Role of O-Acetylation in the Immunogenicity of Bacterial Polysaccharide Vaccines
Abstract
1. Introduction
2. O-acetylation in Conjugate Vaccines
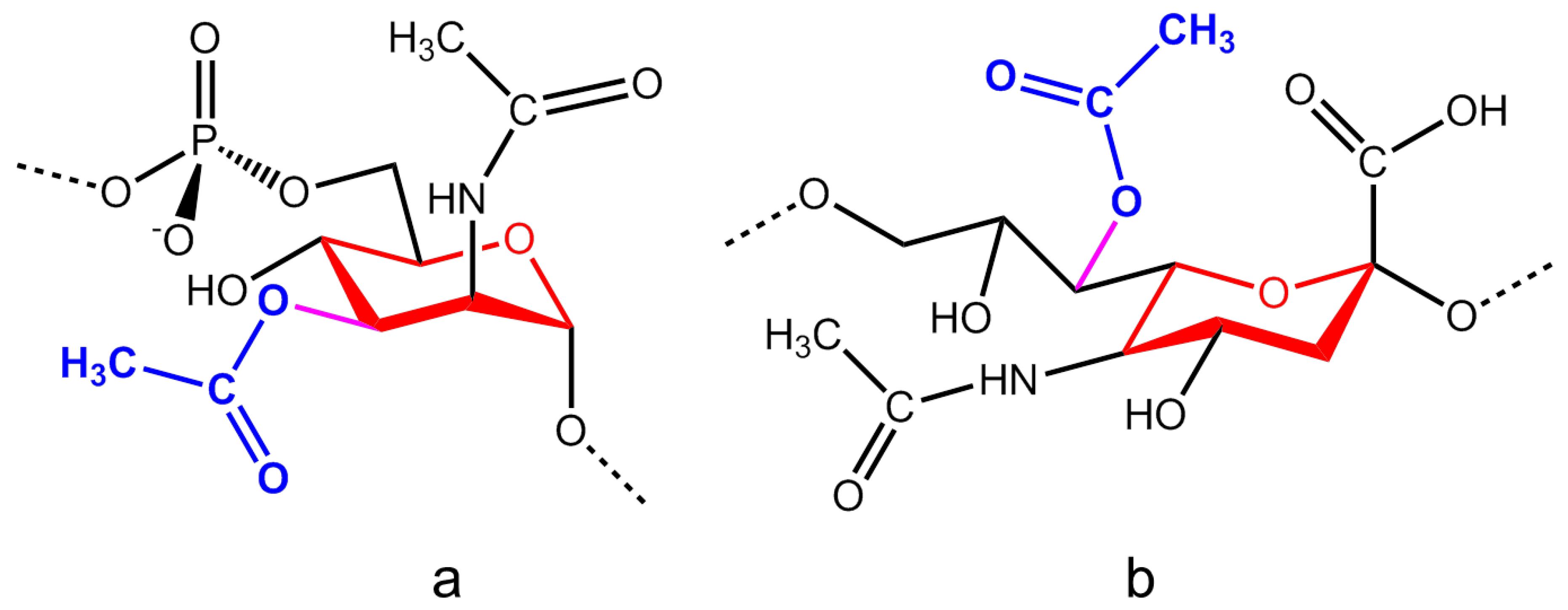
2.1. Neisseria Meningitidis
2.2. Group B Streptococcus
2.3. Streptococcus Pneumoniae
2.4. Salmonella Typhi Vi
2.5. Staphylococcus Aureus
3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Vella, M.; Pace, D. Glycoconjugate vaccines: An update. Expert Opin. Biol. Ther. 2015, 15, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Costantino, P.; Rappuoli, R.; Berti, F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin. Drug Discov. 2011, 6, 1045–1066. [Google Scholar] [CrossRef] [PubMed]
- Jones, C. NMR assays for carbohydrate-based vaccines. J. Pharm. Biomed. Anal. 2005, 38, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Calix, J.J.; Saad, J.S.; Brady, A.M.; Nahm, M.H. Structural characterization of Streptococcus pneumoniae serotype 9A capsule polysaccharide reveals role of glycosyl 6-O-acetyltransferase wcjE in serotype 9V capsule biosynthesis and immunogenicity. J. Biol. Chem. 2012, 287, 13996–14003. [Google Scholar] [CrossRef] [PubMed]
- Calix, J.J.; Brady, A.M.; Du, V.Y.; Saad, J.S.; Nahm, M.H. Spectrum of pneumococcal serotype 11A variants results from incomplete loss of capsule O-acetylation. J. Clin. Microbiol. 2014, 52, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Camilli, R.; Spencer, B.L.; Moschioni, M.; Pinto, V.; Berti, F.; Nahm, M.H.; Pantosti, A. Identification of Streptococcus pneumoniae serotype 11E, serovariant 11Av and mixed populations by high-resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy and flow cytometric serotyping assay (FCSA). PLoS ONE 2014, 9, e100722. [Google Scholar] [CrossRef]
- Jones, C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An. Acad. Bras. Cienc. 2005, 77, 293–324. [Google Scholar] [CrossRef] [PubMed]
- Claus, H.; Borrow, R.; Achtman, M.; Morelli, G.; Kantelberg, C.; Longworth, E.; Frosch, M.; Vogel, U. Genetics of capsule O-acetylation in serogroup C, W-135 and Y meningococci. Mol. Microbiol. 2004, 51, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Bundle, D.R.; Smith, I.C.; Jennings, H.J. Determination of the structure and conformation of bacterial polysaccharides by carbon 13 nuclear magnetic resonance. Studies on the group-specific antigens of Neisseria meningitidis serogroups A and X. J. Biol. Chem. 1974, 249, 2275–2281. [Google Scholar] [PubMed]
- Bröker, M.; Berti, F.; Costantino, P. Factors contributing to the immunogenicity of meningococcal conjugate vaccines. Hum. Vaccin. Immunother. 2016, 12, 1808–1824. [Google Scholar] [CrossRef] [PubMed]
- Gudlavalleti, S.K.; Datta, A.K.; Tzeng, Y.L.; Noble, C.; Carlson, R.W.; Stephens, D.S. The Neisseria meningitidis serogroup A capsular polysaccharide O-3 and O-4 acetyltransferase. J. Biol. Chem. 2004, 279, 42765–42773. [Google Scholar] [CrossRef] [PubMed]
- Bardotti, A.; Averani, G.; Berti, F.; Berti, S.; Carinci, V.; D’Ascenzi, S.; Fabbri, B.; Giannini, S.; Giannozzi, A.; Magagnoli, C.; et al. Physicochemical characterisation of glycoconjugate vaccines for prevention of meningococcal diseases. Vaccine 2008, 26, 2284–2296. [Google Scholar] [CrossRef] [PubMed]
- Harrison, O.B.; Claus, H.; Jiang, Y.; Bennett, J.S.; Bratcher, H.B.; Jolley, K.A.; Corton, C.; Care, R.; Poolman, J.T.; Zollinger, W.D.; et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg. Infect. Dis. 2013, 19, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, T.; Freiberger, F.; Pinto, V.; Romano, M.R.; Black, A.; Litschko, C.; Bethe, A.; Yashunsky, D.; Adamo, R.; Nikolaev, A.; et al. Molecular cloning and functional characterization of components of the capsule biosynthesis complex of Neisseria meningitidis serogroup A: toward in vitro vaccine production. J. Biol. Chem. 2014, 289, 19395–19407. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.S.; Lynn, F.; Lee, C.H.; Frasch, C.E.; Bash, M.C. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect. Immun. 2002, 70, 3707–3713. [Google Scholar] [CrossRef] [PubMed]
- Lupisan, S.; Limkittikul, K.; Sosa, N.; Chanthavanich, P.; Bianco, V.; Baine, Y.; Van der Wielen, M.; Miller, J.M. Meningococcal polysaccharide A O-acetylation levels do not impact the immunogenicity of the quadrivalent meningococcal tetanus toxoid conjugate vaccine: results from a randomized, controlled phase III study of healthy adults aged 18 to 25 years. Clin. Vaccine Immunol. 2013, 20, 1499–1507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Michon, F.; Huang, C.H.; Farley, E.K.; Hronowski, L.; Fusco, P.C. Structure activity studies on group C meningococcal polysaccharide-protein conjugate vaccines: Effect of O-acetylation on the nature of the protective epitope. Dev. Biol. (Basel) 2000, 103, 151–160. [Google Scholar] [PubMed]
- Vodopija, I.; Baklaic, Z.; Hauser, P.; Roelants, P.; André, F.E.; Safary, A. Reactivity and immunogenicity of bivalent (AC) and tetravalent (ACW135Y) meningococcal vaccines containing O-acetyl-negative or O-acetyl-positive group C polysaccharide. Infect. Immun. 1983, 42, 599–604. [Google Scholar] [PubMed]
- Peltola, H.; Safary, A.; Käyhty, H.; Karanko, V.; André, F.E. Evaluation of two tetravalent (ACYW135) meningococcal vaccines in infants and small children: a clinical study comparing immunogenicity of O-acetyl-negative and O-acetyl-positive group C polysaccharides. Pediatrics 1985, 76, 91–96. [Google Scholar] [PubMed]
- Pichichero, M.; Anderson, P.; Gotschlich, E.; Kamm, J.; McMullen, A.; Nielsen, S. Immunogenicity of O-acetyl-negative and -positive polysaccharide vaccines for infections with Neisseria meningitidis group C in infants. J. Infect. Dis. 1985, 152, 850–851. [Google Scholar] [CrossRef] [PubMed]
- Borrow, R.; Findlow, J. Prevention of meningococcal serogroup C disease by NeisVac-C. Expert. Rev. Vaccines 2009, 8, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Fusco, P.C.; Farley, E.K.; Huang, C.H.; Moore, S.; Michon, F. Protective meningococcal capsular polysaccharide epitopes and the role of O acetylation. Clin. Vaccine Immunol. 2007, 14, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Longworth, E.; Fernsten, P.; Mininni, T.L.; Vogel, U.; Claus, H.; Gray, S.; Kaczmarski, E.; Borrow, R. O-acetylation status of the capsular polysaccharides of serogroup Y and W135 meningococci isolated in the UK. FEMS Immunol. Med. Microbiol. 2002, 32, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.K.; Achtman, M.; Alonso, J.M.; Greenwood, B.; Ramsey, M.; Fox, A.; Gray, S.; Kaczmarski, E. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet 2000, 356, 2159. [Google Scholar] [CrossRef]
- Giardina, P.C.; Longworth, E.; Evans-Johnson, R.E.; Bessette, M.L.; Zhang, H.; Borrow, R.; Madore, D.; Fernsten, P. Analysis of human serum immunoglobulin G against O-acetyl-positive and O-acetyl-negative serogroup W135 meningococcal capsular polysaccharide. Clin. Diagn. Lab. Immunol. 2005, 12, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Bohach, G.A.; Shiloach, J.; Norris, S.E.; Freedberg, D.I.; Deobald, C.; Coxon, B.; Robbins, J.B.; Schneerson, R. Conjugates of group A and W135 capsular polysaccharides of Neisseria meningitidis bound to recombinant Staphylococcus aureus enterotoxin C1: preparation, physicochemical characterization, and immunological properties in mice. Infect. Immun. 2005, 73, 7887–7893. [Google Scholar] [CrossRef] [PubMed]
- Gudlavalleti, S.K.; Lee, C.H.; Norris, S.E.; Paul-Satyaseela, M.; Vann, W.F.; Frasch, C.E. Comparison of Neisseria meningitidis serogroup W135 polysaccharide-tetanus toxoid conjugate vaccines made by periodate activation of O-acetylated, non-O-acetylated and chemically de-O-acetylated polysaccharide. Vaccine 2007, 46, 7972–7980. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Nizet, V.; Varki, A. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc. Natl. Acad. Sci. USA 2004, 101, 11123–11128. [Google Scholar] [CrossRef] [PubMed]
- Weiman, S.; Uchiyama, S.; Lin, F.Y.; Chaffin, D.; Varki, A.; Nizet, V.; Lewis, A.L. O-acetylation of sialic acid on group B Streptococcus inhibits neutrophil suppression and virulence. Biochem. J. 2010, 428, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.; Berti, F. Exploring the Group B Streptococcus capsular polysaccharides: the structural diversity provides the basis for development of NMR-based identity assays. J. Pharm. Biomed. Anal. 2014, 98, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pannaraj, P.S.; Edwards, M.S.; Ewing, K.T.; Lewis, A.L.; Rench, M.A.; Baker, C.J. Group B streptococcal conjugate vaccines elicit functional antibodies independent of strain O-acetylation. Vaccine 2009, 27, 4452–4456. [Google Scholar] [CrossRef] [PubMed]
- Carboni, F.; Adamo, R.; Fabbrini, M.; De Ricco, R.; Cattaneo, V.; Brogioni, B.; Veggi, D.; Pinto, V.; Passalacqua, I.; Oldrini, D.; et al. Structure of a protective epitope of group B Streptococcus type III capsular polysaccharide. Proc. Natl. Acad. Sci. USA 2017, 114, 5017–5022. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.B.; Daoust, V.; Carlo, D.J. The specific capsular polysaccharide of Streptococcus pneumoniae type 9V. Can. J. Biochem. 1981, 59, 524–533. [Google Scholar] [CrossRef] [PubMed]
- McNeely, T.B.; Staub, J.M.; Rusk, C.M.; Blum, M.J.; Donnelly, J.J. Antibody responses to capsular polysaccharide backbone and O-acetate side groups of Streptococcus pneumoniae type 9V in humans and rhesus macaques. Infect. Immun. 1998, 66, 3705–3710. [Google Scholar] [PubMed]
- Lugowski, C.; Jennings, H.J. Structural determination of the capsular polysaccharide of Streptococcus pneumoniae type 18C (56). Carbohydr. Res. 1984, 131, 119–129. [Google Scholar] [CrossRef]
- Chang, J.; Serrano, Y.; Garrido, R.; Rodríguez, L.M.; Pedroso, J.; Cardoso, F.; Valdés, Y.; García, D.; Fernández-Santana, V.; Verez-Bencomo, V. Relevance of O-acetyl and phosphoglycerol groups for the antigenicity of Streptococcus pneumoniae serotype 18C capsular polysaccharide. Vaccine 2012, 30, 7090–7096. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, F.G.; Mesenko, M.T.; Martin, D.G.; Perrine, T.D. Physiochemical properties of the Vi antigen before and after mild alkaline hydrolysis. J. Bacteriol. 1967, 94, 1406–1410. [Google Scholar] [PubMed]
- Szewczyk, B.; Taylor, A. Immunochemical properties of Vi antigen from Salmonella typhi Ty2: Presence of two antigenic determinants. Infect. Immun. 1980, 29, 539–544. [Google Scholar] [PubMed]
- Szu, S.C.; Li, X.R.; Stone, A.L.; Robbins, J.B. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect. Immun. 1991, 59, 4555–4561. [Google Scholar] [PubMed]
- Jones, C. Revised structures for the capsular polysaccharides from Staphylococcus aureus Types 5 and 8 components of novel glycoconjugate vaccines. Carbohydr. Res. 2005, 340, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Scully, I.L.; Pavliak, V.; Timofeyeva, Y.; Liu, Y.; Singer, C.; Anderson, A.S. O-acetylation is essential for functional antibody generation against Staphylococcus aureus capsular polysaccharide. Hum. Vaccin. Immunother. 2018, 14, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Shinefield, H.; Black, S.; Fattom, A.; Horwith, G.; Rasgon, S.; Ordonez, J.; Yeoh, H.; Law, D.; Robbins, J.B.; Schneerson, R.; et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 2002, 346, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Fattom, A.I.; Horwith, G.; Fuller, S.; Propst, M.; Naso, R. Development of StaphVAX, a polysaccharide conjugate vaccine against S. aureus infection: from the lab bench to phase III clinical trials. Vaccine 2004, 22, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Fattom, A.; Matalon, A.; Buerkert, J.; Taylor, K.; Damaso, S.; Boutriau, D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum. Vaccin. Immunother. 2015, 11, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Hestrin, S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J. Biol. Chem. 1949, 180, 249–261. [Google Scholar] [PubMed]
- Jones, C.; Lemercinier, X. Use and validation of NMR assays for the identity and O-acetyl content of capsular polysaccharides from Neisseria meningitidis used in vaccine manufacture. J. Pharm. Biomed. Anal. 2002, 30, 1233–1247. [Google Scholar] [CrossRef]
- Lemercinier, X.; Martinez-Cabrera, I.; Jones, C. Use and validation of an NMR test for the identity and O-acetyl content of the Salmonella typhi Vi capsular polysaccharide vaccine. Biologicals 2000, 28, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kao, G.; Tsai, C.M. Quantification of O-acetyl, N-acetyl and phosphate groups and determination of the extent of O-acetylation in bacterial vaccine polysaccharides by high-performance anion-exchange chromatography with conductivity detection (HPAEC-CD). Vaccine 2004, 22, 335–344. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
| Polysaccharide | Repeat Unit |
|---|---|
| Neisseria meningitidis | |
| Group A | →6)-α-d-ManpNAc(3/4OAc)-(1→OPO3→ |
| Group C | →9)-α-d-Neu5Ac(7/8OAc)-(2→ |
| Group W | →6)-α-d-Galp-(1→4)-α-d-Neu5Ac(7/9OAc)-(2→ |
| Group Y | →6)-α-d-Glcp-(1→4)-α-d-Neu5Ac(7/9OAc)-(2→ |
| Group B Streptococcus | |
| Type Ia | →4)-[α-d-Neu5Ac(7/8/9OAc)-(2→3)-β-d-Galp-(1→4)-β-d-GlcpNAc-(1→3)]-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Type Ib | →4)-[α-d-Neu5Ac(7/8/9OAc)-(2→3)-β-d-Galp-(1→3)-β-d-GlcpNAc-(1→3)]-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Type II | →3)-β-d-Glcp-(1→2)-[α-d-Neu5Ac(7/8/9OAc)-(2→3)]-β-d-Galp-(1→4)-β-D-GlcpNAc-(1→3)-[β-d-Galp-(1→6)]-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Type III | →6)-[α-d-Neu5Ac(7/8/9OAc)-(2→3)-β-d-Galp-(1→4)]-β-d-GlcpNAc-(1→3)]-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Type V | →4)-[α-d-Neu5Ac(7/8/9OAc)-(2→3)-β-d-Galp-(1→4)-β-d-GlcpNAc-(1→6)]-α-d-Glcp-(1→4)-[β-d-Glcp-(1→3)]-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Type VI | →6)-[α-d-Neu5Ac(7/8/9OAc)-(2→3)-β-d-Galp-(1→3)]-β-d-Glcp-(1→3)-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Streptococcus pneumoniae | |
| Type 1 | →3)-d-AAT-α-Galp-(1→4)-α-d-GalpA(2/3OAc)-(1→3)-α-d-GalpA-(1→ |
| Type 7F | →6)-[β-d-Galp-(1→2)]-α-d-Galp-(1→3)-β-L-Rhap(2OAc)-(1→4)-β-d-Glcp-(1→3)-[α-d-GlcpNAc-(1→2)-α-L-Rhap-(1→4)]-β-d-GalpNAc-(1→ |
| Type 9V | →4)-α-d-Glcp(2/3OAc)-(1→4)-α-d-GlcpA-(1→3)-α-d-Galp-(1→3)-β-d-ManpNAc(4/6OAc)-(1→4)-β-d-Glcp-(1→ |
| Type 15B | →6)-[α-d-Galp(2/3/4/6OAc)-(1→2)-[Gro-(2→P→3)]-β-d-Galp-(1→2)]-β-d-GlcpNAc-(1→3)-β-d-Galp-(1→4)-β-d-Glcp-(1→ |
| Type 17F | →3)-β-L-Rhap-(1→4)-β-d-Glcp-(1→3)-α-d-Galp-(1→3)-β-L-Rhap(2OAc)-(1→4)-α-L-Rhap-(1→2)-d-Ara-ol-(1→P→ |
| Type 18C | →4)-β-d-Glcp-(1→4)-[α-d-Glcp(6OAc)-(1→2)][Gro-(1→P→3)]-β-d-Galp-(1→4)-α-d-Glcp-(1→3)-β-L-Rhap-(1→ |
| Type 22F | →4)-β-d-GlcpA-(1→4)-[α-d-Glcp-(1→3)]-β-L-Rhap(2OAc)-(1→4)-α-d-Glcp-(1→3)-α-d-Galf-(1→2)-α-L-Rhap-(1→ |
| Type 33F | →3)-β-d-Galp-(1→3)-[α-d-Galp-(1→2)]-α-d-Galp-(1→3)-β-d-Galf-(1→3)-β-d-Glcp-(1→5)-β-d-Galf(2OAc)-(1→ |
| Salmonella enterica | |
| typhi Vi | →)-α-d-GalpNAcA(3OAc)-(1→ |
| Staphylococcus aureus | |
| Type 5 | →4)-β-d-ManpNAcA-(1→4)-α-L-FucpNAc(3OAc)-(1→3)-β-d-FucpNAc-(1→ |
| Type 8 | →3)-β-d-ManpNAcA(4OAc)-(1→3)-α-L-FucpNAc-(1→3)-α-d-FucpNAc-(1→ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berti, F.; De Ricco, R.; Rappuoli, R. Role of O-Acetylation in the Immunogenicity of Bacterial Polysaccharide Vaccines. Molecules 2018, 23, 1340. https://doi.org/10.3390/molecules23061340
Berti F, De Ricco R, Rappuoli R. Role of O-Acetylation in the Immunogenicity of Bacterial Polysaccharide Vaccines. Molecules. 2018; 23(6):1340. https://doi.org/10.3390/molecules23061340
Chicago/Turabian StyleBerti, Francesco, Riccardo De Ricco, and Rino Rappuoli. 2018. "Role of O-Acetylation in the Immunogenicity of Bacterial Polysaccharide Vaccines" Molecules 23, no. 6: 1340. https://doi.org/10.3390/molecules23061340
APA StyleBerti, F., De Ricco, R., & Rappuoli, R. (2018). Role of O-Acetylation in the Immunogenicity of Bacterial Polysaccharide Vaccines. Molecules, 23(6), 1340. https://doi.org/10.3390/molecules23061340





