The Conversion of 5,5′-Bi(1,2,3-dithiazolylidenes) into Isothiazolo[5,4-d]isothiazoles
Abstract
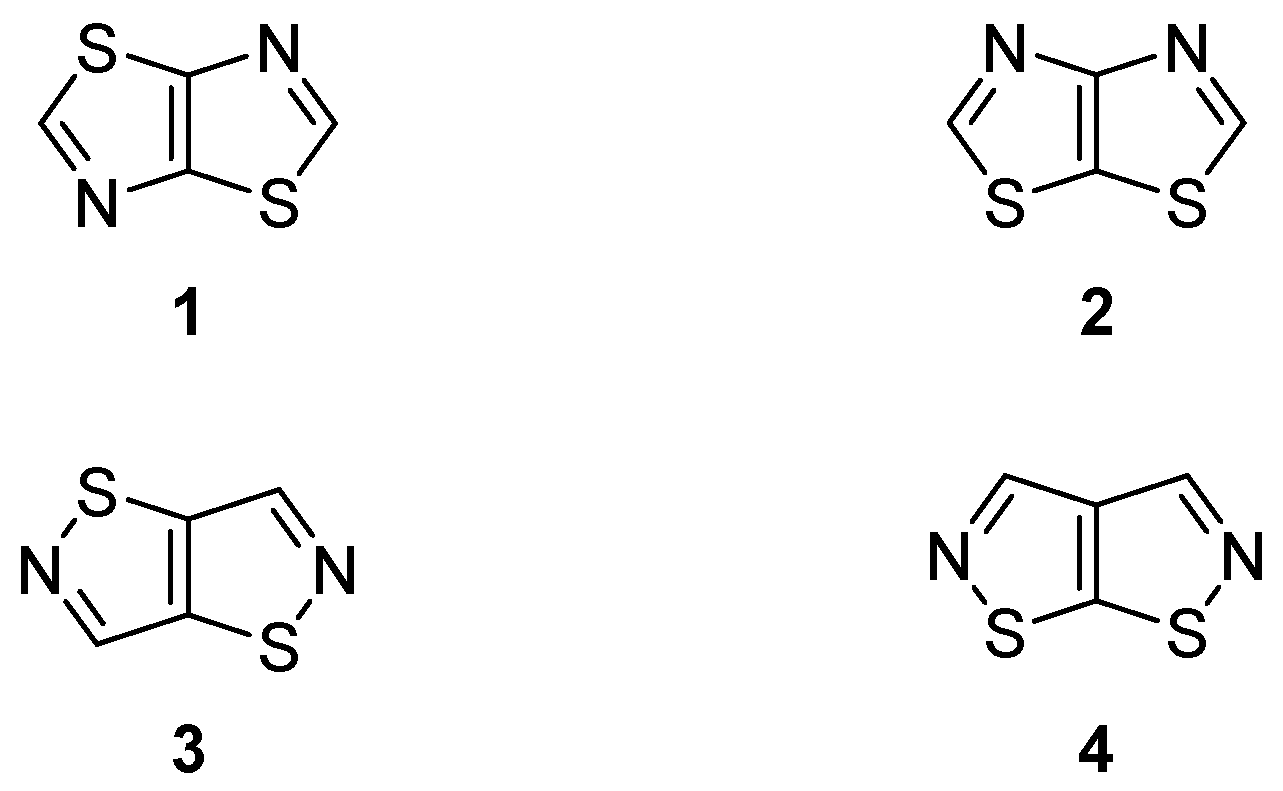
:1. Introduction
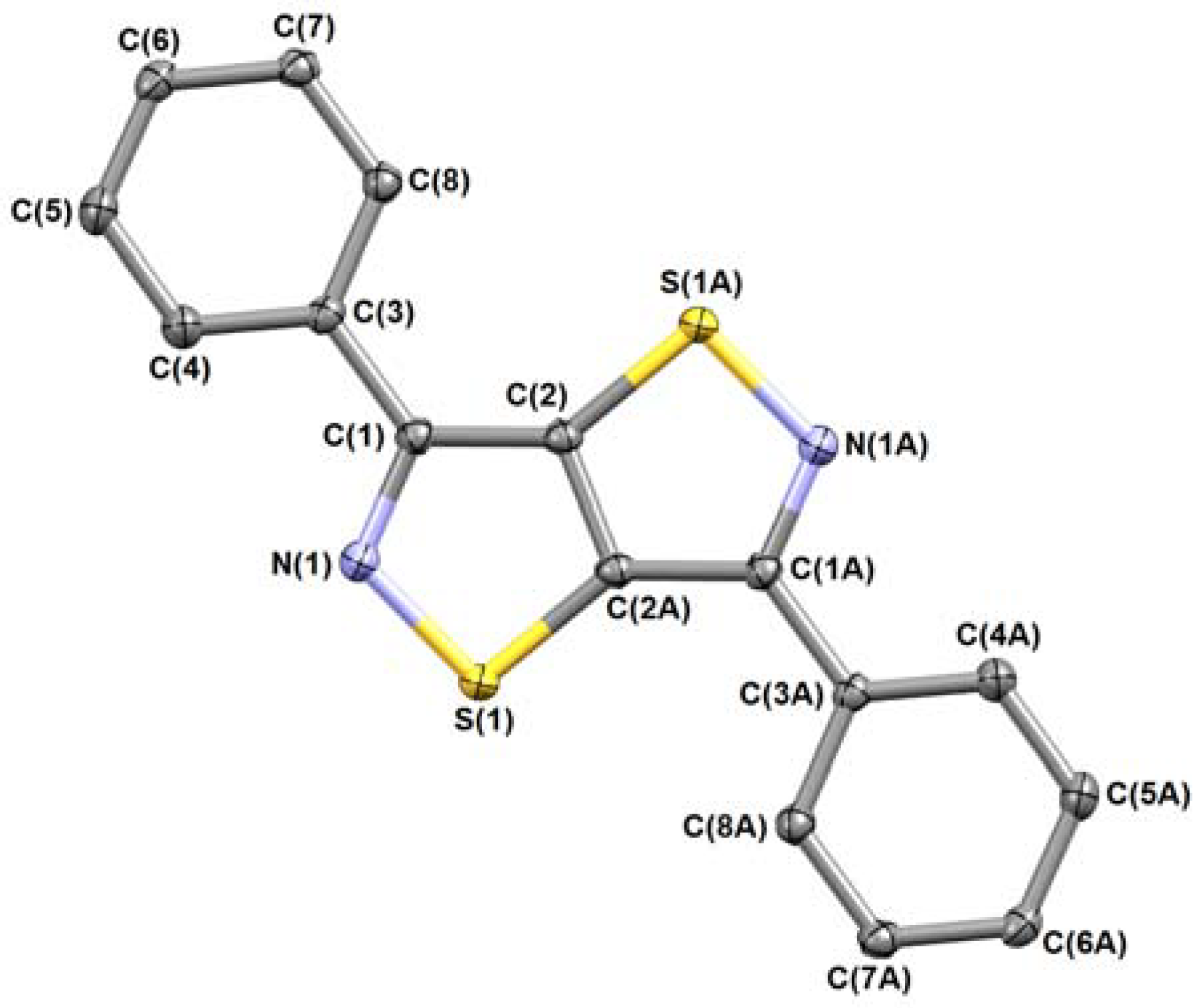
2. Results and Discussion
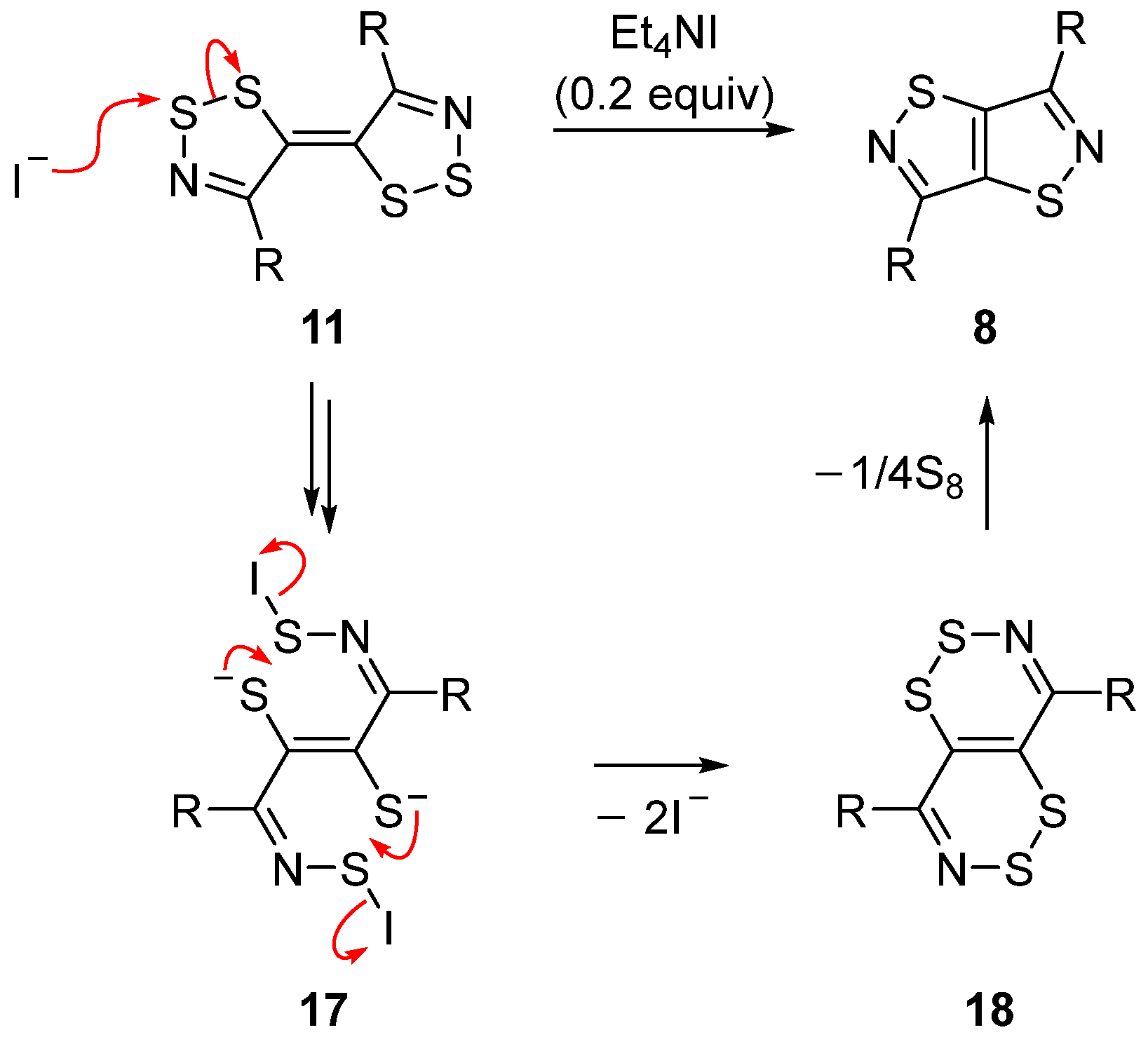
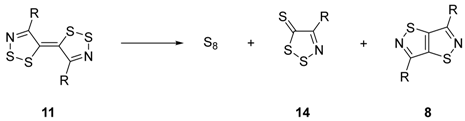
Mechanistic Rationale
3. Materials and Methods
3.1. General Methods and Materials
3.2. (E)-4,4′-Bis(4-bromophenyl)-5,5′-Bi(1,2,3-dithiazolylidene) (11f)
3.3. General Thermolysis Procedure (Method 1)
3.4. General ANRORC Procedure (Method 2)
3.5. Data on Compounds 8a–f
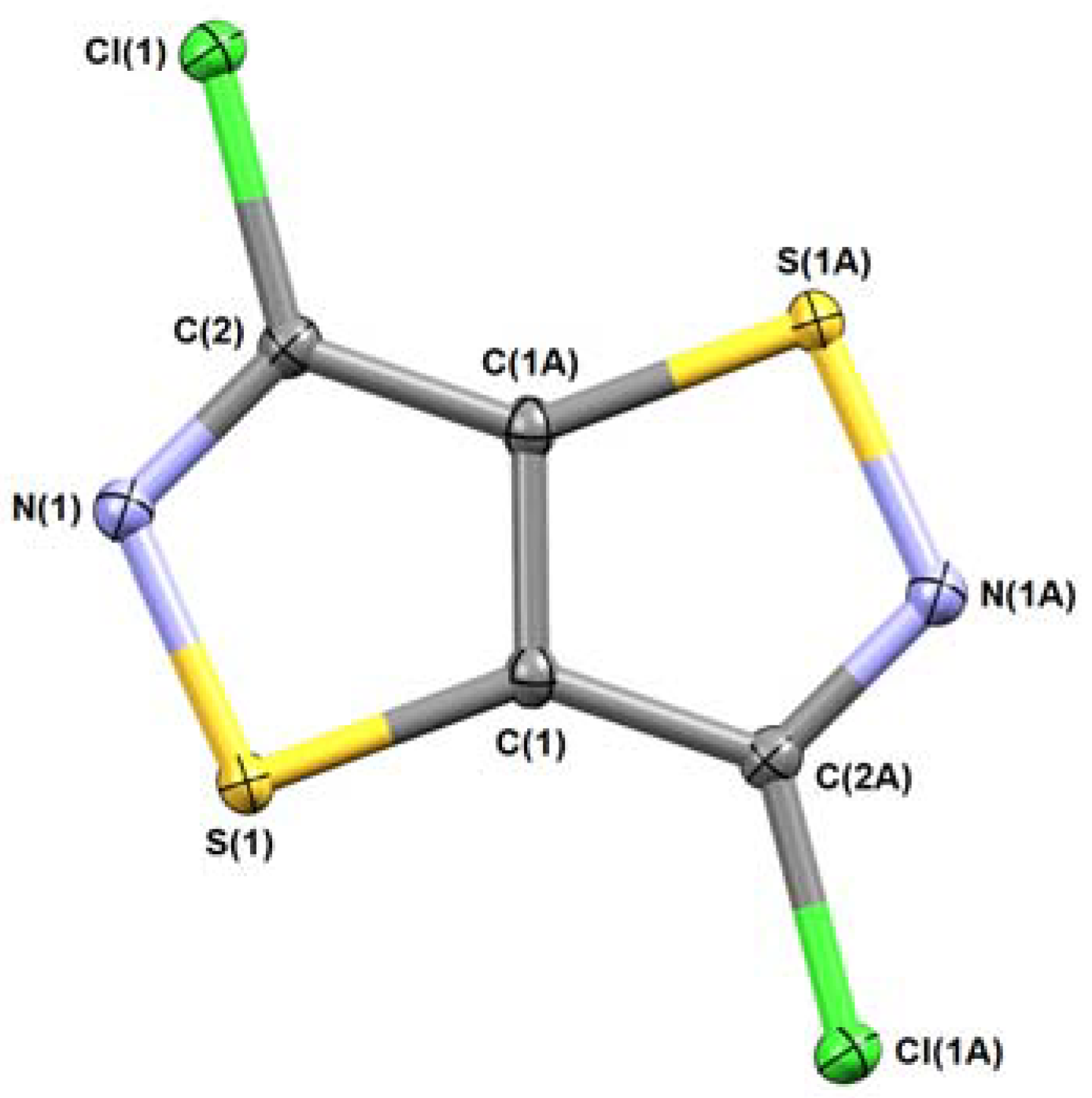
3.5.1. 3,6-Dichloroisothiazolo[5,4-d]isothiazole (8a)
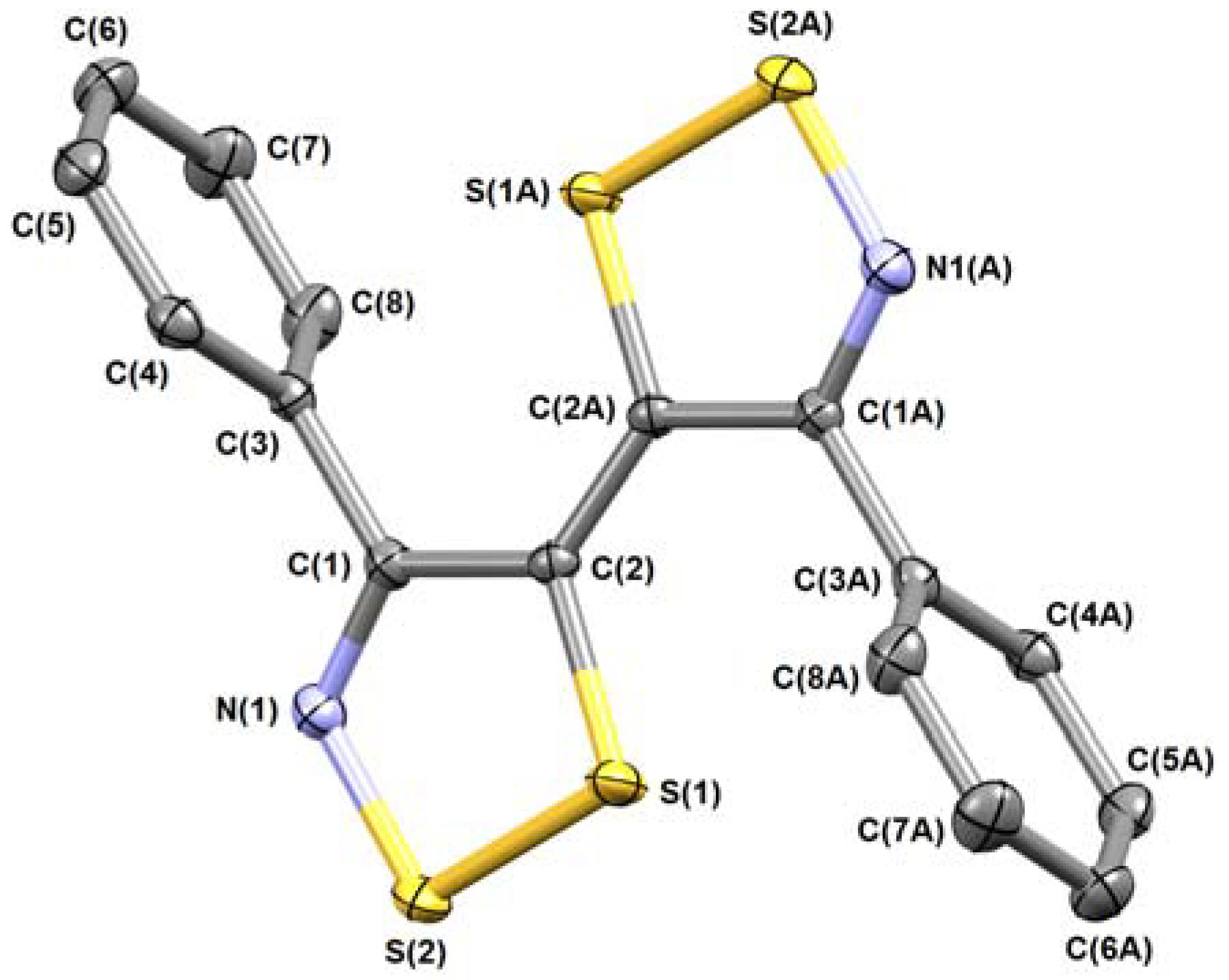
3.5.2. 3,6-Diphenylisothiazolo[5,4-d]isothiazole (8b)
3.5.3. 3,6-Bis(4-fluorophenyl)isothiazolo[5,4-d]isothiazole (8c)
3.5.4. 3,6-Bis(4-methoxyphenyl)isothiazolo[5,4-d]isothiazole (8d)
3.5.5. 3,6-Di(thien-2-yl)isothiazolo[5,4-d]isothiazole (8e)
3.5.6. 3,6-Bis(4-bromophenyl)isothiazolo[5,4-d]isothiazole (8f)
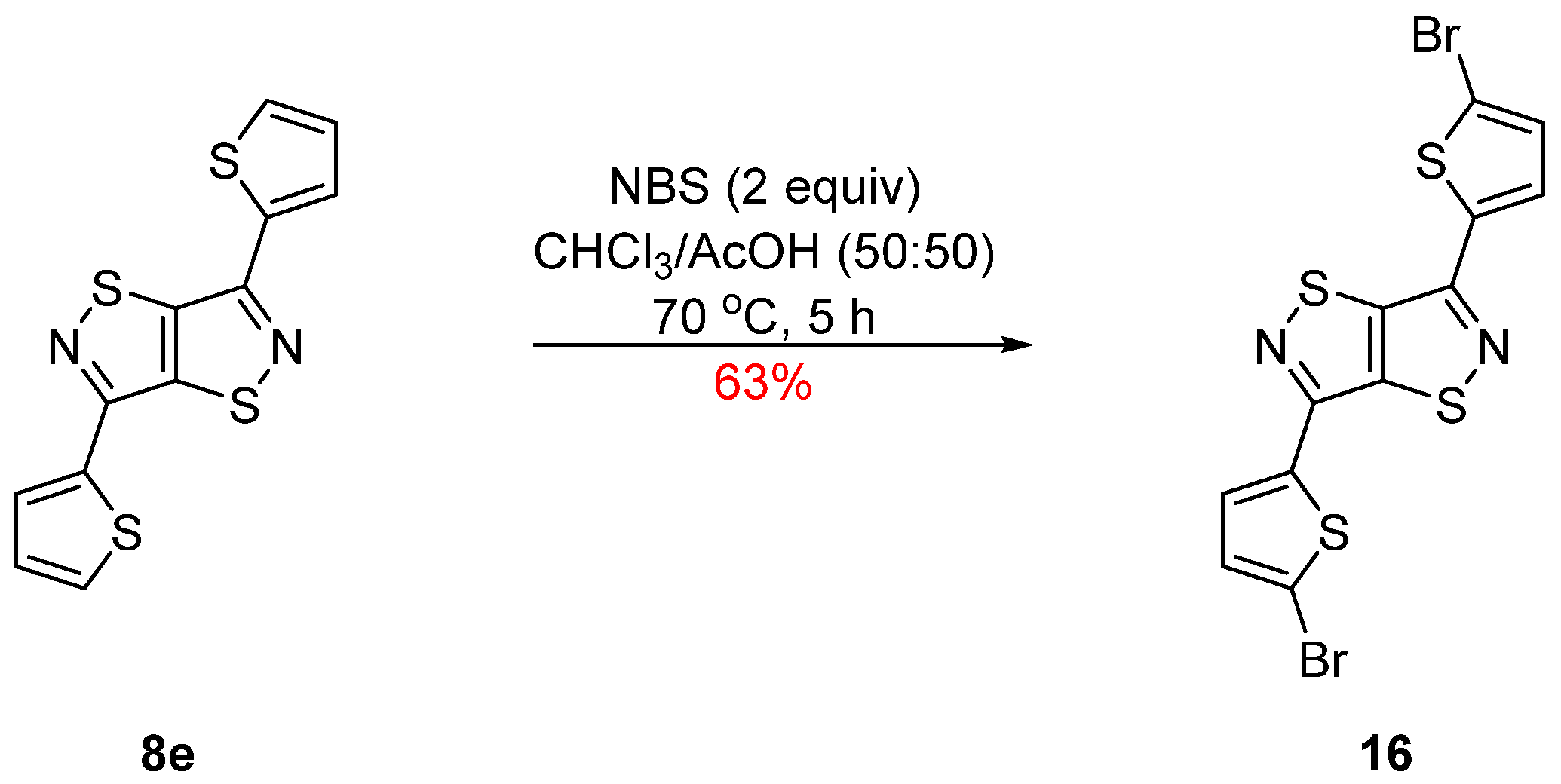
3.6. Bromination of 3,6-Di(thien-2-yl)isothiazolo[5,4-d]isothiazole (16)
3,6-Di(5,5′-dibromothien-2-yl)isothiazolo[5,4-d]isothiazole (16)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Litvinov, V.P. The latest achievements in thienothiophene chemistry. Russ. Chem. Rev. 2005, 74, 217–248. [Google Scholar] [CrossRef]
- Jung, J.W.; Jo, J.W.; Jung, E.H.; Jo, W.H. Recent progress in high efficiency polymer solar cells by rational design and energy level tuning of low bandgap copolymers with various electron-withdrawing units. Org. Electron. 2016, 31, 149–170. [Google Scholar] [CrossRef]
- Nielsen, C.B.; McCulloch, I. Recent advances in transistor performance of polythiophenes. Prog. Polym. Sci. 2013, 38, 2053–2069. [Google Scholar] [CrossRef]
- Johnson, J.R.; Ketcham, R.; Thiazolothiazoles, I. The Reaction of Aromatic Aldehydes with Dithioöxamide. J. Am. Chem. Soc. 1960, 82, 2719–2724. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Bevk, D.; Marin, L.; Lutsen, L.; Vanderzande, D.; Maes, W. Thiazolo[5,4-d]thiazoles-promising building blocks in the synthesis of semiconductors for plastic electronics. RSC Adv. 2013, 3, 11418–11431. [Google Scholar] [CrossRef]
- Mohareb, R.M.; Ibrahim, R.A.; Wardakhan, W.W. Synthesis of pyridine, pyran and thiazole containing thiophene derivatives and their anti-tumor evaluations. Med. Chem. Res. 2016, 25, 2187–2204. [Google Scholar] [CrossRef]
- Zahradník, P.; Magdolen, P.; Zahradník, P. Thiazolo[4,5-d]thiazole—A new domain for potential optoelectronic application. Tetrahedron Lett. 2010, 51, 5819–5821. [Google Scholar] [CrossRef]
- Demydchuk, B.A.; Brovarets, V.S.; Vasilenko, A.N.; Drach, B.S. Preparative conversion of chloralamides to 2,5-diaryl-3a,6a-dihydro-[1,3]thiazolo[4,5-d][1,3]thiazoles with the Lawesson reagent. Heteroat. Chem. 2008, 19, 677–681. [Google Scholar] [CrossRef]
- Beck, G.; Heitzer, H.; Holtschmidt, H. Synthesen von chlorierten S,N-Heterocyclen durch Dehalogenierungsreaktionen mit Schwefel. Synthesis 1985, 1985, 586–591. [Google Scholar] [CrossRef]
- Schäfer, V.H.; Bartho, B.; Gewald, K. Cyclisierungen mit 1-Nitro-2-anilino-äthylenen. J. Prakt. Chem. (Leipzig) 1977, 319, 149–158. [Google Scholar] [CrossRef]
- Beck, G.; Heitzer, H.; Holtschmidt, H. 2,5-Dichloro-thiazolo[4,5-d]thiazole and Process for Making Same. U.S. Patent No. 3,994,913A, 30 November 1976. [Google Scholar]
- Singh, J.M. Studies of the Chemistry of Azole Derivatives. VII. N-Substituted-2-amino-5-keto-thiazolidino[4,5-d]thiazoles. Bull. Chem. Soc. Jpn. 1968, 41, 2183–2184. [Google Scholar] [CrossRef]
- Rees, C.W.; Yue, T.-Y. Simultaneous 1,2-, 1,3- and 1,4-addition of trithiazyl trichloride to a conjugated diene. Chem. Commun. 1998, 1207–1208. [Google Scholar] [CrossRef]
- Kibbel, H.U.; Knebusch, C. 3,6-Bis(acylamino)-3H,6H-isothiazolo[4,3-c][1,2]-isothiazole; Darstellung und Eigenschaften. Z. Chem. 1989, 29, 17–18. [Google Scholar] [CrossRef]
- Yue, T.-Y. Trithiazyl Trichloride in the Synthesis of Sulfur-Nitrogen Heterocyclic Compounds. Ph.D. Thesis, University of London, London, UK, 1995. [Google Scholar]
- Hatchard, W.R. 4-Haloisothiazolo[4,5,d]-3-isothiazolesulfenyl Halides and Sulfenamides. U.S. Patent No. 3,232,935, 1 February 1966. [Google Scholar]
- Hatchard, W.R. 4-Cyanoisothiazolesulfenamides. U.S. Patent No. 3,149,107, 15 September 1964. [Google Scholar]
- Hatchard, W.R. 3, 4-Dihalo isothiazolo[4,5,d]isothiazole and Preparation Thereof. U.S. Patent No. 3,118,901, 21 January 1964. [Google Scholar]
- Konstantinova, L.S.; Bol′shakov, O.I.; Obruchnikova, N.V.; Tonga, A.; Picot, L.; Sopéna, V.; Lanneluc, I.; Sablé, S.; Thiéry, V.; Rakitin, O.A. One-pot synthesis of 5-phenylimino, 5-thieno or 5-oxo-1,2,3-dithiazoles and evaluation of their antimicrobial and antitumor activity. Bioorg. Med. Chem. Lett. 2009, 19, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.V.; Bol′shakov, O.I.; Konstantinova, L.S.; Rakitin, O.A. Synthesis and properties of 4-substituted 5H-1,2,3-dithiazol-5-ylidenes. Russ. Chem Bull. 2009, 58, 437–441. [Google Scholar] [CrossRef]
- Emayan, K.; Rees, C.W. The reaction of acetophenone oximes with disulfur dichloride; 4-aryl-5-arylimino-1,2,3-dithiazoles and pentathiepino[6,7-c]pyrrole. Bull. Soc. Chim. Belg. 1997, 106, 605–611. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Bol′shakov, O.I.; Baranovsky, I.V.; Bogacheva, A.M.; Strunyasheva, V.V.; Rakitin, O.A. A short and efficient synthesis of 5,5′-bi-1,2,3-dithiazoles. Mendeleev Commun. 2015, 25, 427–428. [Google Scholar] [CrossRef]
- Behringer, H.; Meinetsberger, E. 1,1′,2,2′-Tetrathiafulvalene, III. Synthese, Eigenschaften und Reaktionen von 3,3′-Bi(3H-1,2-dithiolylidenen) (1,1′,2,2′-Tetrathiafulvalenen). Liebigs Ann. Chem. 1981, 1928–1959. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Rakitin, O.A. Synthesis and properties of 1,2,3-dithiazoles. Russ. Chem. Rev. 2008, 77, 521–546. [Google Scholar] [CrossRef]
- Khmelnitski, L.I.; Rakitin, O.A. 1,2-Oxa/thia-3-azoles. In Comprehensive Heterocyclic Chemistry II; Storr, R.C., Ed.; Elsevier: Oxford, UK, 1996; Volume 4, pp. 409–432. [Google Scholar]
- Rakitin, O.A. 1,2-Oxa/thia-3-azoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 6, pp. 1–36. [Google Scholar]
- Besson, T.; Dozias, M.J.; Guillard, J.; Rees, C.W. New route to 2-cyanobenzothiazoles via N-arylimino-1,2,3-dithiazoles. J. Chem. Soc. Perkin Trans. 1 1998, 23, 3925–3926. [Google Scholar] [CrossRef]
- English, R.F.; Rakitin, O.A.; Rees, C.W.; Vlasova, O.G. Conversion of imino-1,2,3-dithiazoles into 2-cyanobenzothiazoles, cyanoimidoyl chlorides and diatomic sulfur. J. Chem. Soc. Perkin Trans. 1 1997, 3, 201–206. [Google Scholar] [CrossRef]
- Besson, T.; Rees, C.W. Some chemistry of 4,5-dichloro-1,2,3-dithiazolium chloride and its derivatives. J. Chem. Soc. Perkin Trans. 1 1995, 13, 1659–1662. [Google Scholar] [CrossRef]
- Rees, C.W. Polysulfur-nitrogen heterocyclic chemistry. J. Heterocycl. Chem. 1992, 29, 639–651. [Google Scholar] [CrossRef]
- Van der Plas, H.C. Chapter III SN(ANRORC) Reactions in Azaheterocycles Containing an “Inside” Leaving Group. Adv. Heterocycl. Chem. 1999, 74, 87–151. [Google Scholar] [CrossRef]
- Van der Plas, H.C. Chapter II SN(ANRORC) Reactions in Azines, Containing an “Outside” Leaving Group. Adv. Heterocycl. Chem. 1999, 74, 9–86. [Google Scholar] [CrossRef]
- Van der Plas, H.C. The SN(ANRORC) Mechanism: A New Mechanism for Nucleophilic Substitution. Acc. Chem. Res. 1978, 11, 462–468. [Google Scholar] [CrossRef]
- Deau, E.; Dubouilh-Benard, C.; Levacher, V.; Besson, T. Microwave-assisted synthesis of novel N-(4-phenylthiazol-2-yl)-benzo[d]thiazole-, thiazolo[4,5-b]pyridine-, thiazolo[5,4-b]pyridine- and benzo[d]oxazole-2-carboximidamides inspired by marine topsentines and nortopsentines. Tetrahedron 2015, 70, 5532–5540. [Google Scholar] [CrossRef]
- Koyioni, M.; Manoli, M.; Manolis, M.J.; Koutentis, P.A. Reinvestigating the Reaction of 1H-Pyrazol-5-amines with 4,5-Dichloro-1,2,3-dithiazolium Chloride: A Route to Pyrazolo[3,4-c]isothiazoles and Pyrazolo[3,4-d]thiazoles. J. Org. Chem. 2014, 79, 4025–4037. [Google Scholar] [CrossRef] [PubMed]
- Christoforou, I.C.; Kalogirou, A.S.; Koutentis, P.A. The preparation of dicyano-1,3,4-thiadiazole and tricyanothiazole via 1,2,3-dithiazole chemistry. Tetrahedron 2009, 65, 9967–9972. [Google Scholar] [CrossRef]
- Yarovenko, V.N.; Es′kov, A.A.; Kondrashev, P.A.; Ignatenko, A.V.; Zavarzin, I.V.; Vorontsova, L.G.; Sedishev, I.P.; Krayushkin, M.M.; Starikova, Z.A. Synthesis and Reactivity of N-Vinyl-1,2,3-dithiazolimines. Heterocycles 2005, 65, 1601–1608. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Christoforou, I.C.; Ioannidou, H.A.; Manos, M.J.; Koutentis, P.A. Ring transformation of (4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitriles to 3-haloisothiazole-5-carbonitriles. RSC Adv. 2014, 4, 7735–7748. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A.; Rees, C.W. Reactions of 1,2,3-dithiazoles with halogenated malononitriles. J. Chem. Soc. Perkin Trans. 1 2002, 10, 1236–1241. [Google Scholar] [CrossRef]
- Clarke, D.; Emayan, K.; Rees, C.W. New synthesis of isothiazoles from primary enamines. J. Chem. Soc. Perkin Trans. 1 1998, 1, 77–82. [Google Scholar] [CrossRef]
- Emayan, K.; English, R.F.; Koutentis, P.A.; Rees, C.W. New routes to benzothiophenes, isothiazoles and 1,2,3-dithiazoles. J. Chem. Soc. Perkin Trans. 1 1997, 22, 3345–3350. [Google Scholar] [CrossRef]
- Appel, R.; Janssen, H.; Siray, M.; Knoch, F. Synthese und Reaktionen des 4,5-Dichlor-1,2,3-dithiazolium-chlorids. Eur. J. Inorg. Chem. 1985, 118, 1632–1643. [Google Scholar] [CrossRef]
- Mailey, E.A. 3,4-Dichloroisothiazoles and Process for Making Them. U.S. Patent No. 3,341,547, 12 September 1967. [Google Scholar]
- Christoforou, I.C.; Koutentis, P.A. 3,4,5-Triarylisothiazoles via C–C coupling chemistry. Org. Biomol. Chem. 2007, 5, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Christoforou, I.C.; Koutentis, P.A. New regiospecific isothiazole C–C coupling chemistry. Org. Biomol. Chem. 2006, 4, 3681–3693. [Google Scholar] [CrossRef] [PubMed]
- Christoforou, I.C.; Koutentis, P.A.; Rees, C.W. Regiospecific Suzuki Coupling of 3,5-Dichloro-isothiazole-4-carbonitrile. Org. Biomol. Chem. 2003, 1, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Asquith, C.R.M.; Konstantinova, L.S.; Laitinen, T.; Meli, M.L.; Poso, A.; Rakitin, O.A.; Hofmann-Lehmann, R.; Hilton, S.T. Evaluation of Substituted 1,2,3-Dithiazoles as Inhibitors of the Feline Immunodeficiency Virus (FIV) Nucleocapsid Protein via a Proposed Zinc Ejection Mechanism. ChemMedChem 2016, 11, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Catto, M.; Koutentis, P.A.; Nicolotti, O.; Pochini, L.; Koyioni, M.; Introcaso, A.; Michaelidou, S.S.; Carotti, A.; Indiveri, C. Inactivation of the glutamine/amino acid transporter ASCT2 by 1,2,3-dithiazoles: Proteoliposomes as a tool to gain insights in the molecular mechanism of action and of antitumor activity. Toxicol. Appl. Pharmacol. 2012, 265, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, L.S.; Rakitin, O.A.; Rees, C.W.; Amelichev, S.A. Regioselective synthesis of pentathiepino-fused pyrroles and indoles. Mendeleev Commun. 2004, 14, 91–92. [Google Scholar] [CrossRef]
- Koyioni, M.; Manoli, M.; Koutentis, P.A. Synthesis of Fused 1,2,4-Dithiazines and 1,2,3,5-Trithiazepines. J. Org. Chem. 2014, 79, 9717–9727. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, L.R.; Weber, S.R.; Cook, S.P. Iron-Catalyzed C–N Bond Formation via the Beckmann Rearrangement. Synlett 2015, 26, 331–334. [Google Scholar] [CrossRef]
- Bruker. _computing_publication_material. Bruker SAINT v7.23A. 2005. [Google Scholar]
- Sheldrick, G.M. SADABS v2008/1, Bruker/Siemens Area Detector Absorption Correction Program; Bruker AXS: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
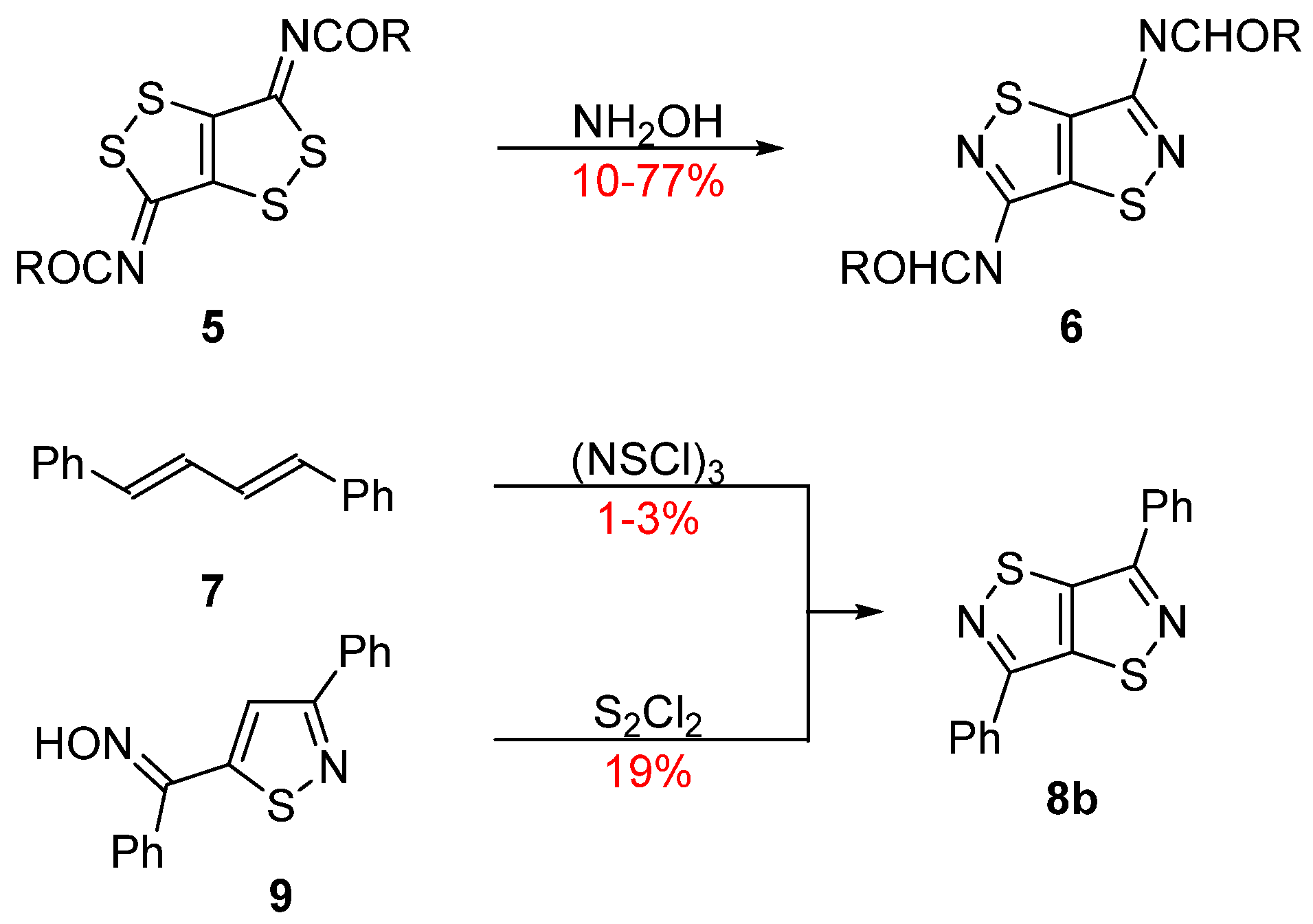


| Entry | R | Temp (°C) | Yield 8 (%) |
|---|---|---|---|
| 1 | Cl | 300 | 8a (8) |
| 2 | Ph | 230 | 8b (96) |
| 3 | 4-FC6H4 | 250 | 8c (83) |
| 4 | 4-MeOC6H4 | 280 | 8d (85) |
| 5 | thien-2-yl | 250 | 8e (90) |
| 6 | 4-BrC6H4 | 270 | 8f (82) |
| Entry | R | mmol | Thiophile | Solvent | Time | Yield 8 | Yields Ph3P = S or 14 |
|---|---|---|---|---|---|---|---|
| (Equiv) | (mL) | (h) | (%) | (%) | |||
| 1 | Ph | 0.05 | — | PhMe (5) | 29 | 8b (76) | |
| 2 | Ph | 0.05 | Ph3P (2) | PhMe (5) | 3 | 8b (81) | Ph3P = S (94) |
| 3 | Ph | 0.05 | Ph3P (4) | PhMe (5) | 2.5 | 8b (82) | Ph3P = S (96) |
| 4 | Ph | 0.05 | Et4NI (0.1) | PhMe (5) | 17 | 8b (78) | |
| 5 | Ph | 0.05 | Et4NI (0.2) | PhMe (5) | 2 | 8b (99) a | |
| 6 | Ph | 0.05 | Et4NI (2) | PhMe (5) | 4 | 8b (98) a | |
| 7 | Ph | 0.05 | Et4NI (0.2) | PhH (5) | 10 | 8b (nr) b | |
| 8 | Ph | 0.05 | Et4NI (0.2) | PhCl (5) | 8 | 8b (72) | 14b (15) [23] |
| 9 | Ph | 0.10 | Et4NI (0.2) | PhMe (5) | 6 | 8b (95) | 14b (5) [23] |
| 10 | Ph | 0.20 | Et4NI (0.2) | PhMe (5) | 13 | 8b (63) | 14b (26) [23] |
| 11 | 4-FC6H4 | 0.05 | Et4NI (0.2) | PhMe (5) | 8 | 8c (69) | 14c (9) [20] |
| 12 | 4-MeOC6H4 | 0.05 | Et4NI (0.2) | PhMe (5) | 6 | 8d (99) | |
| 13 | thien-2-yl | 0.05 | Et4NI (0.2) | PhMe (5) | 7 | 8e (92) | 14e (1) [48] |
| 8a | 8b | 8d | 11b | |
|---|---|---|---|---|
| CCDC | 1840070 | 1840071 | 1840073 | 1840072 |
| Chemical formula | C4Cl2N2S2 | C16H10N2S2 | C18H14N2O2S2 | C16H10N2S4 |
| Formula weight | 211.08 | 294.38 | 354.43 | 358.50 |
| Temperature (K) | 100 | 120 | 120 | 120 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Orthorhombic |
| Space group | C2/c | P21/c | P21/c | Pbca |
| a (Å) | 13.610(3) | 7.9372 (7) | 13.4370 (11) | 6.0657 (3) |
| b (Å) | 3.8300(7) | 5.3085 (5) | 3.9611 (3) | 15.7108 (9) |
| c (Å) | 13.843(3) | 15.6078 (14) | 14.6751 (12) | 16.1959 (9) |
| β (°) | 109.509(3) | 95.2366 (18) | 99.8510 (16) | 90 |
| V (Å3) | 680.2(2) | 654.88 (10) | 769.57 (11) | 1543.42 (14) |
| Z/Z′ | 4/0.5 | 2/0.5 | 2/0.5 | 4/0.5 |
| dcalc (g cm3) | 2.061 | 1.493 | 1.530 | 1.543 |
| µ (Mo Kα) | 14.73 | 8.52 | 8.69 | 8.45 |
| 2Θmax | 58 | 58 | 58 | 58 |
| Reflns. Collected/unique | 3819/907 | 4430/1729 | 8974/2053 | 17,657/2047 |
| Observed reflns [I > 2σ(I)] | 822 | 1556 | 1795 | 1883 |
| Rint (I) | 0.0213 | 0.0213 | 0.0242 | 0.0128 |
| R1 (F2) | 0.0205 | 0.0289 | 0.0308 | 0.0245 |
| wR2 | 0.0510 | 0.0794 | 0.0873 | 0.0541 |
| GOF | 1.082 | 1.044 | 1.035 | 1.003 |
| Δρmin/Δρmax | −0.282/0.418 | −0.202/0.407 | −0.329/0.387 | −0.221/0.417 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinova, L.S.; Baranovsky, I.V.; Strunyasheva, V.V.; Kalogirou, A.S.; Popov, V.V.; Lyssenko, K.A.; Koutentis, P.A.; Rakitin, O.A. The Conversion of 5,5′-Bi(1,2,3-dithiazolylidenes) into Isothiazolo[5,4-d]isothiazoles. Molecules 2018, 23, 1257. https://doi.org/10.3390/molecules23061257
Konstantinova LS, Baranovsky IV, Strunyasheva VV, Kalogirou AS, Popov VV, Lyssenko KA, Koutentis PA, Rakitin OA. The Conversion of 5,5′-Bi(1,2,3-dithiazolylidenes) into Isothiazolo[5,4-d]isothiazoles. Molecules. 2018; 23(6):1257. https://doi.org/10.3390/molecules23061257
Chicago/Turabian StyleKonstantinova, Lidia S., Ilia V. Baranovsky, Vlada V. Strunyasheva, Andreas S. Kalogirou, Vadim V. Popov, Konstantin A. Lyssenko, Panayiotis A. Koutentis, and Oleg A. Rakitin. 2018. "The Conversion of 5,5′-Bi(1,2,3-dithiazolylidenes) into Isothiazolo[5,4-d]isothiazoles" Molecules 23, no. 6: 1257. https://doi.org/10.3390/molecules23061257
APA StyleKonstantinova, L. S., Baranovsky, I. V., Strunyasheva, V. V., Kalogirou, A. S., Popov, V. V., Lyssenko, K. A., Koutentis, P. A., & Rakitin, O. A. (2018). The Conversion of 5,5′-Bi(1,2,3-dithiazolylidenes) into Isothiazolo[5,4-d]isothiazoles. Molecules, 23(6), 1257. https://doi.org/10.3390/molecules23061257








