MAO-A Inhibitory Potential of Terpene Constituents from Ginger Rhizomes—A Bioactivity Guided Fractionation
Abstract
:1. Introduction
2. Results and Discussion
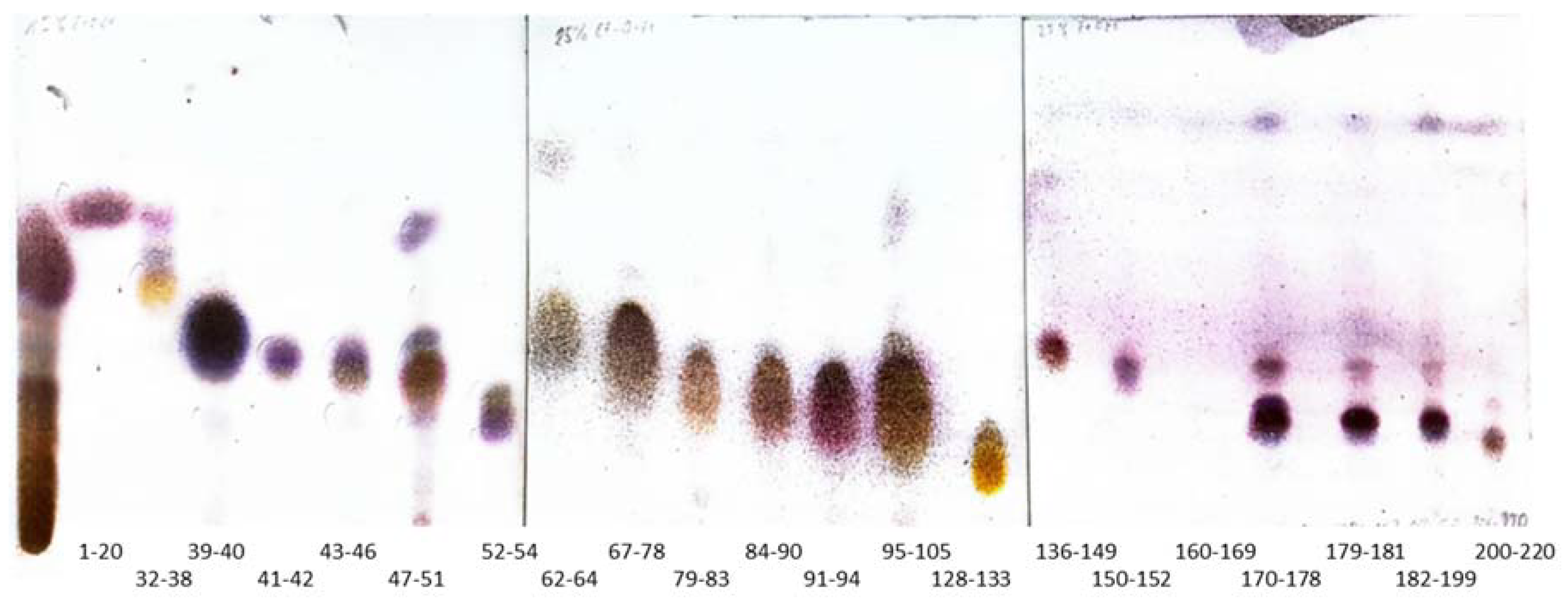
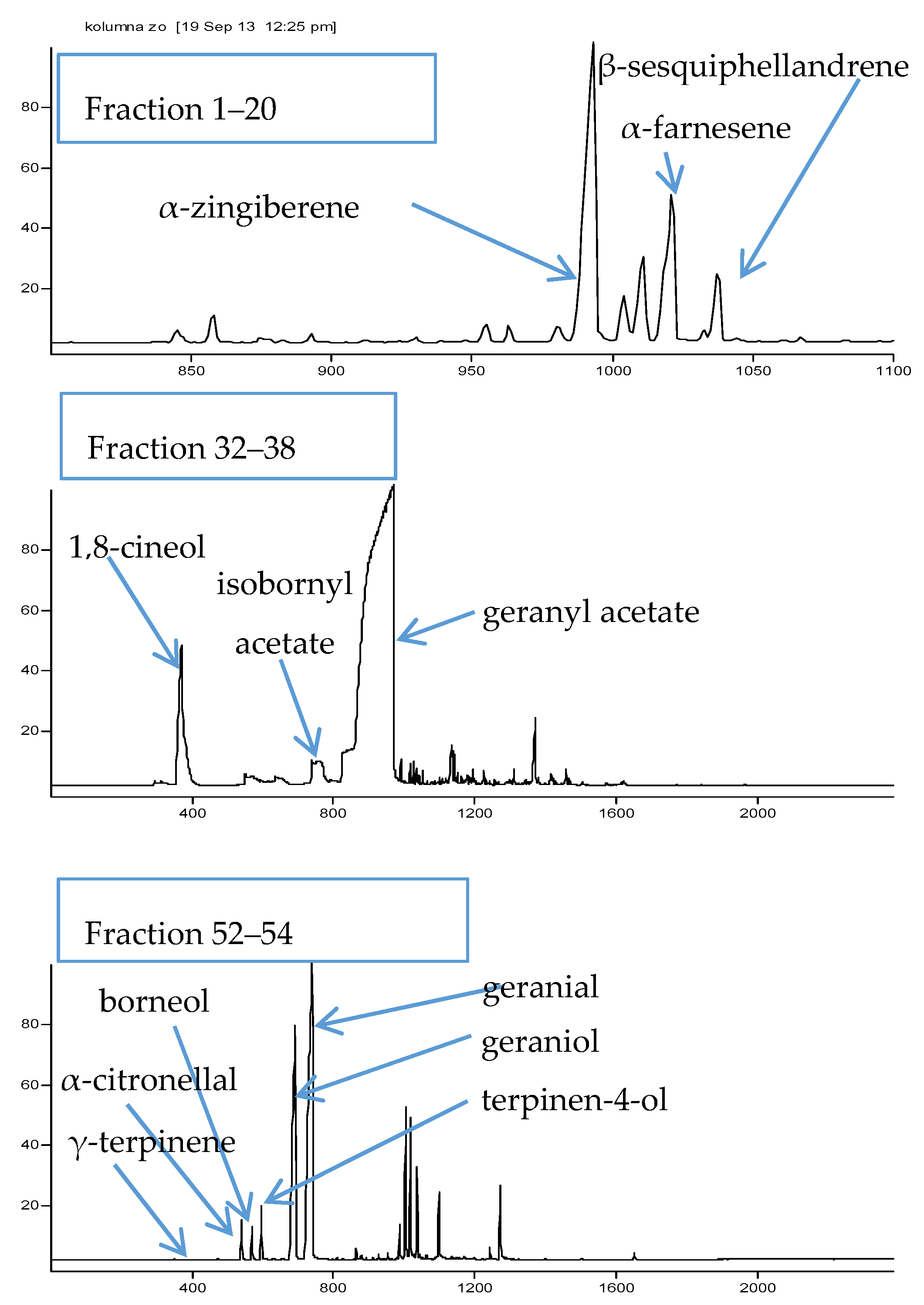
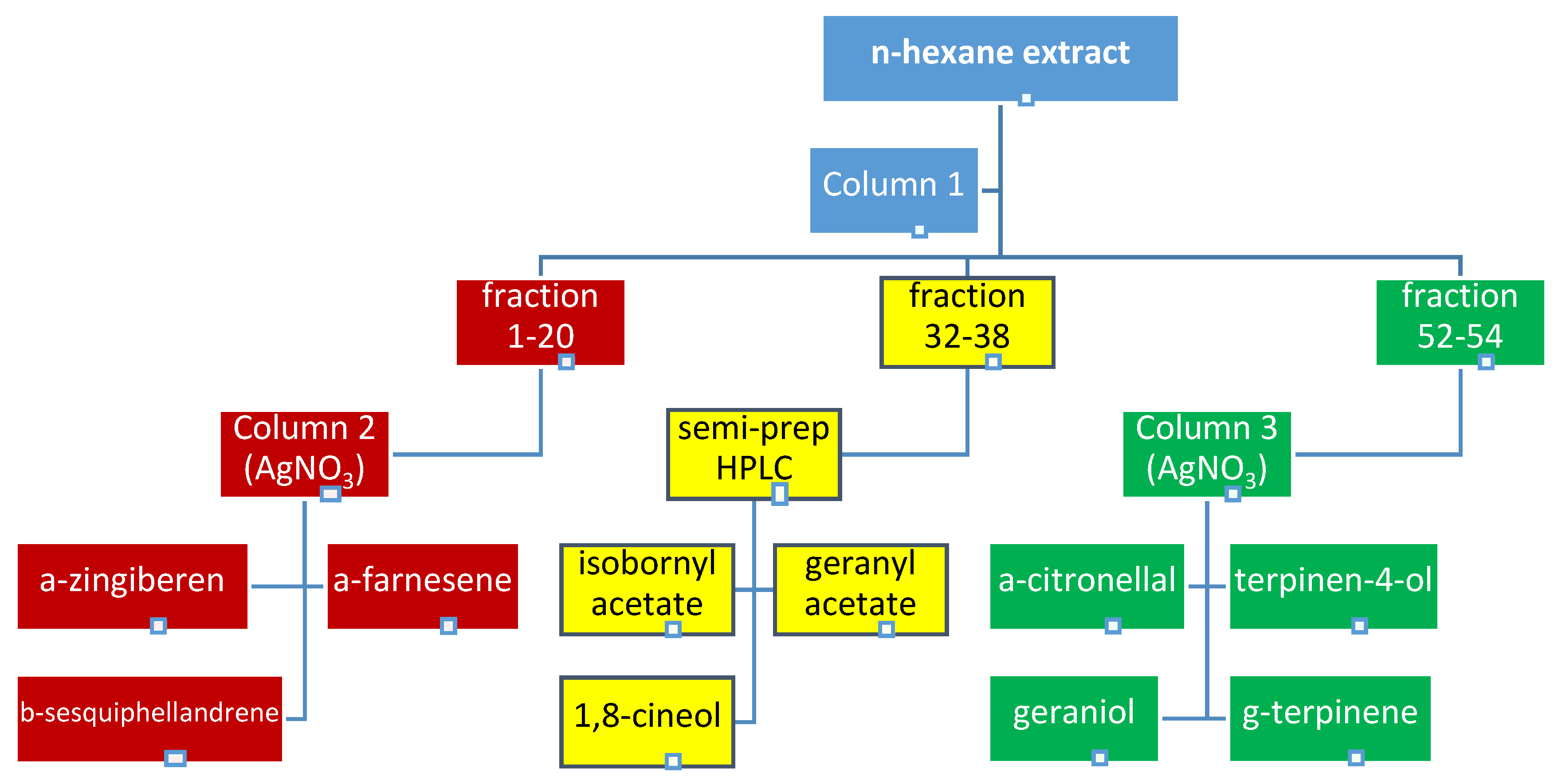
2.1. Bioguided Fractionation of Ginger Extract
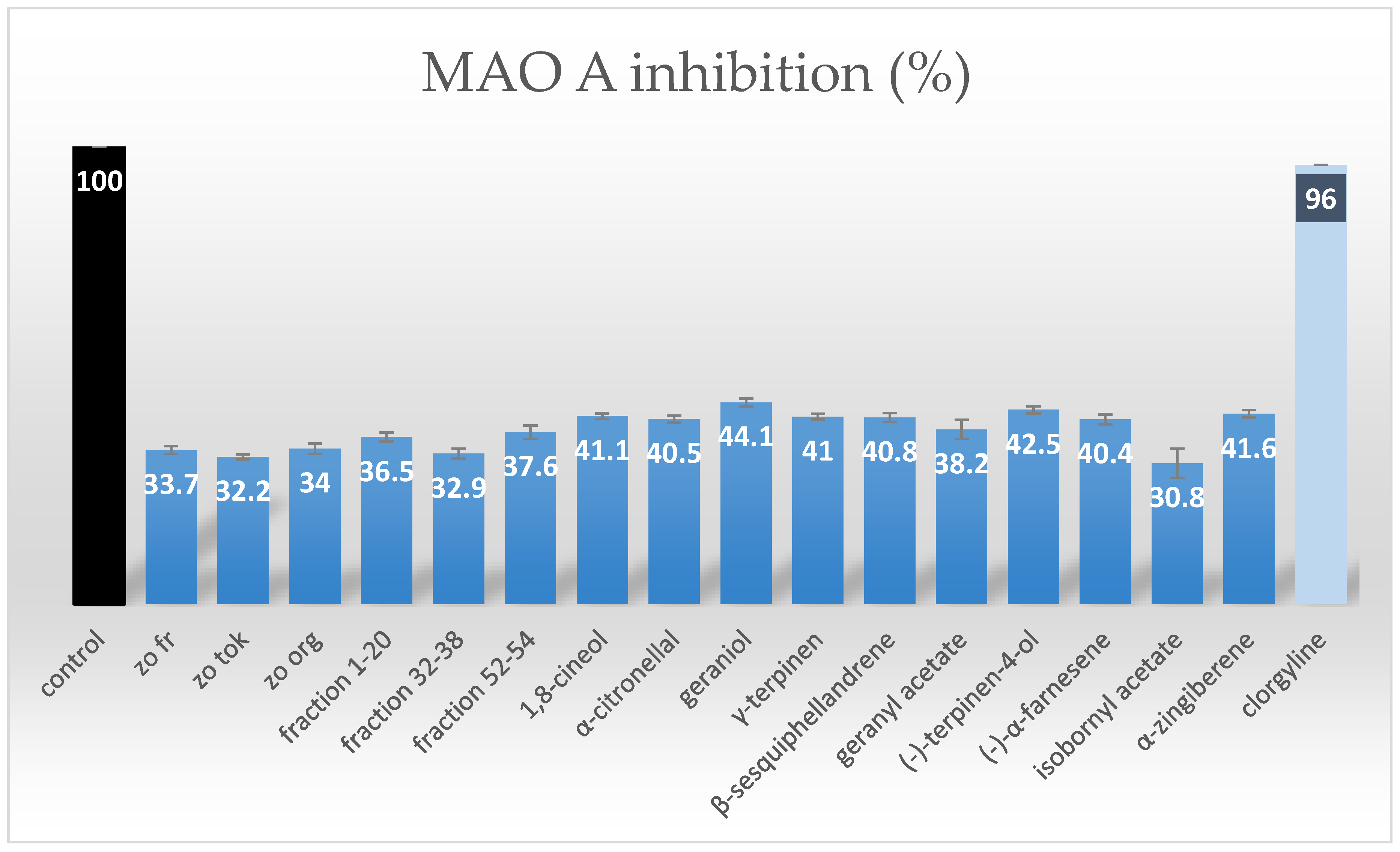
2.2. MAO-A Inhibition Assessment of Purified Terpenes from Ginger Rhizomes
3. Materials and Methods
3.1. Plant Material
3.2. Reagents
3.3. Extraction Protocol
3.4. Fractionation of the Extract
3.4.1. Semi-Prepartive HPLC Purification of Fraction 32–38
3.4.2. The Application of Silver Nitrate Impregnated Silica Gel in the Purification of Terpenes from Fractions 1–20 and 52–58
3.5. GC-MS Analysis of the Obtained Extract and Fractions
3.6. Monoamine Oxidase A (MAO-A) Assay
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mishra, R.K.; Kumar, A.; Kumar, A. Pharmacological activity of Zingiber Officinale. Int. J. Pharm. Chem. Sci. 2012, 1, 1073–1078. [Google Scholar]
- Krup, V.; Hedge, P.L.; Harini, A. Pharmacological activities of turmeric (Curcuma longa L.): A review. J. Homeop. Ayurv. Med. 2013, 2, 133. [Google Scholar] [CrossRef]
- Xia, X.; Pan, Y.; Zhen, O.Y.; Wang, J.; Pan, L.-L.; Zhu, Q.; Huang, J.-J.; Kong, L.-D. Pharmacokinetic-pharmacodynamic modeling of monoamine oxidase a inhibitory activity and behavior improvement by curcumin in the mouse forced swimming test. Chin. J. Nat. Med. 2011, 9, 293–304. [Google Scholar] [CrossRef]
- Li, Y.-Ch.; Wang, F.-M.; Pan, Y.; Qiang, L.-Q.; Cheng, G.; Zhang, W.-Y.; Kong, L.-D. Antidepressant-like effects of curcumin on serotonergic receptor-coupled AC-cAMP pathway in chronic unpredictable mild stress of rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 3, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Cheng, G.; Pan, Y.; Xia, Z.H.; Kong, L.D. Behavioral, neurochemical and neuroendocrine effects of the ethanolic extract from Curcuma longa L. in the mouse forced swimming test. J. Ethnopharmacol. 2007, 2, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food. Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.I.; de Melo, C.T.V.; Vasconcelos, L.F.; de Carvalho, A.M.R.; Sousa, F.C.F. Bioactivity and potential therapeutic benefits of some medicinal plants from the Caatinga (semi-arid) vegetation of Northeast Brazil: a review of the literature. Rev. Bras. Farmacogn. 2012, 22, 193–207. [Google Scholar] [CrossRef]
- Stoilova, I.; Krastanov, A.; Stoyanova, A.; Denev, P.; Gargova, S. Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem. 2007, 102, 764–770. [Google Scholar] [CrossRef]
- Koch, W.; Kukula-Koch, W.; Dziedzic, M.; Głowniak, K.; Asakawa, Y. Influence of thermal processing and in vitro digestion on the antioxidant potential of ginger and ginger containing products. Nat. Prod. Commun. 2016, 11, 1153–1156. [Google Scholar]
- Youdim, M.B.; Bakhle, Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006, 147, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.F.; Isacsson, G.; Bergman, U. Moclobemide. Lancet 1994, 12, 679–680. [Google Scholar] [CrossRef]
- Volz, H.P.; Gleiter, C.H. Monoamine oxidase inhibitors. A perspective on their use in the elderly. Drug Aging 1998, 13, 341–355. [Google Scholar] [CrossRef]
- Mitchell, M. Tranylcypromine. In The Gale Encyclopedia of Mental Disorders; Harris, M., Thackerey, E., Eds.; Gale: Detroit, MI, USA, 2003; Volume 2, pp. 993–995, 13: 978-0787657680. [Google Scholar]
- Finberg, J.P.M. Update on the pharmacology of selective inhibitors of MAO-A and MAO-B: Focus on modulation of CNS monoamine neurotransmitter release. Pharmacol. Ther. 2014, 143, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Guentert, T.W.; Banken, L.; Hilton, S.; Holford, N.H. Moclobemide: Relationships between dose, drug concentration in plasma, and occurrence of adverse events. J. Clin. Psychopharmacol. 1995, 15, 84–94. [Google Scholar] [CrossRef]
- Shulman, K.; Herrmann, N.; Walker, S.E. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs 2013, 27, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Serra, S.; Ferino, G.; Matos, M.J.; Vázquez-Rodríguez, S.; Delogu, G.; Viña, D.; Cadoni, E.; Santana, L.; Uriarte, E. Hydroxycoumarins as selective MAO-B inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Vina, D.; Serra, S.; Lamela, M.; Delogu, G. Herbal natural products as a source of monoamine oxidase inhibitors: A Review. Curr. Top. Med. Chem. 2012, 12, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Armijos, C.; Giardoni, G.; Amay, L.; Lozano, A.; Bracco, F.; Ramirez, J.; Bec, N.; Larroque, C.; Finzi, P.V.; Vidari, G. Phytochemical and ethnomedicinal study of huperzia species used in the traditional medicine of Saraguros in Southern Ecuador; AChE and MAO inhibitory activity. J. Ethnopharmacol. 2016, 193, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Van Diermen, D.; Marston, A.; Bravo, J.; Reist, M.; Carrupt, P.A.; Hostettmann, K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J. Ethnopharmacol. 2009, 122, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Demirkiran, O. Three new benzophenone glycosides with MAO-A inhibitory activity from Hypericum thasium Griseb. Phytochem. Lett. 2012, 5, 700–704. [Google Scholar] [CrossRef]
- Lopez, V.; Les, F.; Iannarelli, R.; Caprioli, C.; Maggi, F. Methanolic extract from red berry-like fruits of Hypericum androsaemum: Chemical characterization and inhibitory potential of central nervous system enzymes. Ind. Crop. Prod. 2016, 94, 363–367. [Google Scholar] [CrossRef]
- Saaby, L.; Rasmussen, H.B.; Jäger, A.K. MAO-A inhibitory activity of quercetin from Calluna vulgaris (L.) Hull. J. Ethnopharmacol. 2009, 121, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Jäger, A.K.; Gauguin, B.; Andersen, J.; Adsersen, A.; Gudiksen, L. Screening of plants used in Danish folk medicine to treat depression and anxiety for affinity to the serotonin transporter and inhibition of MAO-A. J. Ethnopharmacol. 2013, 145, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.D.; Cheng, C.H.K.; Tan, R.X. Inhibition of MAO A and B by some plant-derived alkaloids, phenols and anthraquinones. J. Ethnopharmacol. 2004, 91, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.T.; Stafford, G.I.; Staden, J.; Christensen, S.B.; Jäger, A.K. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. J. Ethnopharmacol. 2008, 117, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Shaemaa, H.A.; Haider, R.M.; Ali, M.A.; Hussein, A.A. The effect on ginger on monoaminoxidase (MAO), and acetyle cholinesterase (Ache) enzyme activity in human serum in vitro. J. Multidiscip. Eng. Sci. Technol. 2016, 2, 854–858. [Google Scholar]
- Peng, F.; Tao, Q.; Wu, X.; Dou, H.; Spencer, S.; Mang, C.; Xu, L.; Sun, L.; Zhao, Y.; Li, H.; et al. Cytotoxic, cytoprotective and antioxidant effects of isolated phenolic compounds from fresh ginger. Fitoterapia 2012, 83, 568–585. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, S.; Bahramsoltani, R.; Rahimi, R.; Abdollahi, M. Clinical risks of St John’s Wort (Hypericum perforatum) coadministration. Expert Opin. Drug Met. 2017, 12, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kukula-Koch, W.; Marzec, Z.; Kasperek, E.; Wyszogrodzka-Koma, L.; Szwerc, W.; Asakawa, Y. Application of chromatographic and spectroscopic methods towards the quality assessment of ginger (Zingiber officinale) rhizomes from ecological plantations. Int. J. Mol. Sci. 2017, 18, 452. [Google Scholar]
- Ravi Kiran, C.; Chakka, A.K.; Padmakumari Amma, K.P.; Nirmala Menon, A.; Sree Kumar, M.M.; Venugopalan, V.V. Essential oil composition of fresh ginger cultivars from North-East India. J. Essent. Oil Res. 2013, 25, 380–387. [Google Scholar] [CrossRef]
- Denyer, C.V.; Jackson, P.; Loakes, D.; Ellis, M.R.; Young, D.A.B. Isolation of antirhinoviral sesquiterpenes from ginger (Zingiber officinale). J. Nat. Prod. 1994, 57, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Zarmouh, N.O.; Messeha, S.S.; Elshami, F.M.; Soliman, K.F.A. Evaluation of the isoflavone genistein as reversible human monoamine oxidase-A and -B inhibitor. Evid. Based Complement. Altern. Med. 2016, 1423052. [Google Scholar] [CrossRef] [PubMed]
- Hiasa, M.; Isoda, Y.; Kishimoto, Y.; Saitoh, K.; Kimura, Y.; Kanai, M.; Shibasaki, M.; Hatakeyama, D.; Kirino, Y.; Kuzuhara, T. Inhibition of MAO-A and stimulation of behavioural activities in mice by the inactive prodrug form of the anti-influenza agent oseltamivir. Br. J. Pharmacol. 2013, 169, 115–129. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the plant material, extracts and collective fractions are available from the authors. |
| Compound | Rt (min) | Retention Index (RI) | No of Scan | Zo Tok (%) | Zo Fresh (%) | Zo Org (%) |
|---|---|---|---|---|---|---|
| ƴ-Terpinen | 12.48 | 1133 | 352 | 2.57 | 3.81 | 4.71 |
| 1,8-Cineol | 12.90 | 1141 | 368 | 0.26 | 0.82 | 1.18 |
| α-Citronellal | 16.55 | 1291 | 549 | 0.29 | 0.11 | 0.28 |
| Borneol | 16.94 | 1306 | 567 | 0.63 | 0.55 | 1.02 |
| Terpinen-4-ol | 17.31 | 1328 | 584 | 0.22 | 0.08 | 0.27 |
| Geraniol | 19.70 | 1420 | 697 | 3.46 | 5.35 | 0.41 |
| Geranial | 20.10 | 1438 | 717 | 12.41 | 5.66 | 7.99 |
| Isobornyl acetate | 20.49 | 1455 | 736 | 0.28 | 0.16 | 0.19 |
| Geranyl acetate | 23.13 | 1575 | 863 | 5.05 | 5.90 | 0.35 |
| α-Zingiberene | 26.10 | 1716 | 1002 | 22.22 | 30.53 | 39.53 |
| (E,E)-α-Farnesene | 26.34 | 1731 | 1016 | 12.49 | 16.28 | 9.27 |
| β-Sesquiphellandrene | 26.76 | 1750 | 1034 | 8.47 | 10.48 | 13.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukula-Koch, W.; Koch, W.; Czernicka, L.; Głowniak, K.; Asakawa, Y.; Umeyama, A.; Marzec, Z.; Kuzuhara, T. MAO-A Inhibitory Potential of Terpene Constituents from Ginger Rhizomes—A Bioactivity Guided Fractionation. Molecules 2018, 23, 1301. https://doi.org/10.3390/molecules23061301
Kukula-Koch W, Koch W, Czernicka L, Głowniak K, Asakawa Y, Umeyama A, Marzec Z, Kuzuhara T. MAO-A Inhibitory Potential of Terpene Constituents from Ginger Rhizomes—A Bioactivity Guided Fractionation. Molecules. 2018; 23(6):1301. https://doi.org/10.3390/molecules23061301
Chicago/Turabian StyleKukula-Koch, Wirginia, Wojciech Koch, Lidia Czernicka, Kazimierz Głowniak, Yoshinori Asakawa, Akemi Umeyama, Zbigniew Marzec, and Takashi Kuzuhara. 2018. "MAO-A Inhibitory Potential of Terpene Constituents from Ginger Rhizomes—A Bioactivity Guided Fractionation" Molecules 23, no. 6: 1301. https://doi.org/10.3390/molecules23061301
APA StyleKukula-Koch, W., Koch, W., Czernicka, L., Głowniak, K., Asakawa, Y., Umeyama, A., Marzec, Z., & Kuzuhara, T. (2018). MAO-A Inhibitory Potential of Terpene Constituents from Ginger Rhizomes—A Bioactivity Guided Fractionation. Molecules, 23(6), 1301. https://doi.org/10.3390/molecules23061301









