receptor 2, and peroxisome proliferator-activated receptors alpha and gamma; inhibiting the expression of leptin and lipocalin-2 (4) Improving lipid metabolism and liver lipotoxicity, and suppressing low-grade inflammation induced by a high saturated fat and cholesterol diet in hyperlipidaemic rats (5) Suppressing increase in lipid droplet accumulation, and free fatty acid and leptin concentrations; reducing lipoprotein lipase and adipose triglyceride lipase levels (6) Inhibiting expression of sterol-regulatory element binding protein-1c, and fatty acid synthase and acetyl-CoA carboxylase induced by high saturated fat and cholesterol diet | [49,50,51,52,53,54] |
| Cardiovascular disease prevention | Dietary fibre, resistant starch, vitamins, phytosterols, phenolic acids, flavonoids, saponins, anthocyanins, and chlorophyll | (1) Reducing blood lipid levels in rats fed high-fat diet, and inhibiting the formation of atherosclerotic plaques and the occurrence of fatty liver (2) Enhancing antioxidative capacity and preventing atherosclerosis | [55] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, N.; Du, Y.; Liu, X.; Chu, C.; Shi, J.; Zhang, H.; Liu, Y.; Zhang, Z. Morphological Characteristics, Nutrients, and Bioactive Compounds of Zizania latifolia, and Health Benefits of Its Seeds. Molecules 2018, 23, 1561. https://doi.org/10.3390/molecules23071561
Yan N, Du Y, Liu X, Chu C, Shi J, Zhang H, Liu Y, Zhang Z. Morphological Characteristics, Nutrients, and Bioactive Compounds of Zizania latifolia, and Health Benefits of Its Seeds. Molecules. 2018; 23(7):1561. https://doi.org/10.3390/molecules23071561
Chicago/Turabian StyleYan, Ning, Yongmei Du, Xinmin Liu, Cheng Chu, John Shi, Hongbo Zhang, Yanhua Liu, and Zhongfeng Zhang. 2018. "Morphological Characteristics, Nutrients, and Bioactive Compounds of Zizania latifolia, and Health Benefits of Its Seeds" Molecules 23, no. 7: 1561. https://doi.org/10.3390/molecules23071561
APA StyleYan, N., Du, Y., Liu, X., Chu, C., Shi, J., Zhang, H., Liu, Y., & Zhang, Z. (2018). Morphological Characteristics, Nutrients, and Bioactive Compounds of Zizania latifolia, and Health Benefits of Its Seeds. Molecules, 23(7), 1561. https://doi.org/10.3390/molecules23071561