Establishment and Phytochemical Analysis of a Callus Culture from Ageratina pichinchensis (Asteraceae) and Its Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Callus Production
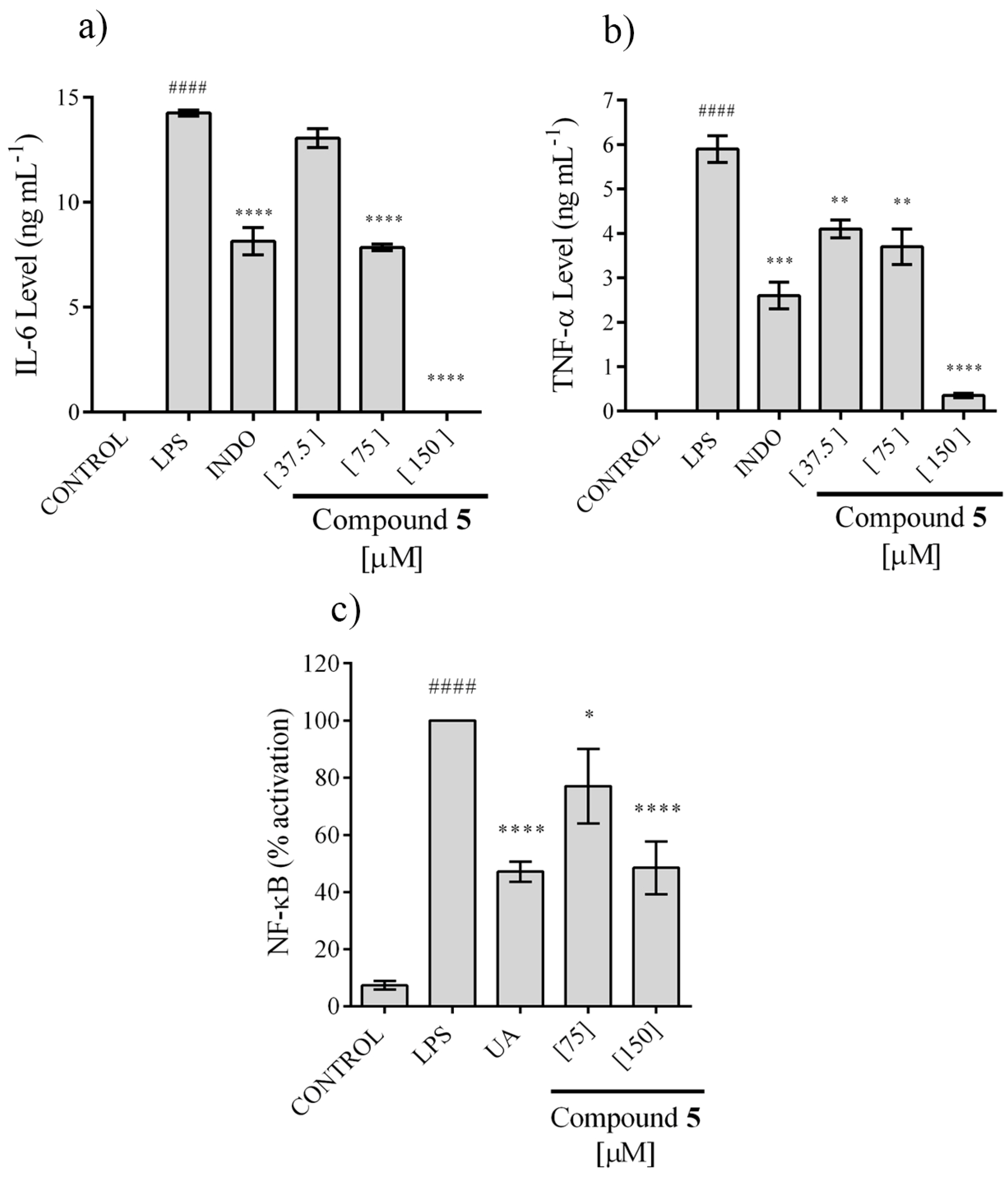
2.2. Anti-Inflammatory Activity
TPA-Induced Auricular Edema in Mice
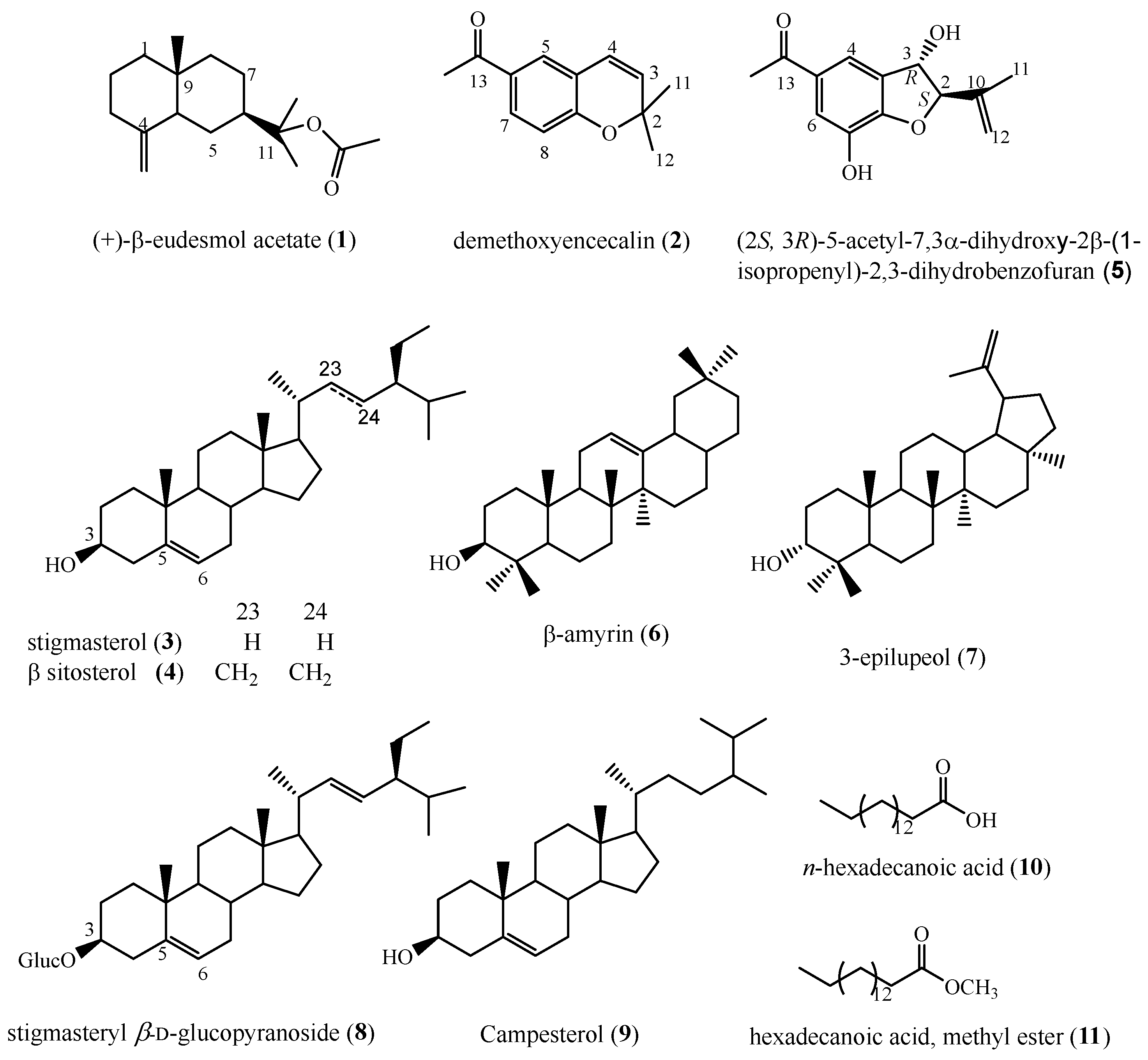
2.3. Chemical Analysis of Callus Biomass
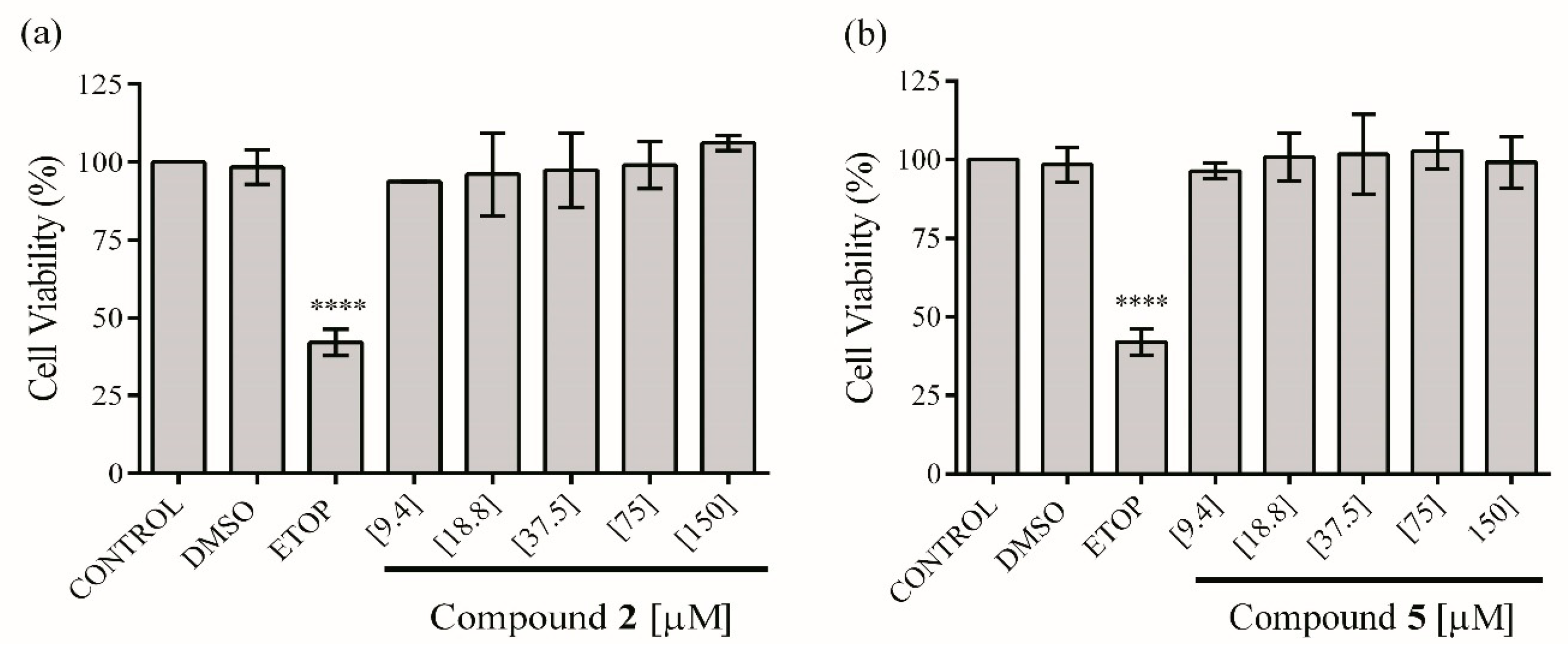
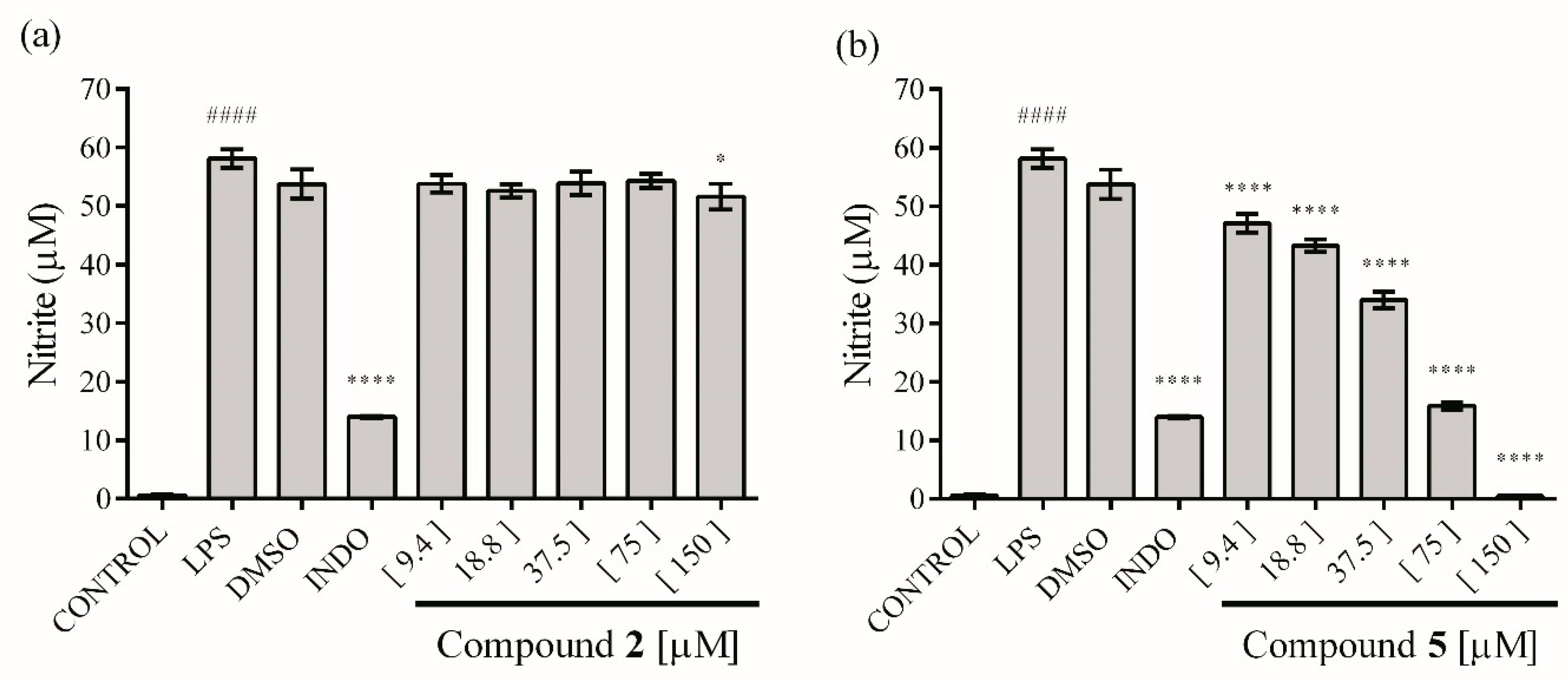
2.4. In Vitro Anti-Inflammatory Activity
2.5. Quantification of 2,3-Dihydrobenzofuran (5) in Wild Plant and Callus Extracts of A. pichinchensis
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Callus Induction and Maintenance
3.4. Extraction and Isolation
3.5. GC/MS Quantification of the Anti-Inflammatory Metabolites 5 and 7
Preparation of Extracts for GC-MS Quantification
3.6. Anti-Inflammatory Experiments
3.6.1. TPA-Induced Mouse Ear Edema
3.6.2. Cell Culture (RAW 264.7)
3.6.3. Assay for Cell Viability (RAW 264.7)
3.6.4. Treatment of Macrophages RAW 264.7 with LPS
3.6.5. Determination of NO Concentration
3.6.6. Measurement of IL-6 and TNF-α
3.6.7. Cells Culture (RAW-Blue)
3.6.8. Determination of NF-κB Activation
3.6.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ríos, M.Y.; Aguilar-Guadarrama, B.; Navarro, V. Two new benzofuranes from Eupatorium aschenbornianum and their antimicrobial activity. Planta Med. 2003, 69, 967–970. [Google Scholar] [PubMed]
- Argueta, A.; Cano, L.; Rodarte, M. Atlas de la Medicina Tradicional Mexicana, Tomo 1–3; Instituto Nacional Indigenista: Mexico City, Mexico, 1994; p. 1786. [Google Scholar]
- Avilés, M.; Suárez, G. Catálogo de Plantas Medicinales. Jardín Etnobotánico; Centro INAH: Cuernavaca, Mexico, 1994; p. 47. [Google Scholar]
- Navarro-García, V.M.; Gonzalez, A.; Fuentes, M.; Aviles, M.; Rios, M.Y.; Zepeda, G.; Rojas, M.G. Antifungical activities of nine traditional Mexican medicinal plants. J. Ethnopharmacol. 2003, 87, 85–88. [Google Scholar] [CrossRef]
- Torres-Barajas, L.; Rojas-Vera, J.; Morales-Méndez, A.; Rojas-Fermín, L.; Lucena, M.; Buitrago, A. Chemical composition and evaluation of antibacterial activity of essential oils of Ageratina jahnii and Ageratina pichinchensis collected in Mérida, Venezuela. Bol. Latinoam. Caribe Plant. Med. Aromat. 2013, 12, 92–98. [Google Scholar]
- Sánchez-Mendoza, M.E.; Reyes-Trejo, B.; Sánchez-Gómez, P.; Rodriguez-Silverio, J.; Castillo-Henkel, C.; Cervantes-Cuevas, H.; Arrieta, J. Bioassay-guided isolation of an anti-ulcer chromene from Eupatorium aschenbornianum: Role of nitric oxide, prostaglandins and sulfydryls. Fitorerapia 2010, 81, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mendoza, M.; Rodriguez-Silverio, J.; Rivero-Cruz, J.F.; Rocha-González, H.; Pineda-Farías, J.; Arrieta, J. Antinociceptive effect and gastroprotective mechanisms of 3,5-diprenyl-4-hydroxyacetophenone from Ageratina pichinchensis. Fitoterapia 2013, 87, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Zamilpa, A.; González-Cortazar, M.; Alonso-Cortés, D.; Jiménez-Ferrer, E.; Nicasio-Torres, P.; Aguilar-Santamaría, L.; Tortoriello, J. Pharmacological and chemical study to identify wound-healing active compounds in Ageratina pichinchensis. Planta Med. 2013, 79, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Romero, O.; Zamilpa, A.; Jiménez, E.; Tortoriello, J. Exploratory study on the effectiveness of a standardized extract from of Ageratina pichinchensis in patients with Chronic venous leg ulcers. Planta Med. 2011, 78, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Romero, O.; Zamilpa, A.; Tortoriello, J. Effectiveness and tolerability of standardized extract from Ageratina pichinchensis in patients with diabetic food ulcer: A randomized, controlled pilot study. Planta Med. 2015, 81, 272–278. [Google Scholar]
- Romero, O.; Zamilpa, A.; Ramos, A.; Alonso, D.; Jiménez, J.; Huerta, M.; Tortoriello, J. Effect on the wound healing process and in vitro cell proliferation by the medical mexican plant Ageratina pichinchensis. Planta Med. 2011, 77, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Zamilpa, A.; Díaz, G.; Tortoriello, J. Pharmacological effect of Ageratina pichinchensis on wound healing in diabetic rats and genotoxicity evaluation. J. Etnopharmacol. 2014, 156, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Gómez, F.; Quijano, L.; Calderón, J.; Perales, A.; Ríos, T. 2,2-Dimethylchromenes from Eupatorium aschembornianum. Phytochemistry 1982, 21, 2095–2097. [Google Scholar] [CrossRef]
- Aguilar-Guadarrama, B.; Navarro, V.; León-Rivera, I.; Ríos, M.Y. Active compounds against tinea pedis dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 23, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Nevagi, R.; Dighe, N.; Dighe, S. Biological and medicinal significance of benzofuran. Eur. J. Med. Chem. 2015, 97, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Dias, T.; Brito, A.; Proenca, F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016, 123, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, X.; Wang, D.; Lu, H. In vitro culture of croftonweed (Ageratina adenophora): Considerable potential for fast and convenient plantlet production. Weed Technol. 2007, 21, 445–452. [Google Scholar] [CrossRef]
- Gutiérrez-Rebolledo, G.; Garduño-Siciliano, L.; García-Rodríguez, R.; Pérez-González, M.; Chávez, M.I.; Bah, M.; Siordia-Reyes, G.; Chamorro-Cevallos, G.; Jiménez-Arellano, M.A. Anti-inflammatory and toxicological evaluation of Moussonia deppeana (Schldl. & Cham) Hanst and verbascoside as a main active metabolite. J. Ethnopharmacol. 2016, 187, 269–280. [Google Scholar] [PubMed]
- Da Silva, K.A.; Paszcuk, A.F.; Passos, G.F.; Silva, E.S.; Bento, A.F.; Meotti, F.C.; Calixto, J.B. Activation of cannabinoid receptors by the pentacyclic triterpene α,β-amyrin. Pain 2011, 152, 1872–1887. [Google Scholar] [CrossRef] [PubMed]
- Chicca, A.; Marazzi, J.; Gertsch, J. The antinociceptive triterpene β-amyrin inhibits 2-arachidonoylglycerol (2-AG) hydrolysis without directly targeting cannabinoid receptors. Br. J. Pharmacol. 2012, 167, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.F.; Cherng, J.Y. Reduction of adhesion molecule production and alteration of eNOS and endothelin-1 mRNA expression in endothelium by Euphorbia hirta L. through its beneficial β-amyrin molecule. Molecules 2014, 19, 10534–10545. [Google Scholar] [CrossRef] [PubMed]
- Romero-Estrada, A.; Maldonado, M.A.; González-Christen, J.; Marquina, B.S.; Garduño-Ramírez, M.L.; Rodríguez-López, V.; Alvarez, L. Anti-inflammatory and antioxidative effects of six pentacyclic triterpenes isolated from the Mexican copal resin of Bursera copallifera. BMC Complement. Altern. Med. 2016, 16, 422. [Google Scholar] [CrossRef] [PubMed]
- Backhouse, N.; Rosales, I.; Apablaza, C.; Goity, I.; Erazo, S.; Negrete, R.; Thedoluz, C.; Rodríguez, J.; Delporte, C. Analgesic, anti-inflammatory and antioxidant properties of Buddleja globosa, Buddlejaceae. J. Ethnopharmacol. 2008, 116, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Antiinflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yuan, J.; Wu, S.; Huang, J.; Xu, X.; Wu, Z.; Gao, H. Anti-inflammation furanoditerpenoids from Caesalpinia minax Hance. Phytochemistry 2015, 117, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Kanwar, R.K.; Burrow, H.; Baratchi, S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr. Med. Chem. 2009, 16, 2373–2394. [Google Scholar] [CrossRef] [PubMed]
- Meckes, M.; Garduño-Ramírez, M.L.; Marquina, S.; Alvarez, L. Iridoides adicionales de la planta medicinal Astianthus viminalis y su actividad hipoglucemiante y antihiperglucemiante. Rev. Soc. Quím. Mexico 2001, 45, 195–199. [Google Scholar]
- Alvarez, L.; Herrera-Arellano, A.; Marquina, S.; Tortoriello, J.; Zamilpa, A.; González, M.; Villarreal, M.L.; Martínez-Rivera, M.A.; López-Villegas, E.D.; Rodríguez-Tovar, A.V.; et al. Anti-mycotic and anti-inflammatory constituents from four Mexican medicinal Solanum species. Curr. Top. Steroid Res. 2009, 6, 89–104. [Google Scholar]
- Mincione, E.; Iavarone, C. Terpenes from Arabian Commiphora myrra. I. Chim. Ind. 1972, 5, 424–425. [Google Scholar]
- Jena, S.; Ray, A.; Banerjee, A.; Sahoo, A.; Nasim, N.; Sahoo, S.; Kar, B.; Patnaik, J.; Panda, P.; Nayak, S. Chemical composition and antioxidant activity of essential oil from leaves and rhizomes of Curcuma angustifolia Roxb. Nat. Prod. Res. 2017, 31, 2188–2191. [Google Scholar] [CrossRef] [PubMed]
- Willuhn, G.; Junior, I.; Wendisch, D. Desmethoxyencecalin and thymol derivatives from Arnica sachalinensis. Planta Med. 1986, 5, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Liu, W.X.; Pei, G.; Ren, H.; Wang, J.; Xu, Q.L.; Xie, H.H.; Wan, F.H.; Tan, J.W. Phenolics from Ageratina adenophora roots and their phytotoxic effects on Arabidopsis thaliana seed germination and seedlind growth. J. Agric. Food Chem. 2013, 61, 11792–11799. [Google Scholar] [CrossRef] [PubMed]
- Torres-Naranjo, M.; Suarez, A.; Gilardoni, G.; Cartuche, L.; Flores, P.; Morocho, V. Chemical constituents of Muehlenbeckia tomnifolia (Kunth) Meisn (Polygonaceae) and its in vitro α-amilase and α-glucosidase inhibitory activities. Molecules 2016, 21, 1461. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, K.; Ham, I.; Choi, H.-Y. Inhibition of lipopolysaccharide induced nitric oxide and protaglandin E2 production by chloroform fraction of Cudrania tricuspidata in RAW 264.7 macrophages. BMC Complement. Altern. Med. 2012, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent anti-inflamatory activity of ursolic acid, a triterpenoid antioxidant, is mediated throgh suppresion of NF-kB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Majumdar, S.; Banerjee, S.; Aggarwal, B.B. Ursolic acid inhibits nuclear factor-kB activation induced by carcinogenic agents through suppression of IƙBα kinase and p65 phosphorylation: Correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003, 63, 4375–4383. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds 1–4, 6, and 7 are available from the authors. |
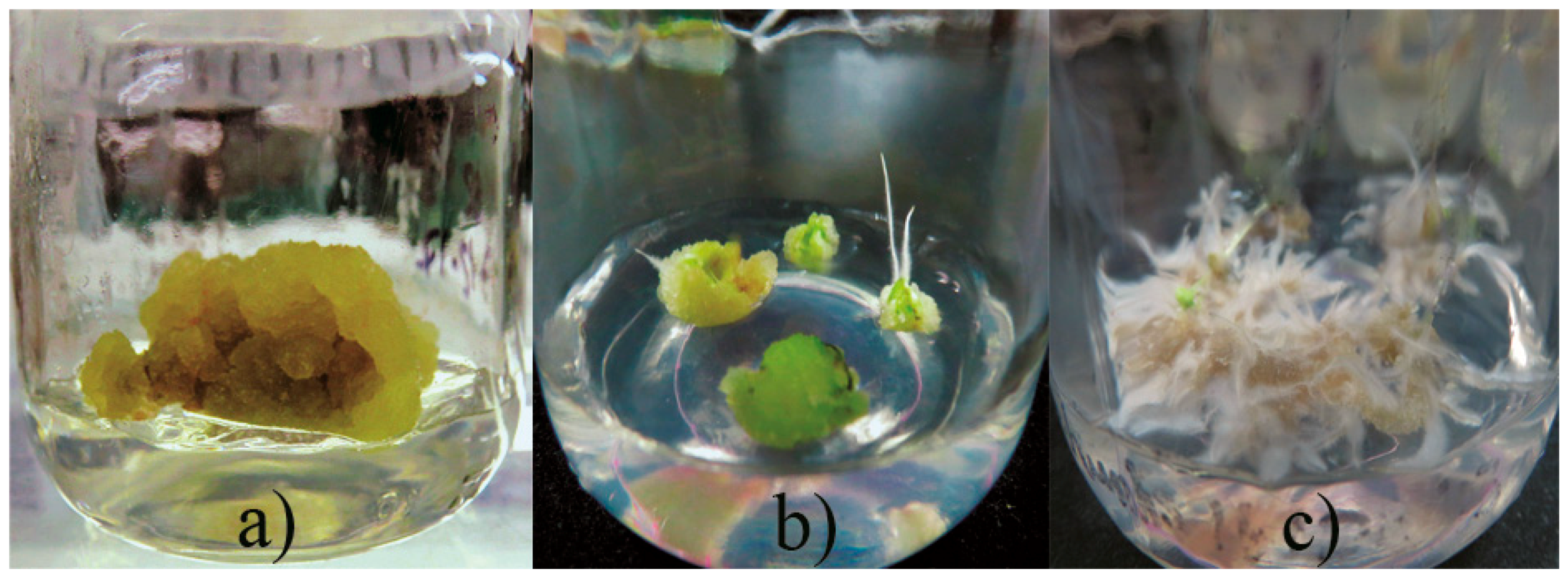
| PGRs (mg L−1) | Callus (%) | Callus with Roots (%) | Roots (%) | |
|---|---|---|---|---|
| NAA | KIN | |||
| 0 | 0 | 0.00 ± 0.00 e | 0.00 ± 0.00 c | 0.00 ± 0.00 d |
| 0 | 0.1 | 0.00 ± 0.00 e | 0.00 ± 0.00 c | 0.00 ± 0.00 d |
| 0 | 1 | 0.00 ± 0.00 e | 28.33 ± 4.19 ab | 33.33 ± 4.35 abc |
| 0 | 2 | 0.00 ± 0.00 e | 21.67 ± 5.03 bc | 51.67 ± 10.40 a |
| 0.1 | 0 | 0.00 ± 0.00 e | 0.00 ± 0.00 c | 0.00 ± 0.00 d |
| 0.1 | 0.1 | 0.00 ± 0.00 e | 20.00 ± 4.45 bc | 36.67 ± 7.52 abc |
| 0.1 | 1 | 0.00 ± 0.00 e | 33.33 ± 6.37 ab | 51.67 ± 8.40 a |
| 0.1 | 2 | 0.00 ± 0.00 e | 31.67 ± 5.23 ab | 40.00 ± 6.25 ab |
| 1 | 0 | 30.00 ± 8.66 d | 0.00 ± 0.00 c | 0.00 ± 0.00 d |
| 1 | 0.1 | 81.67 ± 7.64 a | 7.50 ± 1.15 c | 0.00 ± 0.00 d |
| 1 | 1 | 41.67 ±7.64 bcd | 36.67 ± 4.04 ab | 21.66 ± 3.45 bcd |
| 1 | 2 | 5.00 ± 0.61 e | 50.00 ± 6.35 a | 35.16 ± 7.22 abc |
| 2 | 0 | 33.33 ± 5.77 cd | 0.00 ± 0.00 c | 0.00 ± 0.00 d |
| 2 | 0.1 | 51.67 ±12.58 b | 33.33 ± 3.84 ab | 15.00 ± 4.25 cd |
| 2 | 1 | 48.33 ± 4.16 bc | 20.00 ± 2.54 bc | 0.00 ± 0.00 d |
| 2 | 2 | 10.00 ± 1.11 e | 30.00 ± 3.76 ab | 38.33 ± 2.89 ab |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ramos, M.; Bahena, S.M.; Romero-Estrada, A.; Bernabé-Antonio, A.; Cruz-Sosa, F.; Gonzálesssz-Christen, J.; Acevedo-Fernández, J.J.; Perea-Arango, I.; Alvarez, L. Establishment and Phytochemical Analysis of a Callus Culture from Ageratina pichinchensis (Asteraceae) and Its Anti-Inflammatory Activity. Molecules 2018, 23, 1258. https://doi.org/10.3390/molecules23061258
Sánchez-Ramos M, Bahena SM, Romero-Estrada A, Bernabé-Antonio A, Cruz-Sosa F, Gonzálesssz-Christen J, Acevedo-Fernández JJ, Perea-Arango I, Alvarez L. Establishment and Phytochemical Analysis of a Callus Culture from Ageratina pichinchensis (Asteraceae) and Its Anti-Inflammatory Activity. Molecules. 2018; 23(6):1258. https://doi.org/10.3390/molecules23061258
Chicago/Turabian StyleSánchez-Ramos, Mariana, Silvia Marquina Bahena, Antonio Romero-Estrada, Antonio Bernabé-Antonio, Francisco Cruz-Sosa, Judith Gonzálesssz-Christen, Juan José Acevedo-Fernández, Irene Perea-Arango, and Laura Alvarez. 2018. "Establishment and Phytochemical Analysis of a Callus Culture from Ageratina pichinchensis (Asteraceae) and Its Anti-Inflammatory Activity" Molecules 23, no. 6: 1258. https://doi.org/10.3390/molecules23061258






