Design and Efficacy of Nanogels Formulations for Intranasal Administration
Abstract
:1. Introduction
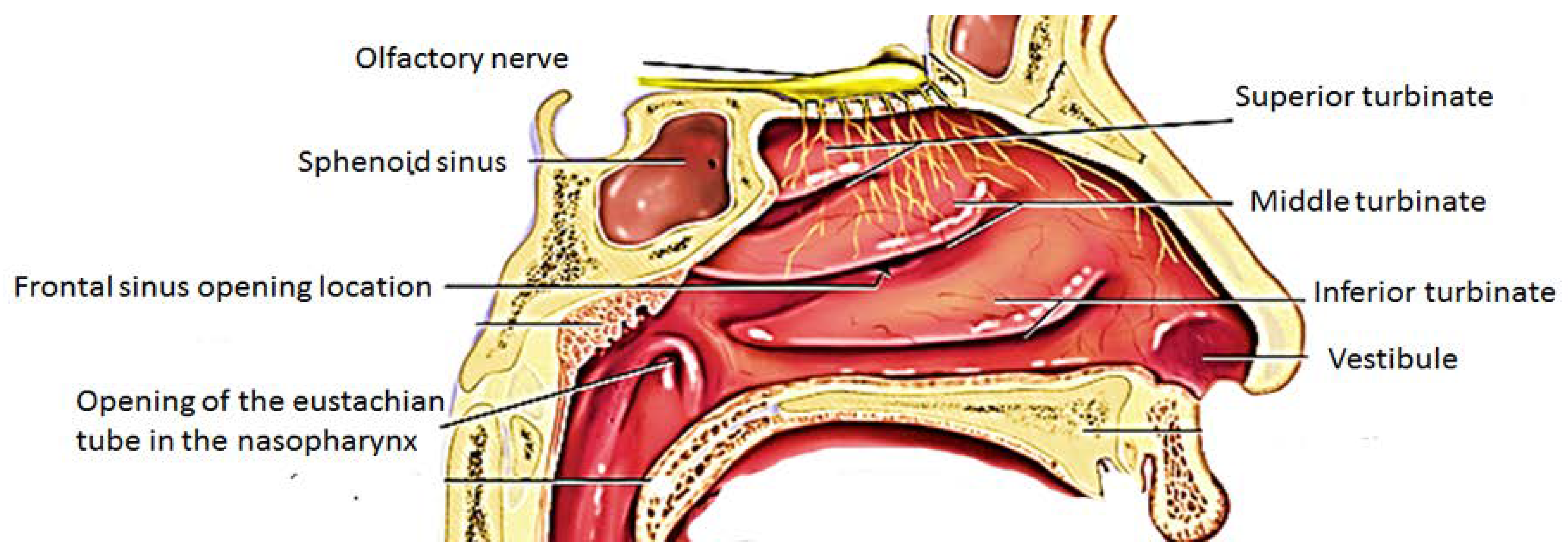
2. Anatomy of the Nose and Mechanism of Drug Uptake from the Nose-to-the Brain
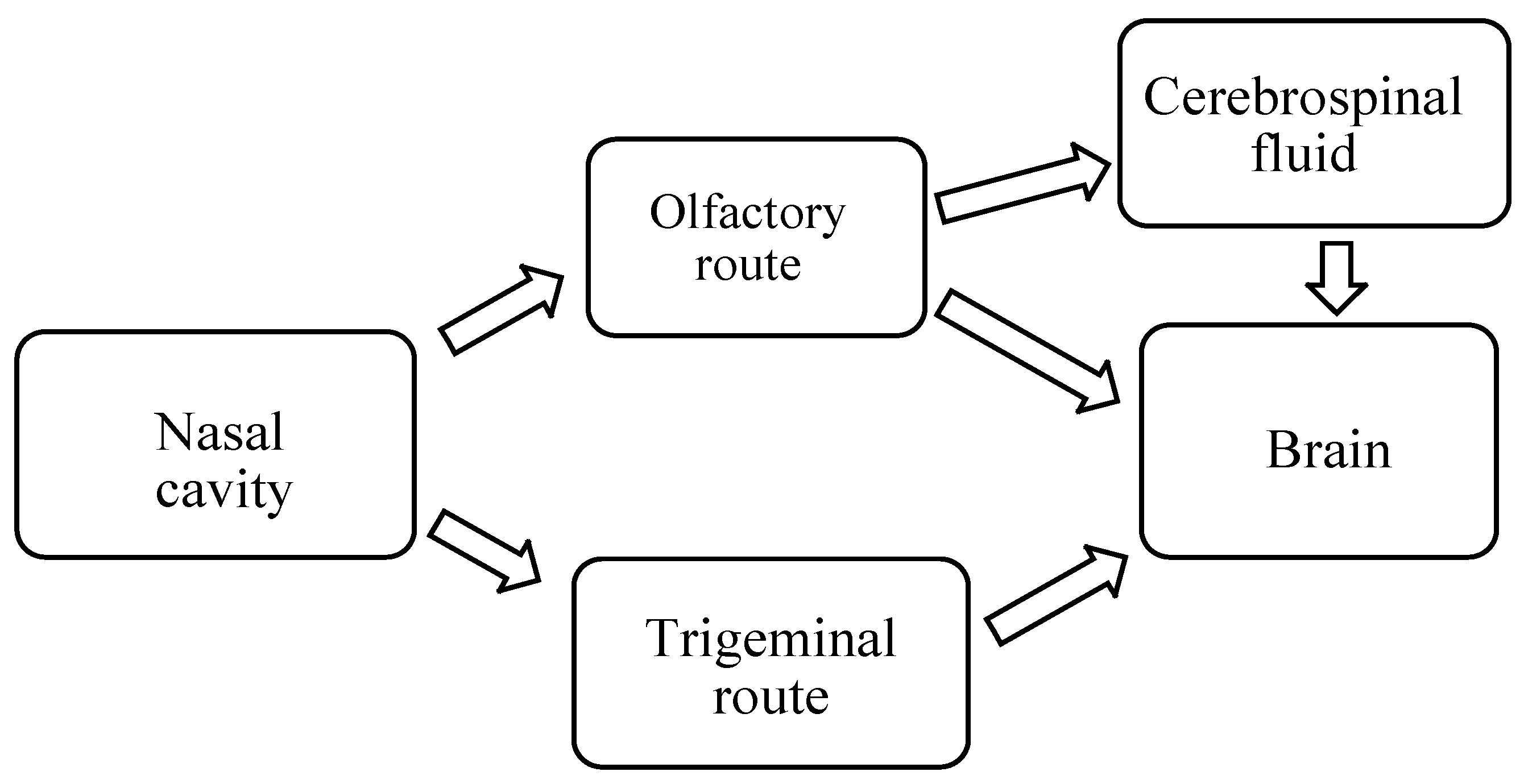
2.1. Mechanism of Drug Delivery from Nose-to-Brain
2.1.1. Olfactory Nerve Pathway
2.1.2. Trigeminal Nerve Pathway
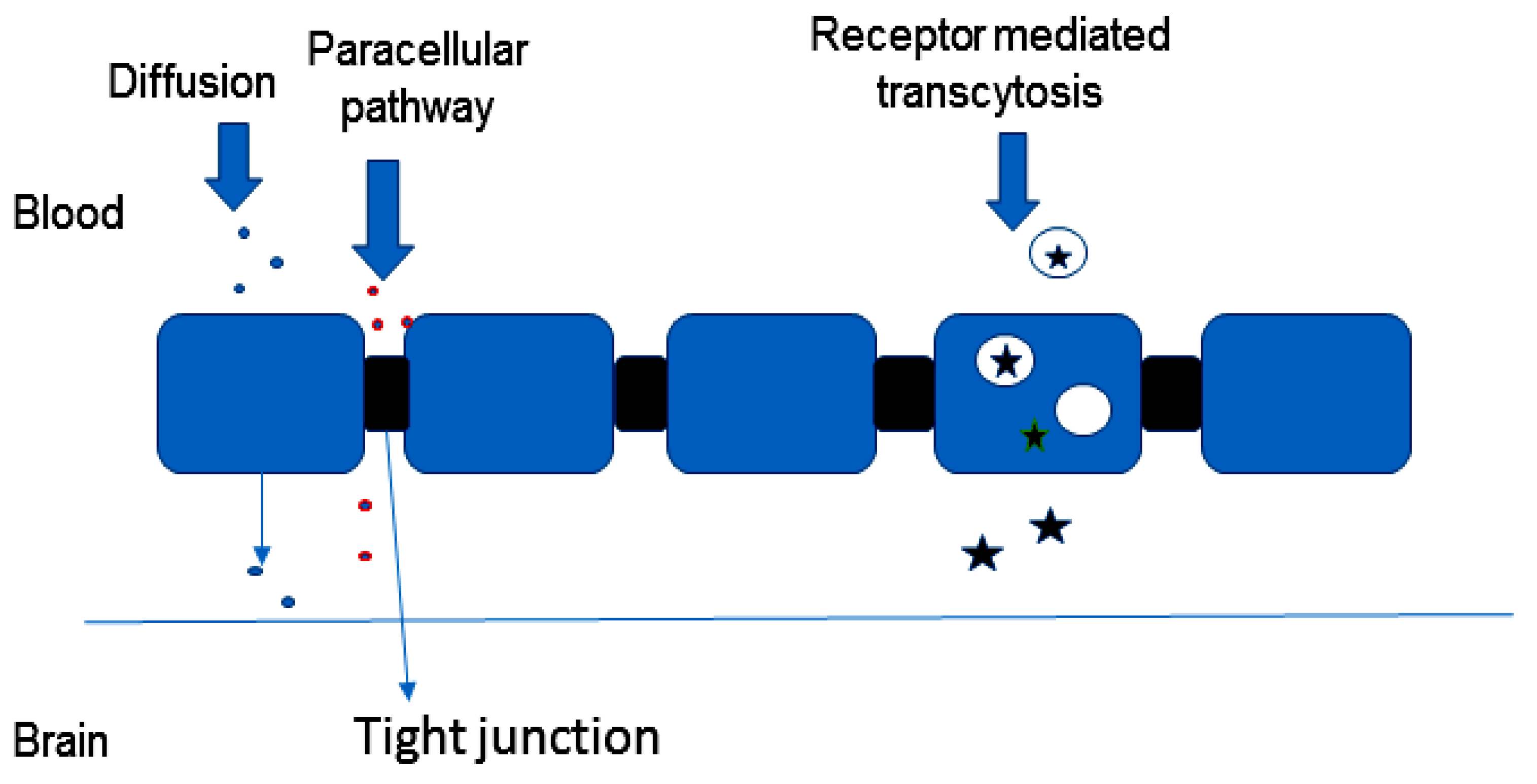
2.1.3. Other Pathways
3. Nanogels Designed for Drug Delivery to the Brain
4. Preparation of Nanogels
4.1. Nanogels Application for Intranasal Administration
4.1.1. Nanogels Application for Intranasal Administration of Anti-Alzheimer Drugs
4.1.2. Nanogels Application for Intranasal Administration of Anti-Schizophrenia Drug
4.1.3. Nanogels Application for Intranasal Administration of Anti-Migraine
4.1.4. Nanogels for Intranasal Delivery of Anti-Depression Drug
4.2. Nanogels Application for Intranasal Delivery of Anti-Hypertensive Drug
4.3. Nanogels Application for Intranasal Administration of Anticancer Drugs
4.4. Nanogel Application for Intranasal Delivery of Anti-HIV Drug
4.5. Nanogels Application as Vaccine Delivery System for Intranasal Administration
4.5.1. Nanogels as Vaccine Delivery System for Intranasal Administration for the Prevention of Bacterial Respiratory Infection Caused by Streptococcus pneumoniae
4.5.2. Nanogels Application as Vaccine Carrier for Intranasal Administration for the Prevention of Viral Respiratory Disease
4.6. Nanogels Application as Vaccine Delivery System for Intranasal Administration of Vaccine for the Prevention of Obesity
4.7. Nanogels as Vaccine Delivery System for Veterinary Applications
4.7.1. Nanogels Application as Vaccine for Intranasal Administration for the Prevention of Encephalomyelitis
4.7.2. Nanogels Application as Vaccine for Intranasal Administration for the Prevention of Mouth to Foot Disease
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wyatt, H.R. Update on treatment strategies for obesity. J. Clin. Endocrinol. Metab. 2013, 98, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, H.D. Drug targets for obesity and depression: From serotonin to leptin. Curr. Drug Targets 2016, 17, 1282–1291. [Google Scholar] [CrossRef]
- Comerma-Steffensen, S.; Grann, M.U.; Andersen, C.; Rungby, J.; Simonsen, U. Cardiovascular effects of current and future anti-obesity drugs. Curr. Vasc. Pharmacol. 2014, 12, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; et al. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the global burden of disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Neurological Diseases and Disorders Health Impacts of Climate Change. Environmental Health Perspectives and the National Institute of Environmental Health Sciences 22 April 2010. Available online: https://www.niehs.nih.gov/health/materials/a_human_health_perspective_on_climate_change_full_report_508.pdf (accessed on 28 May 2017).
- Neurological Testing and Treatment. Available online: http://www.mountsinai.org/patient-care/service-areas/neurology/treatment (accessed on 29 December 2017).
- Upadhyay, R.K. Drug delivery systems, CNS protection, and the blood brain barrier. BioMed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.S. Treatment of neurological and psychiatric disorders with deep brain stimulation; raising hopes and future challenges. Basic Clin. Neurosci. 2013, 4, 266–270. [Google Scholar] [PubMed]
- Xu, X.; Warrington, A.E.; Bieber, A.J.; Rodriguez, M. Enhancing CNS repair in neurological disease. CNS Drugs 2011, 25, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.I.; Sandeman, S.R.; et al. Nano carriers for drug transport across the blood-brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.B.; Zurita-Turk, M.; Saraiva, T.D.; De Castro, C.P.; Souza, B.M.; Agresti, P.M.; Lima, F.A.; Pfeiffer, V.N.; Azevedo, M.S.; Rocha, C.S.; et al. DNA vaccines approach: From concepts to applications. World J. Vaccin. 2014, 4, 50–71. [Google Scholar] [CrossRef]
- Luke, J.M.; Simon, G.G.; Soderholm, J.; Errett, J.S.; August, J.T.; Gale, M.; Hodgson, C.P.; Williams, J.A. Coexpressed RIG-I agonist enhances humoral immune response to influenza virus DNA vaccine. J. Virol. 2011, 85, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Guy, B. The perfect mix: Recent progress in adjuvant research. Nat. Rev. Microbiol. 2007, 5, 505–517. [Google Scholar] [PubMed]
- Ferreira, S.A.; Gama, F.M.; Vilanova, M. polymeric nanogels as vaccine delivery systems. Nanomedicine 2013, 9, 159–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, Z.; Patel, B.; Patel, S.; Pardeshi, C. Nose to brain targeted drug delivery bypassing the blood-brain barrier: An overview. Drug Invent. Today 2012, 4, 610–615. [Google Scholar]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Ghori, M.U.; Mahdi, M.H.; Smith, A.M.; Conway, B.R. Nasal drug delivery systems: An Overview. Am. J. Pharmacol. Sci. 2015, 3, 110–119. [Google Scholar]
- Rassu, G.; Soddu, E.; Cossu, M.; Gavini, E.; Giunchedi, P.; Dalpiaz, A. Particulate formulations based on chitosan for nose-to-brain delivery of drugs. A review. J. Drug Deliv. Sci. Technol. 2016, 32, 77–87. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., II. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.S.; Desale, S.S.; Bronich, T.N. An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Kersey, F.R.; Merkel, T.J.; Perry, J.L.; Napier, M.E.; DeSimone, J.M. Effect of aspect ratio and deformability on nanoparticle extravasation through nanopores. Langmuir 2012, 28, 8773–8781. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2013, 13, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Mura, J.S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Akiyoshi, K. Current advances in self-assembled nanogel delivery systems for immunotherapy. Adv. Drug Deliv. Rev. 2015, 95, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.V.; Bronich, T.K.; Kabanov, A.V. Nanosized cationic hydrogels for drug delivery: Preparation, properties and interactions with cells. Adv. Drug Deliv. Rev. 2002, 54, 135–147. [Google Scholar] [CrossRef]
- Raemdonck, K.; Demeester, J.; De Smedt, S. Advanced nanogel engineering for drug delivery. Soft Matter. 2009, 5, 707–715. [Google Scholar] [CrossRef]
- Vinogradov, S.V.; Zeman, A.D.; Batrakova, E.V.; Kabanov, A.V. Polyplex nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J. Control. Release 2005, 107, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, G.R.; Lyon, L.A. Microgel translocation through pores under confinement. Angew. Chem. Int. Ed. 2010, 49, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Z.; Li, Y.; Ella-Menye, J.R.; Bai, T.; Jiang, S.Y. Softer zwitterionic nanogels for longer circulation and lower splenic accumulation. ACS Nano. 2012, 6, 6681–6686. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.; Asadian-Birjand, M.; Balach, J. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.K.; Pradhan, A.; Banerjee, R.; Bahadur, D. Dual pH and temperature stimuli-responsive magnetic nanohydrogels for thermo-chemotherapy. J. Nanosci. Nanotechnol. 2014, 14, 4082–4089. [Google Scholar] [CrossRef] [PubMed]
- Water, J.J.; Kim, Y.; Maltesen, M.J.; Franzyk, H.; Foged, C.; Nielsen, H.M. Hyaluronic acid-based nanogels produced by microfluidics-facilitated self-assembly improves the safety profile of the cationic host defense peptide novicidin. Pharm. Res. 2015, 32, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Malhotra, S.; Molina, M.; Haag, R. Micro-and nanogels with labile crosslinks from synthesis to biomedical applications. Chem. Soc. Rev. 2015, 44, 1948–1973. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.R.; Kumar, P.A.; Raj, D.K.; James, N.R. Nanogels based on alginic aldehyde and gelatin by inverse miniemulsion technique: Synthesis and characterization. Carbohydr. Polym. 2015, 119, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A. In situ-based gels for nose to brain delivery for the treatment of neurological diseases. Pharmaceutics 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B. Nose to brain drug delivery: A recent update. J. Formul. Sci. Bioavailab. 2017, 1, 2. [Google Scholar]
- Kvq, L.; Nguyen, L.T. Environmental factors in Alzheimer’s and Parkinson’s diseases. J. Alzheimers Dis. Parkinsonism 2013, 3, 12. [Google Scholar]
- Šimić, G.; Babić, L.M.; Wray, S.; Harrington, C.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; De Silva, R.; Di Giovanni, G.; et al. Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Feldman, E.L. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp. Mol. Med. 2015, 47, e149. [Google Scholar] [CrossRef] [PubMed]
- Kroner, Z. The relationship between Alzheimer’s disease and diabetes: Type 3 diabetes? Altern. Med. Rev. 2009, 14, 373–379. [Google Scholar] [PubMed]
- Liu, Y.; Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J. Pathol. 2011, 225, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, Y.; Mao, Y.F.; Zheng, T.; Jiang, Y.; Yan, Y.; Yin, X.; Zhang, B. Long-term treatment with intranasal insulin ameliorates cognitive impairment, tau hyperphosphorylation, and microglial activation in a streptozotocin-induced Alzheimer’s rat model. Sci. Rep. 2017, 7, 45971. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Ditta, L.A.; Sabatino, M.A.; Militello, V.; San Biagio, P.L.; Di Giacinto, M.L.; Cristaldi, L.; Nuzzo, D.; Dispenza, C.; Giacomazza, D.; et al. Ionizing radiation-engineered nanogels as insulin nanocarriers for the development of a new strategy for the treatment of Alzheimer’s disease. Biomaterials 2016, 80, 179–194. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.M.; Wands, J.R. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: Relevance to Alzheimer’s disease. J. Alzheimer’s Dis. 2005, 7, 45–61. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P.; Baldassano, S.; Caruana, L.; Messina, E.; Marino Gammazza, A.; Cappello, F.; Mulè, F.; Di Carlo, M. Insulin resistance as common molecular denominator linking obesity to Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 723–735. [Google Scholar] [CrossRef] [PubMed]
- M de la Monte, S. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 35–66. [Google Scholar] [CrossRef]
- Picone, P.; Sabatino, M.A.; Ditta, L.A.; Amato, A.; San Biagio, P.L.; Mulè, F.; Giacomazza, D.; Dispenza, C.; Di Carlo, M. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J. Control. Release 2018, 270, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Claxton, A.; Baker, L.D.; Hanson, A.; Trittschuh, E.H.; Cholerton, B.; Morgan, A.; Callaghan, M.; Arbuckle, M.; Behl, C.; Craft, S. Long-acting intranasal insulin Detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J. Alzheimers Dis. 2015, 44, 897–906. [Google Scholar] [PubMed]
- Picone, P.; Giacomazza, D.; Vetri, V.; Carrotta, R.; Militello, V.; San Biagio, P.L.; Di Carlo, M. Insulin-activated Akt rescues ab oxidative stress-induced cell death by orchestrating molecular trafficking. Aging Cell 2011, 10, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, N.; Sabatino, M.A.; Przybytniak, G.; Kaluska, I.; Bondì, M.L.; Bulone, D.; Alessi, S.; Spadaro, G.; Dispenza, C. High-energy radiation processing, a smart approach to obtain PVP-graft-AA nanogels. Radiat. Phys. Chem. 2014, 9, 76–79. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: Optimization, biological efficacy, and potential toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef] [PubMed]
- Llorca, P.M.; Pereira, B.; Jardri, R.; Chereau-Boudet, I.; Brousse, G.; Misdrahi, D.; Fénelon, G.; Tronche, A.M.; Schwan, R.; Lançon, C.; Marques, A. Hallucinations in schizophrenia and Parkinson’s disease: an analysis of sensory modalities involved and the repercussion on patients. Sci. Rep. 2016, 6, 38152. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and treatment options. Pharm. Ther. 2014, 39, 638–645. [Google Scholar]
- Baltzley, S.; Mohammad, A.; Malkawi, A.H.; Al-Ghananeem, A.M. Intranasal drug delivery of olanzapine-loaded chitosan nanoparticles. AAPS PharmSciTech. 2014, 15, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef]
- Weatherall, M.W. The diagnosis and treatment of chronic migraine. Ther. Adv. Chronic Dis. 2015, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Mei, N.; Feng, L.; Jiang, X.G. Hydrophilic nasal gel of lidocaine hydrochloride. Arzneimittelforsch. 2009, 59, 635–640. [Google Scholar] [PubMed]
- Shah, B.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Intranasal delivery of venlafaxine loaded nanostructured lipid carrier: Risk assessment and QbD based optimization. J. Drug Deliv. Sci. Technol. 2016, 33, 37–50. [Google Scholar] [CrossRef]
- Lenox, R.H.; Frazer, A. Mechanism of action of antidepressants and mood stabilizers. In Neuropsychopharmacology: The Fifth Generation of Progress; Davis, K.L., Charney, D., Nemeroff, C.C., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2008; pp. 1138–1156. [Google Scholar]
- Dange, S.M.; Kamble, M.S.; Bhalerao, K.K.; Chaudhari, P.D.; Bhosale, A.V.; Nanjwade, B.K.; Shinde, S.A. Formulation and evaluation of venlafaxine nanostructured lipid carriers. J. Bionanosci. 2014, 8, 81–89. [Google Scholar] [CrossRef]
- Haque, S.; Md, S.; Sahni, J.K.; Ali, J.; Baboota, S. Development and evaluation of brain targeted intranasal alginate nanoparticles for treatment of depression. J. Psychiatr. Res. 2014, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A.; Gori, S.; Shive, M.; Desrosiers, E.; Buschmann, D. Monolithic gelation of chitosan solutions via enzymatic hydrolysis of urea. Carbohydr Polym. 2006, 64, 419–424. [Google Scholar] [CrossRef]
- Singh, D.; Rashid, M.; Hallan, S.S.; Mehra, N.K.; Prakash, A.; Mishra, N. Pharmacological evaluation of nasal delivery of selegiline hydrochloride-loaded thiolated chitosan nanoparticles for the treatment of depression. Artif. Cells Nanomed. Biotechnol. 2016, 44, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Sheraz, M.A.; Ahsan, S.F.; Khan, M.F.; Ahmed, S.; Ahmad, I. Formulations of amlodipine: A review. J. Pharm. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Kamble, M.S.; Dange, S.M.; Bhalerao, K.K.; Bhosale, A.V.; Nanjwade, B.K.; Shinde, S.A.; Chaudhari, P.D. Development and evaluation of amlodipine besylate nanogel. J. Bionanosci. 2015, 9, 22–27. [Google Scholar] [CrossRef]
- Shahnaz, G.; Vetter, A.; Barthelmes, J.; Rahmat, D.; Laffleur, F.; Iqbal, J.; Perera, G.; Schlocker, W.; Dünnhaput, S.; Augustijns, P.; et al. Thiolated chitosan nanoparticles for the nasal administration of leuprolide: Bioavailability and pharmacokinetic characterization. Int. J. Pharm. 2012, 428, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Persad, R. Leuprorelin acetate in prostate cancer: A European update. Int. J. Clin. Pract. 2002, 56, 389–396. [Google Scholar] [PubMed]
- Palmberger, T.F.; Augustijns, P.; Vetter, A.; Bernkop-Schnürch, A. Safety assessment of thiolated polymers: Effect on ciliary beat frequency in human nasal epithelial cells. Drug Dev. Ind. Pharm. 2011, 37, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Vetter, A.; Martien, R.; Bernkop-Schnürch, A. Thiolated polycarbophil as an adjuvant for permeation enhancement in nasal delivery of antisense oligonucleotides. J. Pharm. Sci. 2010, 99, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Vetter, A.; Bernkop-Schnürch, A. Nasal delivery of antisense oligonucleotides: In vitro evaluation of a thiomer/glutathione microparticulate delivery system. J. Drug Target. 2010, 18, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Gavegnano, C.D.M.; Bassit, L.; Hurwitz, S.J.; North, T.W.; Schinazi, R.F. Cellular pharmacology and potency of HIV-1 nucleoside analogs in primary human macrophages. Antimicrob. Agents Chemother. 2012, 57, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Marban, C.; Forouzanfar, F.; Ait-Ammar, A.; Fahmi, F.; El Mekdad, H.; Daouad, F.; Rohr, O.; Schwartz, C. Targeting the brain reservoirs: Toward an HIV cure. Front. Immunol. 2016, 7, 397. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.A.; Cherry, C.L.; Bell, J.E.; McLean, C.A. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am. J. Pathol. 2011, 179, 1623–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desplats, P.; Dumaop, W.; Smith, D.; Adame, A.; Everall, I.; Letendre, S.; Ellis, R.; Cherner, M.; Grant, I.; Masliah, E. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology 2013, 80, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghananeem, A.M.; Saeed, H.; Florence, R.; Yokel, R.A.; Malkawi, A.H. Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; an attractive route against infections caused by AIDS viruses. J. Drug Target. 2010, 18, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mukkur, T.K.; Benson, H.A.; Chen, Y. Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. J. Pharm. Sci. 2009, 98, 812–843. [Google Scholar] [CrossRef] [PubMed]
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; John, J.A.; Ekberg, J.A.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens penetrating the central nervous system: Infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev. 2014, 27, 691–726. [Google Scholar] [CrossRef] [PubMed]
- Infections of the nervous system. Available online: http://neuropathology-web.org/chapter5/ chapter5aSuppurative.html (accessed on 21 March 2018).
- Van Sorge, N.M.; Doran, K.S. Defense at the border: The blood–brain barrier versus bacterial foreigners. Future Microbiol. 2012, 7, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Dockrell, D.H.; Whyte, M.K.; Mitchell, T.J. Pneumococcal pneumonia: Mechanisms of infection and resolution. Chest 2012, 142, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Zar, H.J.; Madhi, S.A.; Aston, S.J.; Gordon, S.B. Pneumonia in low and middle income countries: Progress and challenges. Thorax 2013, 68, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Kong, I.G.; Sato, A.; Yuki, Y.; Nochi, T.; Takahashi, H.; Sawada, S.; Mejima, M.; Kurokawa, S.; Okada, K.; Sato, S.; et al. Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae. Infect. Immun. 2013, 81, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y.; Yuki, Y.; Katakai, Y.; Harada, N.; Takahashi, H.; Takeda, S.; Mejima, M.; Joo, S.; Kurokawa, S.; Sawada, S.; et al. Nanogel-based pneumococcal surface protein a nasal vaccine induces microRNA-associated Th17 cell responses with neutralizing antibodies against Streptococcus pneumoniae in macaques. Mucosal Immunol. 2015, 8, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Yuki, Y.; Kong, I.; Sato, A.; Nochi, T.; Mejima, M.; Kurokawa, S.; Hiroiwa, T.; Fukuyama, Y.; Sawada, S.; Takahashi, H.; et al. Adjuvant-free nanogel-based PspA nasal vaccine for the induction of protective immunity against Pneumococcus. J. Immunol. 2012, 188, 166–167. [Google Scholar]
- Webster, R.G.; Govorkova, E.A. Continuing challenges in influenza. Ann. N. Y. Acad. Sci. 2014, 1323, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A. Polymeric therapeutic delivery systems for the treatment of infectious diseases. Ther. Deliv. 2017, 8, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Nochi, T.; Yuki, Y.; Takahashi, H.; Sawada, S.I.; Mejima, M.; Kohda, T.; Harada, N.; Kong, I.G.; Sato, A.; Kataoka, N.; et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 2010, 9, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, D.; Taniai, M.; Ariyasu, H.; Taniguchi, M.; Aga, M.; Ariyasu, T.; Ohta, T.; Fukuda, S. Cholesteryl pullulan encapsulated TNF-α nanoparticles are an effective mucosal vaccine adjuvant against influenza virus. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Sajoux, I.; Bellon, A.; Vidal, J. Challenges in treatment of obesity in the elderly. Endocrinol. Metab. Int. J. 2017, 5, 00135. [Google Scholar] [CrossRef]
- Bessesen, D.H.; Van Gaal, L.F. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018, 6, 237–248. [Google Scholar] [CrossRef]
- Villareal, D.T.; Apovian, C.M.; Kushner, R.F.; Klein, S. Obesity in older adults: Technical review and position statement of the American society for nutrition and NAASO, the obesity society. Am. J. Clin. Nutr. 2005, 82, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Azegami, T.; Yuki, Y.; Sawada, S.; Mejima, M.; Ishige, K.; Akiyoshi, K.; Itoh, H.; Kiyono, H. Nanogel-based nasal ghrelin vaccine prevents obesity. Mucosal Immunol. 2017, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vizcarra, J.A.; Kirby, J.D.; Kim, S.K.; Galyean, M.L. Active immunization against ghrelin decreases weight gain and alters plasma concentrations of growth hormone in growing pigs. Domest. Anim. Endocrinol. 2007, 33, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, E.P.; Iwasaki, S.; Moss, J.A.; Chang, J.; Otsuji, J.; Inoue, K.; Meijler, M.M.; Janda, K.D. Vaccination against weight gain. Proc. Natl. Acad. Sci. USA 2006, 103, 13226–13231. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jou, W.; Gavrilova, O.; Hall, K.D. Persistent diet-induced obesity in male C57BL/6 mice resulting from temporary obesigenic diets. PLoS ONE 2009, 4, e5370. [Google Scholar] [CrossRef] [PubMed]
- Debache, K.; Kropf, C.; Schütz, C.A.; Harwood, L.J.; Käuper, P.; Monney, T.; Rossi, N.; Laue, C.; McCullough, K.C.; Hemphill, A. Vaccination of mice with chitosan nanogel-associated recombinant NcPDI against challenge infection with Neospora caninum tachyzoites. Parasite Immunol. 2011, 33, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Kamali, A.; Naseri, Z.; Movassaghi, A.R.; Razmi, G.R.; Naseri, Z. Histopathological and molecular study of Neospora caninum infection in bovine aborted foetuses. Asian Pac. J. Trop. Biomed. 2014, 4, 990–994. [Google Scholar] [CrossRef]
- Jamal, S.M.; Belsham, G.J. Foot-and-mouth disease: Past, present and future. Vet. Res. 2013, 44, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight-Jones, T.J.; Rushton, J. The economic impacts of foot and mouth disease-What are they, how big are they and where do they occur? Prev. Vet. Med. 2013, 112, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Çokçalışkan, C.; Özyörük, F.; Gürsoy, R.N.; Alkan, M.; Günbeyaz, M.; Arca, H.Ç.; Uzunlu, E.; Şenel, S. Chitosan-based systems for intranasal immunization against foot-and-mouth disease. Pharm. Dev. Technol. 2014, 19, 181–188. [Google Scholar] [CrossRef] [PubMed]

| Application | Polymer | Drug/Vaccine | Biological Outcomes | References |
|---|---|---|---|---|
| Alzheimer disease | Poly(N-vinyl pyrrolidone) | Insulin | Protection of the loaded insulin from protease degradation. Enhanced uptake of insulin in the brain. | [44,48] |
| Alzheimer disease | Chitosan | Piperine | In vivo studies showed that the formulation enhanced the cognitive functions of the mice. | [52] |
| Schizophrenia | Chitosan | olanzapine | Good nasal absorption of the drug. | [55] |
| Migraine | Hydroxypropyl methyl cellulose | lidocaine hydrochloride | The drug targeting index of olfactory and ventricle after nasal gel was enhanced compared to the nasal spray. | [58] |
| Depression | Venlafaxine | The prolonged duration of action of the loaded drug. | [61] | |
| Depression | Alginate | Venlafaxine | Increased permeation of drug and the mucosal absorption. | [62] |
| Depression | Chitosan | Selegiline | Reduced oxidative stress and restoration of the activity of the mitochondrial complex in vivo. | [64] |
| Hypertension | Chitosan | Amlodipine besylate | The formulation did not exhibit drug toxicity on the sheep nasal mucosa in vitro. | [66] |
| Cancer | Chitosan | Leuprolide acetate | Sustained drug release. | [67] |
| HIV | Chitosan | Didanosine | The drug concentration in the cerebrospinal fluid, olfactory bulb and brain in vivo was high. | [76] |
| S. pneumoniae infection | Pullulan | pneumococcal surface protein A antigen | formulation induced humoral and cellular immune responses. | [83,84,85] |
| Influenza | Pullulan | Clostridium botulinum type-A neurotoxin | The vaccine did not accumulate in the brain. | [88] |
| Influenza | Pulllulan | tumor necrosis factor-α | The formulation induced systemic IgG1 and mucosal IgA antibodies. It was effective in protecting the mice against a lethal challenge of A/PR/8/34 (H1N1) influenza virus. | [89] |
| Obesity | Pullulan | Ghrelin-pneumococcal surface protein A | The vaccine did not alter food intake in immunized mice. Peroxisome proliferator-activated receptor gamma expression in adipocytes was increased in the mice immunized with the formulation. | [93] |
| Veterinary application (Encephalomyelitis) | Chitosan and alginate | recombinant NcPDI. | The formulation protected the mice against the disease. | [97] |
| Mouth to foot disease | Chitosan | inactivated foot to mouth disease virion | The formulation prevented the viral infection in upper respiratory mucosa by inducing IgA responses. | [101] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aderibigbe, B.A.; Naki, T. Design and Efficacy of Nanogels Formulations for Intranasal Administration. Molecules 2018, 23, 1241. https://doi.org/10.3390/molecules23061241
Aderibigbe BA, Naki T. Design and Efficacy of Nanogels Formulations for Intranasal Administration. Molecules. 2018; 23(6):1241. https://doi.org/10.3390/molecules23061241
Chicago/Turabian StyleAderibigbe, Blessing A., and Tobeka Naki. 2018. "Design and Efficacy of Nanogels Formulations for Intranasal Administration" Molecules 23, no. 6: 1241. https://doi.org/10.3390/molecules23061241
APA StyleAderibigbe, B. A., & Naki, T. (2018). Design and Efficacy of Nanogels Formulations for Intranasal Administration. Molecules, 23(6), 1241. https://doi.org/10.3390/molecules23061241





