Profile of Polyphenolic and Essential Oil Composition of Polish Propolis, Black Poplar and Aspens Buds
Abstract
:1. Introduction
2. Results
2.1. Composition of Essential Oils of Propolis and Its Plant Precursor
2.2. Identification of Compounds Present in 70EE and UPLC-PDA-MS Profile of Propolis and Populus Buds
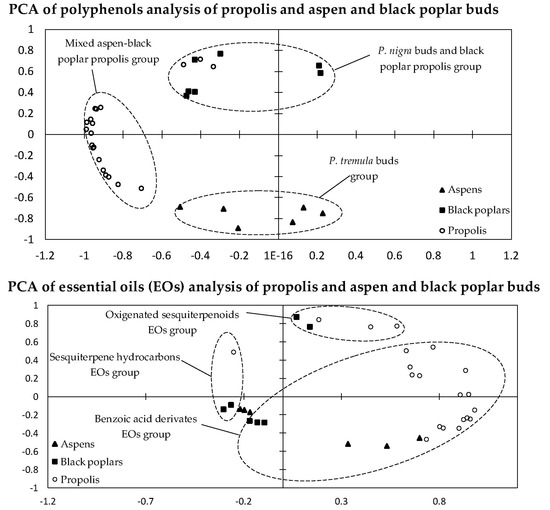
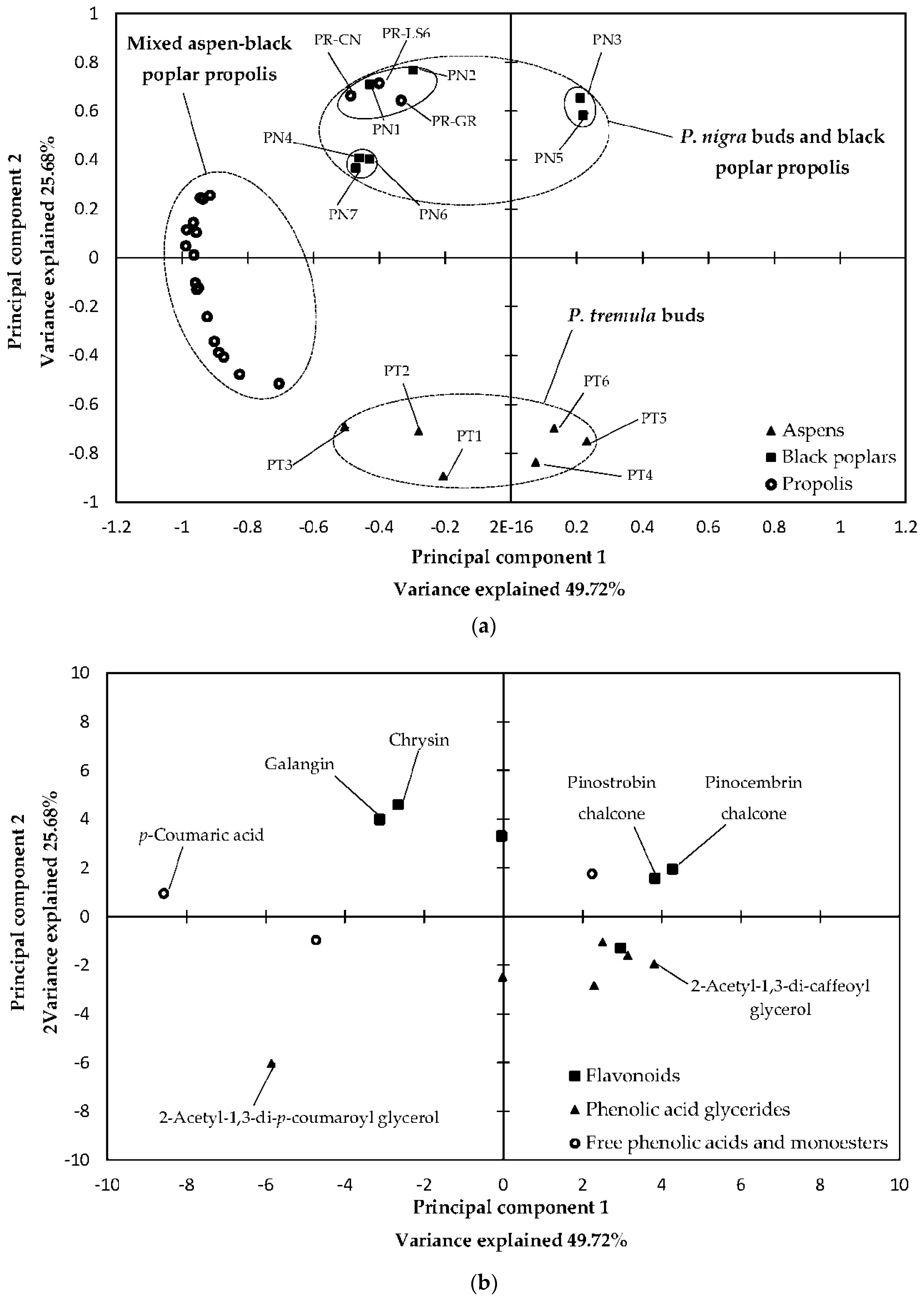
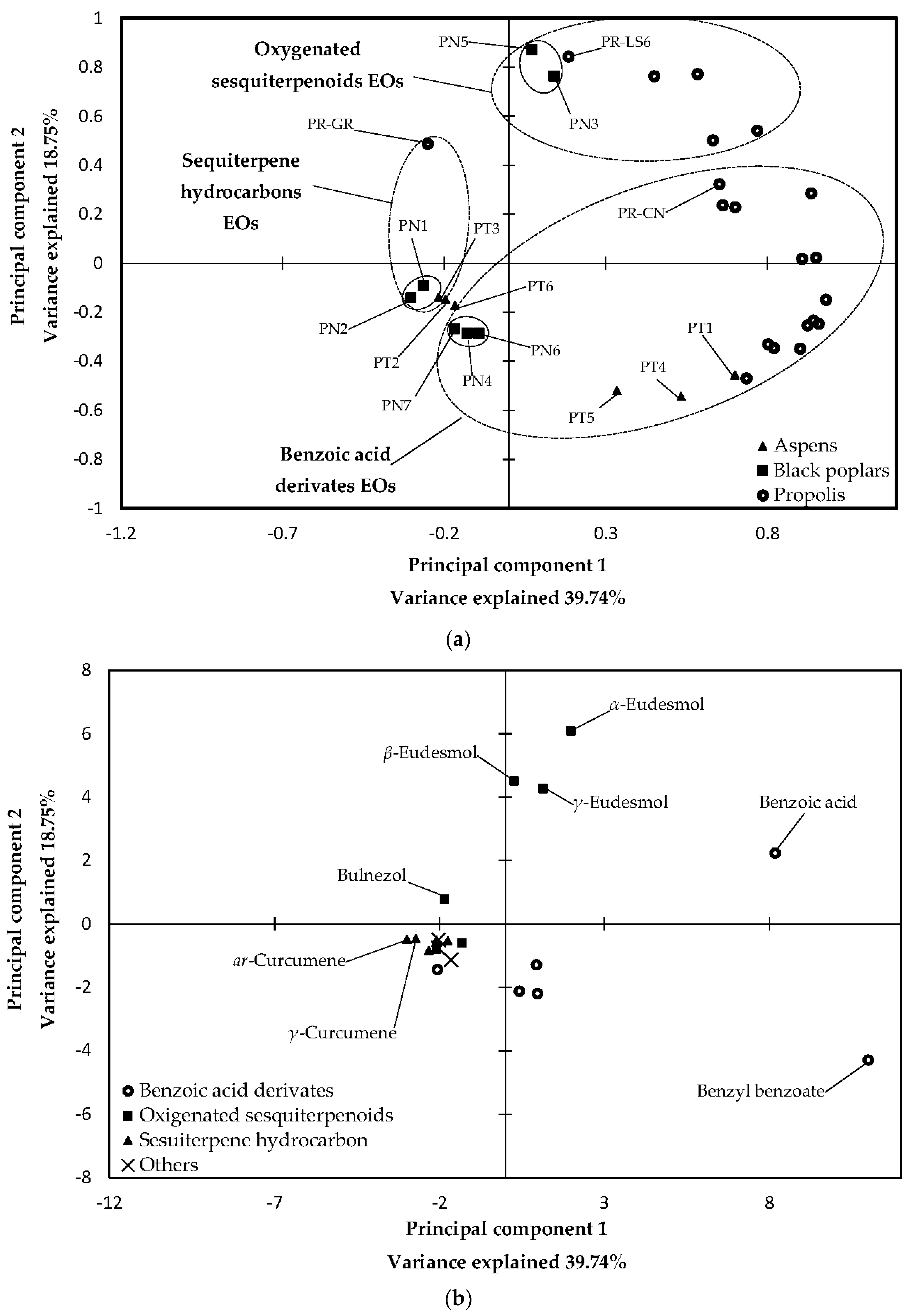
2.3. Statistical Analysis of 70% Ethanol in Water Extracts and Essential Oils
3. Discussion
3.1. The Composition of Populus spp. and Propolis EOs
3.2. The Composition of Populus spp. and Propolis 70EE
3.3. Statistical Analysis
4. Materials and Methods
4.1. Research Materials, Reagents and Standards
4.2. Isolation and Analysis of Essential Oils (EOs)
4.3. Preparation and UPLC-PDA-MS Analysis of 70% Ethanol in Water Extract (70EE)
4.4. Statistical Analysis of 70EE and EOS Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bankova, V.S. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.S.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 28, 28. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Szczepaniak, L.; Bakier, S. Rapid GC/MS determination of botanical precursors of Eurasian propolis. Food Chem. 2014, 142, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Bakier, S.; Pirożnikow, E.; Zambrzycka, M.; Swiecicka, I. Selective behaviour of honeybees in acquiring European propolis plant precursors. J. Chem. Ecol. 2016, 42, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Bertrams, J.; Müller, B.M.; Kunz, N.; Kammerer, D.R.; Stintzing, F.C. Phenolic compounds as marker compounds for botanical origin determination of German propolis samples based on TLC and TLC-MS. J. Appl. Bot. Food Qual. 2013, 86, 143–153. [Google Scholar]
- Popova, M.; Trusheva, B.; Khismatullin, R.; Gavrilova, N.; Legotkina, G.; Lyapunov, J.; Bankova, V.S. The triple botanical origin of Russian propolis from the Perm Region, its phenolic content and antimicrobial activity. Nat. Prod. Commun. 2013, 8, 617–620. [Google Scholar]
- Popova, M.; Giannopoulou, E.; Skalicka-Woźniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bęben, K.; Antosiewicz, B.; et al. Characterization and biological evaluation of propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Ristivojević, P.; Janakiev, T.; Berić, T.; Trifković, J.; Milojković-Opsenica, D.; Stanković, S. Phenolic profiles and antimicrobial activity of various plant resins as potential botanical sources of Serbian propolis. Ind. Crops Prod. 2016, 94, 856–871. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Gašić, U.; Andrić, F.; Nedić, N.; Tešić, Ž.; Milojković-Opsenica, D. Ultrahigh-performance Liquid Chromatography and Mass Spectrometry (UHPLC-LTQ/Orbitrap/MS/MS) Study of Phenolic Profile of Serbian Poplar Type Propolis. Phytochem. Anal. 2015, 26, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Mastelić, J. Volatile compounds from leaf-buds of Populus nigra L. (Salicaceae). Phytochemistry 2003, 63, 109–113. [Google Scholar] [CrossRef]
- Blande, J.D.; Tiiva, P.; Oksanen, E.; Holopainen, J.K. Emission of herbivore-induced volatile terpenoids from two hybrid aspen (Populus tremula × tremuloides) clones under ambient and elevated ozone concentrations in the field. Glob. Chang. Biol. 2007, 13, 2538–2550. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Chi, D. Chemical constituents of essential oil from bark of ten species of Populus. For. Res. 2007, 100, 267–271. [Google Scholar]
- Castro, M.L.; Cury, J.A.; Rosalen, P.L.; Alencar, S.M.; Ikegaki, M.; Duarte, S.; Koo, H. Propolis from southeastern and northeastern of Brazil: The influence of seasonality in antibacterial activity and phenolic composition. Quim. Nova 2007, 30, 1512–1516. [Google Scholar] [CrossRef]
- Daugsch, A.; Moraes, C.S.; Fort, P.; Park, Y.K. Brazilian red propolis—Chemical composition and botanical origin. Evid. Based Complement. Alternat. Med. eCAM 2008, 5, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Navarro, M.; Ruiz-Bustos, P.; Valencia, D.; Robles-Zepeda, R.; Ruiz-Bustos, E.; Virués, C.; Hernandez, J.; Domínguez, Z.; Velazquez, C. Antibacterial activity of Sonoran propolis and some of its constituents against clinically significant Vibrio species. Foodborne Pathog. Dis. 2013, 10, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem. Toxicol. 2010, 48, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Kędzia, B. Pochodzenie propolisu w świetle teorii i badań naukowych. Herba Pol. 2008, 54, 179–186. [Google Scholar]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef]
- Bankova, V.S.; Popova, M.; Bogdanov, S.; Sabatini, A.-G. Chemical composition of European propolis expected and unexpected results. Z. Naturforsch. C 2002, 57, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, W.; Wollenweber, E.; Scaysbrook, T.; Whatley, F.R. Esters of caffeic acid with aliphatic alcohols in bud exudate of Populus nigra. Z. Naturforsch. C 1988, 43, 795–798. [Google Scholar]
- Greenaway, W.; May, J.; Scaysbrook, T.; Whatley, F.R. Compositions of bud and leaf exudates of some Populus species compared. Z. Naturforsch. C 1992, 47, 329–334. [Google Scholar]
- Isidorov, V.A.; Brzozowska, M.; Czyżewska, U.; Glinka, L. Gas chromatographic investigation of phenylpropenoid glycerides from aspen (Populus tremula L.) buds. J. Chromatogr. A 2008, 1198–1199, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-P.; Zheng, H.-Q.; Liu, G.; Hu, F.-L. Development and validation of HPLC method for determination of salicin in poplar buds: Application for screening of counterfeit propolis. Food Chem. 2011, 127, 345–350. [Google Scholar] [CrossRef]
- Trudić, B.; Anđelković, B.; Orlović, S.; Tešević, V.; Pilipović, A.; Cvetković, M.; Stanković, J. HPLC/MS-TOF Analysis of Surface Resins from Three Poplar Clones Grown in Serbia. South-East Eur. For. 2016, 7, 129–133. [Google Scholar] [CrossRef]
- Abreu, I.N.; Ahnlund, M.; Moritz, T.; Albrectsen, B.R. UHPLC-ESI/TOFMS determination of salicylate-like phenolic glycosides in Populus tremula leaves. J. Chem. Ecol. 2011, 37, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-P.; Zheng, H.-Q.; Hu, F.-L. Extraction, Partial Characterization, and Storage Stability of β-Glucosidase from Propolis. J. Food Sci. 2011, 76, C75–C79. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, T.; Białobok, S.; Bugała, W.; Hejnowicz, A.; Jakuszewski, T.; Jankiewicz, J.N.; Obmiński, Z.; Siwecki, R.; Środoń, A.; Surmiński, J.; et al. Nasze Drzewa Leśne: Topole Populus L. (Our Forest Trees Poplars: Populus L.), 1st ed.; PWN: Warszawa, Poland, 1973; Volume 12, pp. 15–19, 81. ISSN 0137-6438. [Google Scholar]
- Šiler, B.; Skorić, M.; Mišić, D.; Kovačević, B.; Jelić, M.; Patenković, A.; Kurbalija-Novičić, Z. Variability of European Black poplar (Populus nigra L.) in the Danube Basin, 1st ed.; Public Enterprise “Vojvodinašume”: Novi Sad, Croatia, 2014; pp. 93–95. ISBN 978-86-906665-4-6. [Google Scholar]
- Imdorf, A.; Bogdanov, S.; Ochoa, I.R.; Calderone, N.W. Use of essential oils for the control of Varroa jacobsoni Oud. in honey bee colonies. Apidologie 1999, 30, 209–228. [Google Scholar] [CrossRef]
- Milojković Opsenica, D.; Ristivojević, P.; Trifković, J.; Vovk, I.; Lušić, D.; Tešić, Z. TLC Fingerprinting and pattern recognition methods in the assessment of authenticity of poplar-type propolis. J. Chromatogr. Sci. 2016, 54, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.G.; Pereira, A.P.; Estevinho, L.M. Comparative study of different Portuguese samples of propolis: Pollinic, sensorial, physicochemical, microbiological characterization and antibacterial activity. Food Chem. Toxicol. 2012, 50, 4246–4253. [Google Scholar] [CrossRef] [PubMed]
- Çelemli, O.G.; Hatjina, F.; Charistos, L.; Schiesser, A.; Özkirim, A. More insight into the chemical composition of Greek propolis; differences and similarities with Turkish propolis. Z. Naturforsch. C 2013, 68, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Anastasiadou, P.; Papadopoulos, A.; Machera, K. Revisiting Greek propolis: Chromatographic analysis and antioxidant activity study. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E.; Ristivojevic, P.; Chernetsova, E.S. Combined multivariate data analysis of high-performance thin-layer chromatography fingerprints and direct analysis in real time mass spectra for profiling of natural products like propolis. J. Chromatogr. A 2014, 1328, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Katircioǧlu, H.; Mercan, N. Antimicrobial activity and chemical compositions of Turkish propolis from different regions. Afr. J. Biotechnol. 2006, 11, 1151–1153. [Google Scholar]
- Torres, R.; Lopes, J.; Neto, J.; Citó, A. The volatile constituents of propolis from Piaui. Quim. Nova 2008, 31, 479–485. [Google Scholar] [CrossRef]
- Cheng, H.; Qin, Z.H.; Guo, X.F.; Hu, X.S.; Wu, J.H. Geographical origin identification of propolis using GC-MS and electronic nose combined with principal component analysis. Food Res. Int. 2013, 51, 813–822. [Google Scholar] [CrossRef]
- Kaškoniene, V.; Kaškonas, P.; Maruška, A.; Kubiliene, L. Chemometric analysis of volatiles of propolis from different regions using static headspace GC-MS. Cent. Eur. J. Chem. 2014, 12, 736–746. [Google Scholar] [CrossRef]
- Hage, S.; Morlock, G.E. Bioprofiling of Salicaceae bud extracts through high-performance thin-layer chromatography hyphenated to biochemical, microbiological and chemical detections. J. Chromatogr. A 2017, 1490, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.A.; Guerreiro, M.C. Characterization of Brazilian green propolis throughout the seasons by headspace GC/MS and ESI-MS. J. Sci. Food Agric. 2012, 30, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Szumny, A.; Figiel, A.; Gutiérrez-Ortíz, A.; Carbonell-Barrachina, A.A. Composition of rosemary essential oil (Rosmarinus officinalis) as affected by drying method. J. Food Eng. 2010, 91, 253–260. [Google Scholar] [CrossRef]
- Shi, H.; Yang, H.; Zhang, X.; Sheng, Y.; Huang, H.; Yu, L. Isolation and Characterization of Five Glycerol Esters from Wuhan Propolis and Their Potential Anti-Inflammatory Properties. J. Agric. Food Chem. 2012, 60, 10041–10047. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yang, H.; Zhang, X.; Yu, L.L. Identification and quantification of phytochemical composition and anti-inflammatory and radical scavenging properties of methanolic extracts of Chinese propolis. J. Agric. Food Chem. 2012, 60, 12403–12410. [Google Scholar] [CrossRef] [PubMed]
- StatSoft Inc. I. Internetowy Podręcznik Statystyki. (The First Internet Handbook of Statistic, In Polish). Available online: https://www.statsoft.pl/textbook/stathome.html (accessed on 24 January 2018).
Sample Availability: Samples of the compounds are not available from the authors. |
| Component | Black Poplars (Populus nigra L.) | Aspens (Populus tremula L.) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PN1 | PN2 | PN3 | PN4 | PN5 | PN6 | PN7 | PT1 | PT2 | PT3 | PT4 | PT5 | PT6 | |
| Benzyl alcohol | 3.22 | 4.19 | 0.07 | 0.99 | 0.06 | 1.03 | 0.75 | 1.03 | 0.29 | 1.79 | 4.23 | 7.04 | 15.1 |
| 2-Phenylethanol | 0.34 | 4.25 | tr | 0.25 | - | 1.06 | 1.00 | - | - | - | 1.28 | 3.69 | 34.5 |
| Benzoic acid | tr | tr | - | - | - | 0.12 | 0.05 | - | - | - | 0.08 | 0.07 | - |
| Eugenol | 0.20 | 0.28 | tr | - | tr | 0.05 | 0.05 | 0.30 | 0.50 | 0.20 | 2.28 | 3.78 | 14.2 |
| cis-β-Cariophyllen | - | 0.26 | 0.23 | - | 1.18 | 0.11 | 0.08 | 0.32 | 18.76 | 24.0 | tr | 0.09 | - |
| α-Guaien | - | - | - | - | - | - | - | - | 6.91 | 10.2 | - | - | - |
| Prenyl benzoate | - | - | - | 21.6 | - | 20.3 | 19.8 | - | - | - | - | - | - |
| ar-Curcumen | 27.2 | 8.92 | - | 0.51 | - | 8.62 | 9.14 | - | - | - | - | - | - |
| γ-Curcumen | 9.50 | 9.32 | - | 2.29 | 3.30 | 3.79 | 11.6 | - | - | - | - | - | - |
| δ-Cadinen | 7.74 | 12.0 | 1.78 | 4.24 | 0.40 | 0.33 | 0.45 | 4.79 | 0.53 | 0.77 | 0.30 | 0.39 | 0.68 |
| trans-Nerolidol | 0.06 | 0.07 | - | 17.3 | - | 0.61 | 0.31 | - | - | 0.10 | - | - | - |
| Cariophyllene oxide | 0.06 | 0.19 | - | - | - | - | - | 0.22 | 18.1 | 12.0 | - | - | - |
| γ-Eudesmol | - | - | 9.90 | - | 14.4 | 0.65 | 0.47 | - | - | - | - | - | - |
| β-Eudesmol | - | - | 16.00 | - | 19.3 | 0.65 | 0.12 | - | - | - | - | - | - |
| α-Eudesmol | - | - | 49.3 | - | 22.2 | 1.18 | 1.30 | - | - | - | - | - | - |
| Bulnezol | - | - | - | 0.69 | 10.1 | 3.89 | 3.68 | - | - | - | - | - | - |
| Benzyl benzoate | 0.26 | 0.20 | - | 3.65 | - | 4.47 | 3.83 | 45.45 | 1.12 | 0.50 | 26.4 | 13.5 | 0.62 |
| Salicyl benzoate | 0.07 | 0.08 | - | 7.20 | - | 12.7 | 10.6 | 7.02 | - | - | 16.0 | 11.7 | 0.23 |
| trans-Benzyl cinnamate | - | - | - | - | - | - | - | - | - | - | 26.8 | 24.7 | 1.83 |
| Aliphatic component | 0.49 | 0.73 | 0.05 | 0.06 | 0.06 | 2.38 | 1.79 | 3.41 | 3.40 | 1.84 | 4.48 | 10.5 | 16.2 |
| Component | Polish Propolis | Foreign Propolis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR-NW1 | PR-NW2 | PR-ŚL1 | PR-ŚL2 | PR-SZ1 | PR-SZ2 | PR-SZ3 | PR-SZ4 | PR-LS1 | PR-LS2 | PR-LS3 | PR-LS4 | PR-LS5 | PR-LS6 | PR-S1 | PR-S2 | PR-GR | PR-CN | |
| Benzyl alcohol | 8.38 | 3.67 | 5.60 | 8.47 | 1.73 | 2.84 | 1.26 | 2.77 | 2.68 | 6.39 | 6.19 | 0.87 | 1.46 | 4.93 | 6.48 | 3.23 | 1.13 | 1.39 |
| 2-Phenylethanol | 0.68 | 0.12 | 0.59 | 0.30 | 0.50 | 0.38 | 0.23 | 0.65 | 0.21 | 1.14 | 1.30 | 0.43 | 0.44 | 2.31 | 1.22 | 0.33 | 5.83 | 0.21 |
| Benzoic acid | 8.52 | 16.7 | 1.82 | 0.78 | 13.3 | 33.0 | 20.7 | 42.2 | 13.1 | 12.1 | 12.4 | 5.23 | 3.75 | 1.57 | 12.8 | 30.2 | - | 47.1 |
| Eugenol | 0.20 | 0.11 | 0.11 | 0.14 | 0.06 | 0.22 | 0.13 | 0.11 | 0.25 | 0.13 | 0.20 | 0.08 | 0.11 | 0.23 | 0.20 | 0.12 | 0.28 | 0.39 |
| cis-β-Cariophyllen | 1.27 | 0.62 | 0.13 | 0.44 | 0.21 | 0.34 | 0.40 | 0.44 | - | tr | 0.09 | 0.43 | 0.43 | 0.11 | 0.16 | 0.07 | - | - |
| α-Guaien | 1.27 | 0.38 | 0.22 | 0.33 | 0.18 | 0.41 | 0.38 | 0.24 | - | - | 0.47 | 0.67 | - | 0.11 | 0.30 | 0.41 | 2.21 | - |
| Prenyl benzoate | tr | tr | tr | - | tr | - | - | - | - | - | 0.65 | 3.29 | 3.26 | tr | 3.85 | 0.36 | - | - |
| ar-Curcumen | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 11.1 | 1.41 |
| γ-Curcumen | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 6.77 | 0.84 |
| δ-Cadinen | 2.42 | 0.71 | 0.31 | 0.66 | 0.72 | 0.78 | 1.07 | 0.79 | 0.67 | 0.50 | 0.55 | 1.62 | 2.17 | 2.33 | 0.55 | 0.29 | 0.86 | 0.44 |
| trans-Nerolidol | 0.40 | 0.13 | 0.16 | 0.24 | 0.38 | 0.22 | 0.36 | 0.23 | - | 0.29 | - | 0.15 | 0.19 | 0.06 | 0.17 | 0.30 | - | 0.26 |
| Cariophyllene oxide | 1.96 | 1.36 | 0.75 | 0.50 | 1.24 | 1.24 | 1.44 | 0.67 | 0.98 | 1.04 | 1.29 | 2.29 | 2.12 | 0.52 | 5.60 | 0.52 | - | - |
| γ-Eudesmol | 0.53 | 3.03 | 2.37 | 1.14 | 8.77 | 2.84 | 8.71 | 3.56 | 3.23 | 5.33 | 6.96 | 14.8 | 11.3 | 16.9 | 1.17 | 3.16 | 5.56 | 5.35 |
| β-Eudesmol | 0.32 | 1.59 | 1.82 | 0.33 | 9.50 | 2.58 | 8.49 | 2.60 | 1.29 | 0.57 | 5.24 | 10.1 | 9.91 | 18.7 | 1.22 | 0.66 | 5.02 | 5.18 |
| α-Eudesmol | - | 0.82 | 4.38 | 1.49 | 9.29 | 4.10 | 9.60 | 4.25 | 3.46 | 9.60 | 10.6 | 16.8 | 10.9 | 17.8 | 3.08 | 4.59 | 6.63 | 6.72 |
| Bulnezol | - | - | - | - | 2.54 | 1.15 | 1.30 | 0.90 | - | - | - | 3.86 | 2.84 | - | - | - | 4.80 | - |
| Benzyl benzoate | 11.89 | 28.6 | 26.4 | 23.6 | 2.65 | 6.29 | 7.38 | 5.80 | 19.6 | 20.5 | 13.7 | 7.40 | 11.8 | 1.28 | 17.6 | 19.3 | - | 6.26 |
| Salicyl benzoate | 5.42 | 0.55 | 3.33 | 3.75 | 1.09 | 2.04 | 2.43 | 1.42 | 3.74 | 1.74 | 3.29 | 1.38 | 3.95 | 0.22 | 5.01 | 2.42 | - | 0.67 |
| trans-Benzyl cinnamate | 0.68 | 3.91 | 5.52 | 8.97 | 1.80 | 3.28 | 2.72 | 4.62 | 3.97 | 1.33 | 3.69 | 0.41 | 1.21 | 0.18 | 3.45 | 4.93 | - | 0.41 |
| Aliphatic component | - | 0.91 | 0.09 | 0.20 | 0.89 | 1.05 | 1.10 | 0.95 | - | 0.57 | 0.88 | 0.18 | 0.28 | - | 0.91 | 0.77 | - | 0.47 |
| Chemical Component Group | Black Poplars (Populus nigra L.) | Aspens (Populus tremula L.) | Polish Propolis | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PN1 | PN2 | PN3 | PN4 | PN5 | PN6 | PN7 | PT1 | PT2 | PT3 | PT4 | PT5 | PT6 | PR-NW1 | PR-NW2 | PR-ŚL1 | PR-ŚL2 | PR-SZ1 | PR-SZ2 | PR-SZ3 | PR-SZ4 | |
| Monoterpenes | 0.2 | 0.2 | 0.1 | 0.8 | 0.1 | 0.0 | 0.0 | tr | 0.1 | 0.2 | 1.9 | 3.4 | - | 0.3 | tr | 0.1 | 0.1 | 1.0 | 3.4 | 1.7 | 0.9 |
| Sum of sesquiterpenes | 90.5 | 85.5 | 99.2 | 45.5 | 96.7 | 33.5 | 41.9 | 36.8 | 91.4 | 92.3 | 3.4 | 5.2 | 3.0 | 48.6 | 31.0 | 28.6 | 31.4 | 63.1 | 29.4 | 51.5 | 28.7 |
| Sesuiterpenes hydrocarbons | 73.3 | 66.6 | 16.2 | 20.1 | 10.8 | 20.7 | 30.2 | 19.1 | 45.3 | 57.7 | 2.2 | 3.2 | 1.8 | 13.5 | 3.8 | 2.2 | 3.5 | 4.4 | 4.4 | 6.9 | 3.9 |
| Oxygenated sesquiterpenoids | 17.2 | 18.9 | 83.0 | 25.4 | 85.9 | 12.9 | 11.7 | 17.7 | 46.0 | 34.5 | 1.2 | 2.0 | 1.2 | 35.1 | 27.2 | 26.4 | 27.8 | 58.7 | 25.0 | 44.7 | 24.8 |
| Benzoic acid derivates | 5.1 | 5.1 | 0.1 | 49.3 | 1.2 | 56.5 | 50.4 | 54.9 | 1.5 | 3.8 | 76.7 | 59.9 | 21.8 | 37.0 | 54.4 | 44.0 | 47.3 | 21.1 | 47.9 | 35.1 | 57.6 |
| Phenylethan and propan derivates | 1.1 | 4.8 | tr | 1.1 | tr | 2.4 | 2.1 | tr | - | tr | 1.3 | 3.7 | 34.5 | 2.4 | 2.3 | 2.7 | 2.2 | 1.8 | 2.6 | 1.2 | 1.9 |
| Phenols | 0.4 | 0.6 | tr | - | 0.2 | 0.1 | 0.1 | 0.6 | 0.6 | 0.3 | 6.5 | 9.1 | 15.5 | 1.7 | 3.7 | 11.1 | 4.8 | 3.5 | 7.1 | 3.1 | 3.9 |
| Aliphatic components | 2.3 | 3.3 | 0.1 | 0.3 | 0.2 | 6.2 | 4.8 | 7.4 | 6.2 | 3.1 | 8.9 | 17.2 | 23.3 | 4.8 | 6.2 | 9.3 | 10.2 | 6.4 | 7.7 | 6.4 | 5.5 |
| Others | 0.4 | 0.6 | 0.6 | 3.0 | 1.7 | 1.2 | 0.8 | 0.3 | 0.3 | 0.4 | 1.3 | 1.6 | 1.9 | 5.4 | 2.4 | 4.3 | 4.0 | 3.1 | 1.8 | 1.0 | 1.6 |
| Amount of EOs ** | 1.0 | 0.7 | 4.3 | 1.5 | 1.0 | 0.5 | 1.2 | 0.4 | 0.2 | tr | 0.1 | 0.1 | 0.1 | 1.1 | 1.2 | 2.8 | 0.7 | 1.1 | 0.8 | 1.8 | 1.4 |
| Chemical Component Group | Polish Propolis | Foreign Propolis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR-LS1 | PR-LS2 | PR-LS3 | PR-LS4 | PR-LS5 | PR-LS6 | PR-S1 | PR-S2 | PR-MR | PR-NSW | PR-GR | PR-CN | |
| Monoterpenes | tr | - | - | - | 0.2 | 0.2 | 0.2 | - | - | 0.1 | 0.1 | - |
| Sum of sesquiterpenes | 41.2 | 42.8 | 39.8 | 74.6 | 64.9 | 77.5 | 26.6 | 19.4 | 14.4 | 36.6 | 84.8 | 31.6 |
| Sesuiterpenes hydrocarbons | 2.0 | 1.6 | 3.7 | 13.2 | 13.6 | 9.3 | 4.9 | 2.1 | 3.6 | 5.2 | 46.9 | 8.1 |
| Oxygenated sesquiterpenoids | 39.2 | 41.2 | 36.1 | 61.4 | 51.3 | 68.2 | 21.6 | 17.3 | 10.8 | 31.4 | 37.9 | 23.6 |
| Benzoic acid derivates | 44.0 | 43.2 | 42.2 | 20.5 | 27.5 | 10.1 | 53.7 | 62.6 | 70.7 | 44.1 | 1.9 | 57.2 |
| Phenylethan and propan derivates | 0.7 | 2.3 | 2.8 | 0.8 | 1.1 | 4.2 | 3.3 | 0.8 | 0.8 | 1.5 | 11.2 | 3.0 |
| Phenols | 6.7 | 4.3 | 5.6 | 1.2 | 2.0 | 1.3 | 4.3 | 11.0 | 7.9 | 9.2 | 0.8 | 2.0 |
| Aliphatic components | 2.8 | 4.6 | 8.1 | 2.1 | 3.4 | 5.5 | 10.4 | 5.0 | 2.9 | 5.7 | 0.2 | 4.3 |
| Others | 4.6 | 2.9 | 1.6 | 0.9 | 1.0 | 1.3 | 1.6 | 1.3 | 3.3 | 2.9 | 1.1 | 1.8 |
| Amount of EOs ** | 1.2 | 0.8 | 0.4 | 0.9 | 0.8 | 0.5 | 0.1 | 0.7 | 0.1 | 0.3 | 1.5 | 0.7 |
| Component | Black Poplars (Populus nigra L.) | Aspens (Populus tremula L.) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PN1 | PN2 | PN3 | PN4 | PN5 | PN6 | PN7 | PT1 | PT2 | PT3 | PT4 | PT5 | PT6 | |
| p-Coumaric acid | 3.22 | 2.65 | 0.14 | 14.66 | 1.77 | 13.91 | 13.43 | 3.49 | 1.67 | 4.37 | 2.51 | 1.15 | 1.65 |
| ** 1,3-Di-p-coumaroylglycerol | - | - | - | - | - | - | - | 6.68 | 12.98 | 3.79 | 1.47 | 1.37 | 2.88 |
| 2-Acetyl-1,3-di-caffeoylglycerol | - | - | - | - | - | - | - | 5.50 | 0.47 | 0.31 | 10.46 | 17.30 | 17.37 |
| Acetyl-caffeoyl-p-coumaroylglycerol | - | - | - | - | - | - | - | 10.97 | 4.19 | 3.88 | 13.83 | 17.74 | 16.93 |
| 2-Acetylo-3-caffeoyl-1-feruloylglycerol | - | - | - | - | - | - | - | 6.48 | 0.92 | 0.25 | 12.28 | 13.50 | 5.52 |
| Chrysin | 13.02 | 14.11 | 4.97 | 1.47 | 8.08 | 2.58 | 1.69 | - | - | - | - | - | - |
| Sakuranetin | - | 0.26 | - | 0.71 | 0.26 | 0.18 | 0.39 | 5.01 | 13.10 | 9.35 | 6.47 | 6.16 | 5.85 |
| Pinocembrin chalcone | - | 4.27 | 18.07 | 1.76 | 21.72 | - | - | - | - | - | - | - | - |
| Galangin | 15.25 | 15.61 | 10.90 | 4.46 | 7.20 | 2.82 | 3.22 | - | - | - | - | - | - |
| Pinobanksin-3-O-acetate | 7.25 | 7.22 | 11.36 | 1.48 | 5.87 | 2.21 | 2.44 | - | - | - | - | - | - |
| ** 2-Acetyl-1,3-di-p-coumaroylglycerol | - | - | - | - | - | - | - | 22.21 | 37.91 | 37.74 | 13.74 | 11.82 | 9.63 |
| ** 2-Acetyl-p-3-coumaroyl-1-feruloylglycerol | - | - | - | - | - | - | - | 10.19 | 7.19 | 6.49 | 14.07 | 10.34 | 2.02 |
| p-Coumaric acid benzyl ester | 2.95 | 0.46 | - | 1.12 | - | 1.58 | 2.13 | 1.93 | 2.02 | 16.39 | 1.08 | 0.35 | 6.78 |
| Pinostrobin chalcone | - | 0.94 | 10.64 | 5.72 | 10.77 | 6.25 | 1.73 | - | - | - | - | - | - |
| p-Coumaric acid cinnamic ester | - | - | - | 5.72 | 0.62 | 8.55 | 10.29 | - | - | - | - | - | - |
| Component | Polish Propolis | Foreign Propolis | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR-NW1 | PR-NW2 | PR-ŚL1 | PR-ŚL2 | PR-SZ1 | PR-SZ2 | PR-SZ3 | PR-SZ4 | PR-LS1 | PR-LS2 | PR-LS3 | PR-LS4 | PR-LS5 | PR-LS6 | PR-S1 | PR-S2 | PR-MR | PR-NSW | PR-GR | PR-CN | |
| p-Coumaric acid | 8.62 | 8.96 | 8.65 | 8.48 | 6.87 | 8.81 | 12.91 | 9.13 | 11.88 | 9.66 | 9.95 | 6.38 | 12.17 | 2.09 | 11.62 | 11.24 | 9.42 | 9.59 | 0.10 | 6.48 |
| ** 1,3-Di-p-coumaroylglycerol | 1.53 | 2.44 | 2.22 | 3.75 | 0.92 | 1.06 | 0.79 | 0.87 | 1.53 | 0.86 | 0.89 | 0.57 | 0.76 | 0.07 | 0.89 | 1.02 | 0.63 | 1.39 | - | 0.11 |
| 2-Acetyl-1,3-di-caffeoylglycerol | 0.50 | 0.71 | 0.45 | 0.54 | 0.18 | 0.23 | 0.09 | 0.06 | 0.68 | 0.21 | 0.29 | 0.74 | 0.32 | - | 0.36 | 0.24 | 0.59 | 0.36 | - | - |
| Acetyl-caffeoyl-p-coumaroylglycerol | 2.38 | 3.08 | 1.93 | 2.44 | 1.39 | 1.47 | 1.10 | 1.12 | 2.55 | 0.92 | 1.08 | 1.70 | 1.01 | - | 1.23 | 1.34 | 1.76 | 1.95 | - | - |
| 2-Acetylo-3-caffeoyl-1-feruloylglycerol | 1.24 | 1.61 | 1.05 | 1.21 | 0.53 | 0.53 | 0.40 | 0.28 | 1.37 | 0.62 | 0.75 | 1.05 | 0.77 | - | 0.91 | 0.85 | 1.14 | 1.02 | - | - |
| Chrysin | 4.98 | 3.25 | 4.58 | 3.38 | 5.98 | 5.54 | 6.55 | 6.91 | 1.78 | 6.81 | 7.24 | 4.59 | 3.89 | 11.85 | 4.71 | 2.02 | 4.39 | 7.37 | 15.25 | 5.82 |
| Sakuranetin | 0.60 | 0.86 | 0.58 | 0.68 | 1.14 | 0.90 | 0.50 | 0.67 | 1.07 | 1.61 | 0.57 | 0.55 | 0.84 | - | 0.56 | 1.01 | 0.43 | 0.57 | - | - |
| Pinocembrin chalcone | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Galangin | 3.38 | 2.34 | 3.05 | 2.30 | 5.92 | 6.10 | 7.10 | 7.13 | 2.08 | 5.65 | 5.60 | 5.95 | 3.20 | 12.23 | 4.39 | 3.19 | 2.29 | 5.04 | 10.59 | 6.08 |
| Pinobanksin-3-O-acetate | 1.24 | 1.15 | 1.82 | 1.21 | 3.92 | 3.43 | 4.37 | 4.93 | 1.18 | 2.96 | 4.20 | 3.94 | 1.63 | 8.92 | 2.88 | 1.72 | 1.92 | 1.94 | 4.04 | 8.73 |
| ** 2-Acetyl-1,3-di-p-coumaroylglycerol | 11.41 | 14.95 | 12.16 | 13.34 | 6.52 | 7.32 | 5.54 | 6.00 | 11.12 | 5.43 | 7.25 | 4.52 | 7.60 | 0.50 | 6.33 | 8.31 | 5.87 | 9.61 | - | 0.84 |
| ** 2-Acetyl-p-3-coumaroyl-1-feruloylglycerol | 4.45 | 5.68 | 6.03 | 5.21 | 2.62 | 2.69 | 2.21 | 2.43 | 4.44 | 2.84 | 2.99 | 2.53 | 3.00 | - | 3.32 | 3.72 | 3.28 | 3.95 | - | 0.20 |
| p-Coumaric acid benzyl ester | 10.71 | 1.10 | 9.67 | 11.39 | 5.83 | 6.37 | 5.12 | 5.55 | 10.51 | 7.63 | 6.89 | 4.39 | 9.01 | 2.63 | 8.97 | 9.31 | 5.33 | 5.35 | 2.18 | 3.37 |
| Pinostrobin chalcone | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| p-Coumaric acid cinnamic ester | 0.12 | 0.13 | 0.25 | 0.22 | - | 0.41 | 0.22 | - | 0.34 | 0.32 | 0.98 | 2.76 | 1.66 | 0.59 | 2.42 | 0.80 | - | 0.22 | - | 7.90 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okińczyc, P.; Szumny, A.; Szperlik, J.; Kulma, A.; Franiczek, R.; Żbikowska, B.; Krzyżanowska, B.; Sroka, Z. Profile of Polyphenolic and Essential Oil Composition of Polish Propolis, Black Poplar and Aspens Buds. Molecules 2018, 23, 1262. https://doi.org/10.3390/molecules23061262
Okińczyc P, Szumny A, Szperlik J, Kulma A, Franiczek R, Żbikowska B, Krzyżanowska B, Sroka Z. Profile of Polyphenolic and Essential Oil Composition of Polish Propolis, Black Poplar and Aspens Buds. Molecules. 2018; 23(6):1262. https://doi.org/10.3390/molecules23061262
Chicago/Turabian StyleOkińczyc, Piotr, Antoni Szumny, Jakub Szperlik, Anna Kulma, Roman Franiczek, Beata Żbikowska, Barbara Krzyżanowska, and Zbigniew Sroka. 2018. "Profile of Polyphenolic and Essential Oil Composition of Polish Propolis, Black Poplar and Aspens Buds" Molecules 23, no. 6: 1262. https://doi.org/10.3390/molecules23061262
APA StyleOkińczyc, P., Szumny, A., Szperlik, J., Kulma, A., Franiczek, R., Żbikowska, B., Krzyżanowska, B., & Sroka, Z. (2018). Profile of Polyphenolic and Essential Oil Composition of Polish Propolis, Black Poplar and Aspens Buds. Molecules, 23(6), 1262. https://doi.org/10.3390/molecules23061262