Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables
Abstract
1. Introduction
2. Results and Discussion
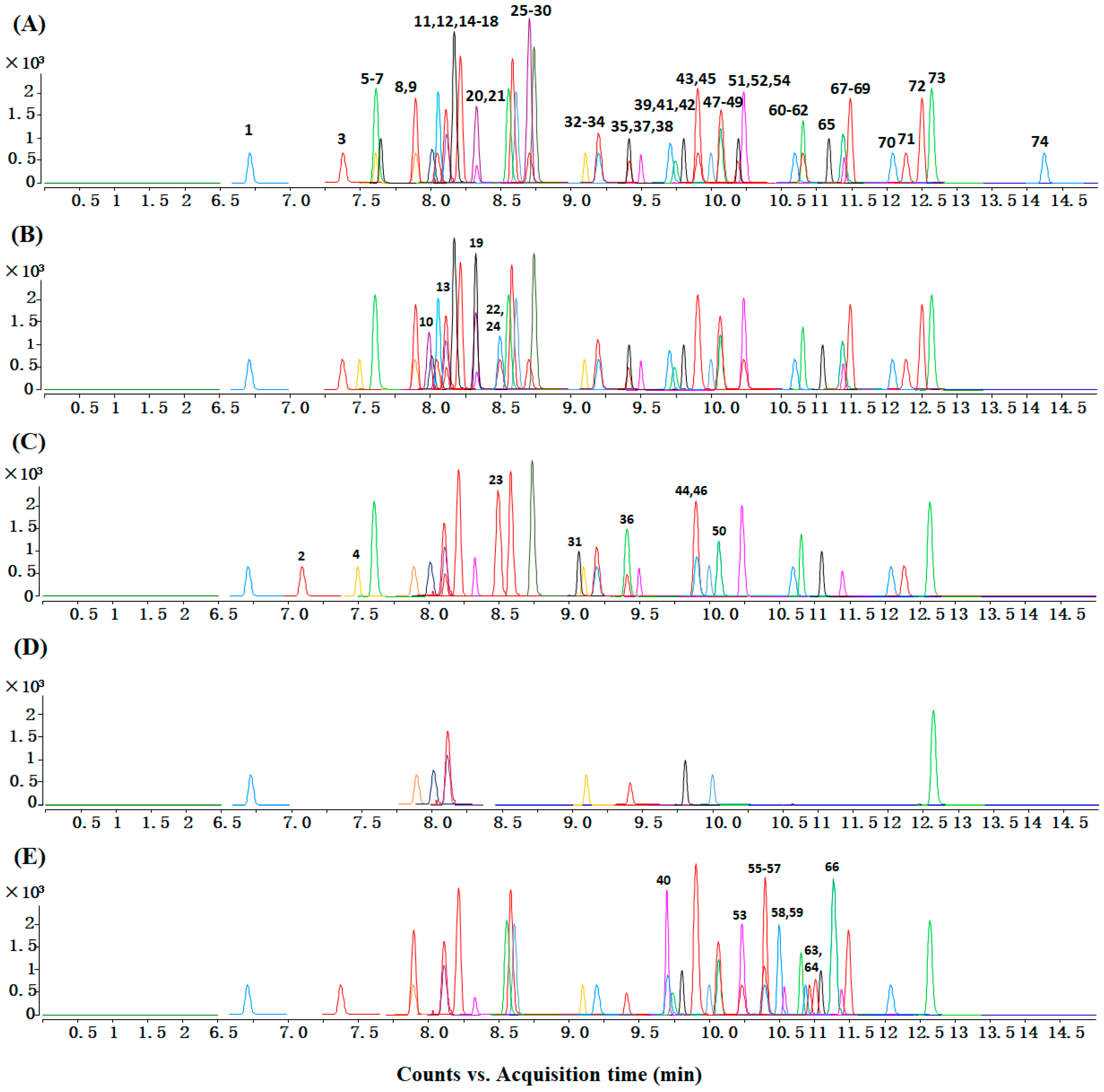
2.1. Phenolic Compounds Identification
2.2. Method Validation
2.3. Phenolic Compounds Profiling of 12 Cruciferous Vegetables
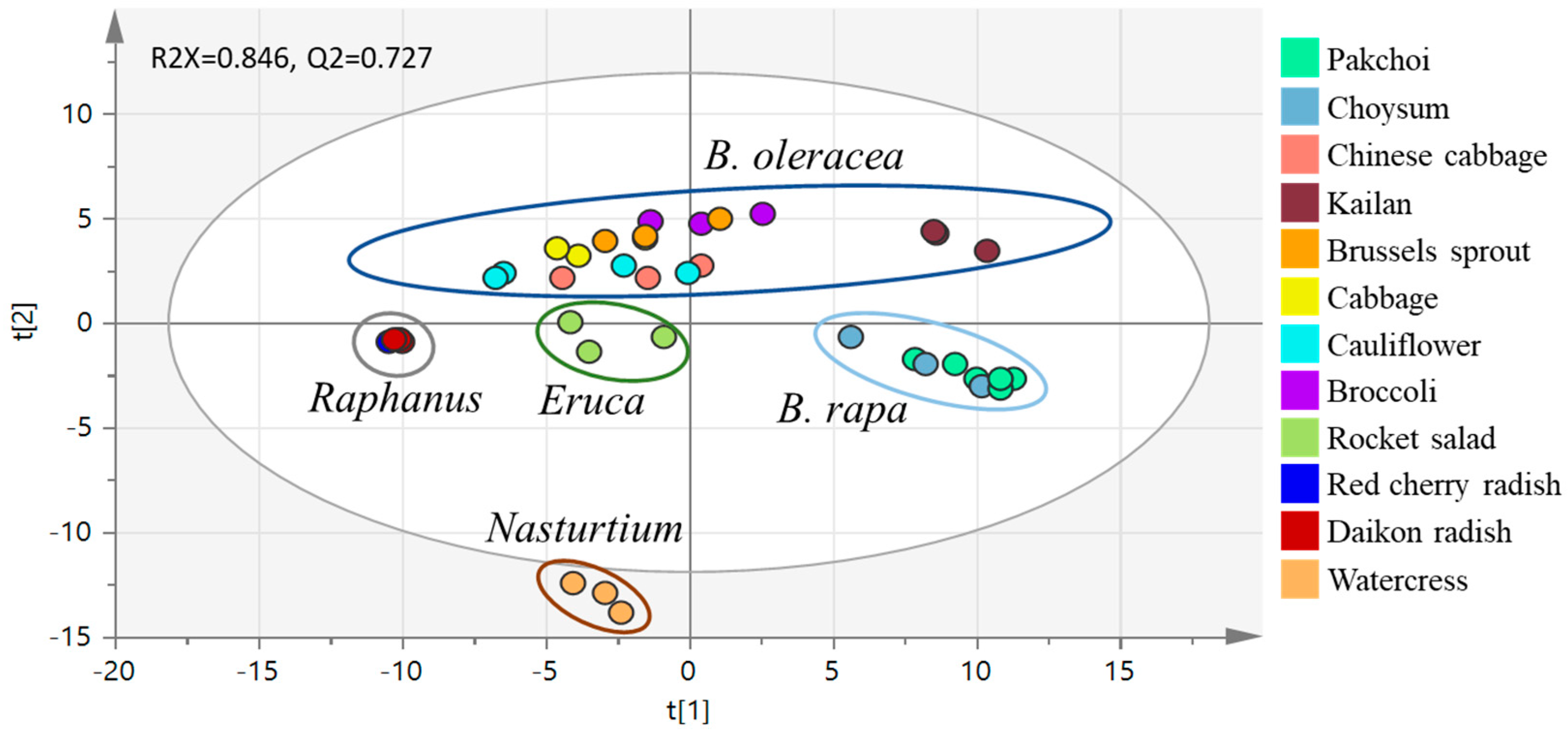
2.4. PCA Analysis
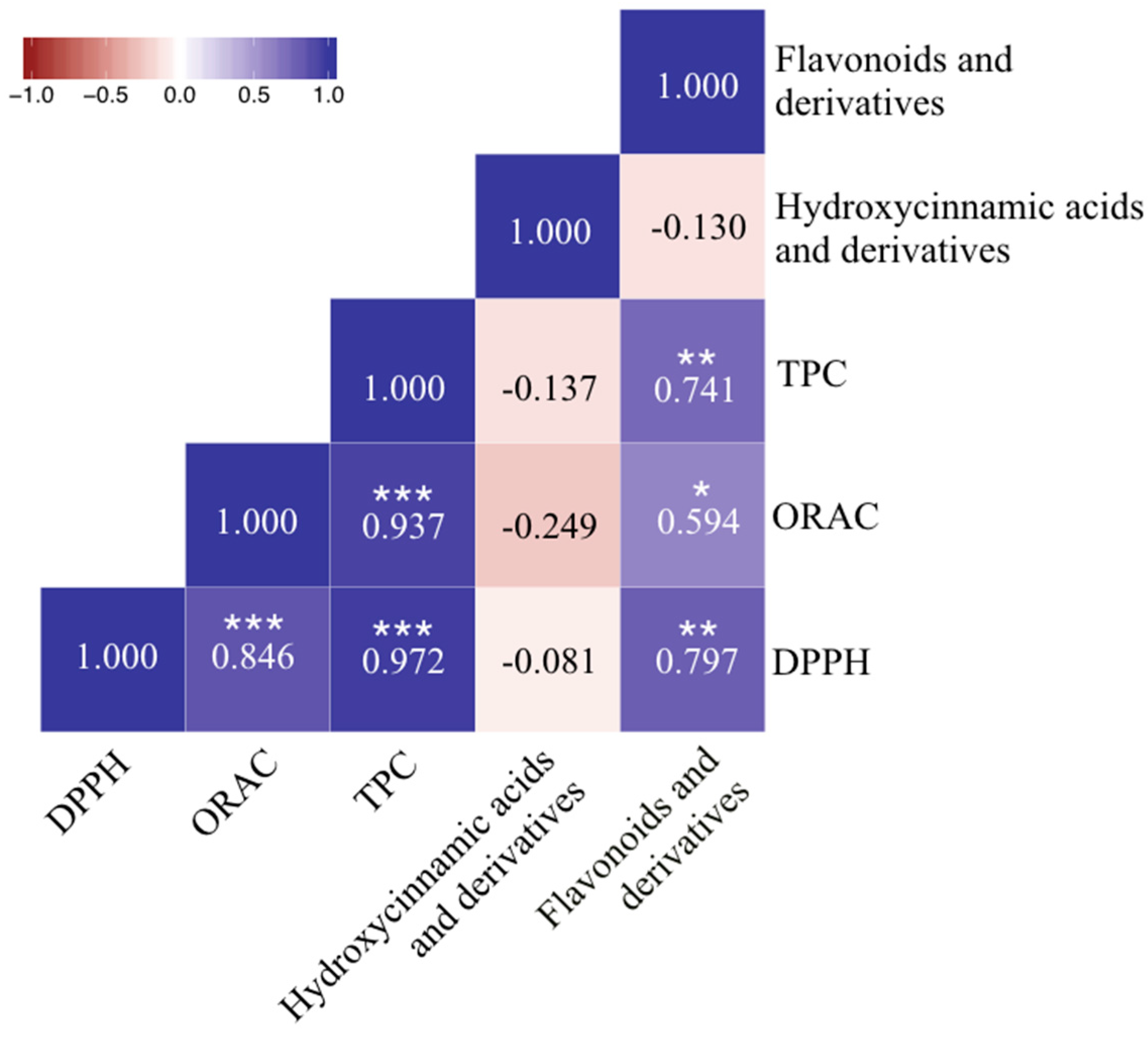
2.5. DPPH, ORAC and TPC Assays and Its Relations to Phenolic Compounds
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Collection and Preparation
3.3. Standards Preparation
3.4. Phenolic Compounds Extraction
3.5. Phenolic Compounds Identification and Quantification
3.6. Method Validation for Phenolic Compounds Chromatographic Analysis
3.7. Sample Preparation for DPPH, ORAC and Total Phenolic Content Assays
3.8. DPPH Radical Scavenging Activity
3.9. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.10. Total Phenolic Content (TPC) Assay
3.11. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Manchali, S.; Murthy, K.N.C.; Patil, B.S. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Soengas, P.; Sotelo, T.; Velasco, P.; Cartea, M.E. Antioxidant properties of Brassica vegetables. Funct. Plant Sci. Biotechnol. 2011, 5, 43–55. [Google Scholar]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Francisco, M.; Moreno, D.A.; Cartea, M.E.; Ferreres, F.; García-Viguera, C.; Velasco, P. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr. A 2009, 1216, 6611–6619. [Google Scholar] [CrossRef] [PubMed]
- Kusznierewicz, B.; Bartoszek, A.; Wolska, L.; Drzewiecki, J.; Gorinstein, S.; Namieśnik, J. Partial characterization of white cabbages (Brassica oleracea var. capitata f. alba) from different regions by glucosinolates, bioactive compounds, total antioxidant activities and proteins. LWT Food Sci. Technol. 2008, 41, 1–9. [Google Scholar] [CrossRef]
- Singh, J.; Upadhyay, A.K.; Prasad, K.; Bahadur, A.; Rai, M. Variability of carotenes, vitamin C, E and phenolics in Brassica vegetables. J. Food Compos. Anal. 2007, 20, 106–112. [Google Scholar] [CrossRef]
- Lin, L.; Harnly, J.M. Phenolic component profiles of mustard greens, yu choy, and 15 other brassica vegetables. J. Agric. Food Chem. 2010, 58, 6850–6857. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Harnly, J.M. Identification of the phenolic components of collard greens, kale, and Chinese broccoli. J. Agric. Food Chem. 2009, 57, 7401–7408. [Google Scholar] [CrossRef] [PubMed]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Baenas, N.; Moreno, D.A.; García-Viguera, C. Selecting sprouts of Brassicaceae for optimum phytochemical composition. J. Agric. Food Chem. 2012, 60, 11409–11420. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xiao, Z.; Lin, L.-Z.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMSn. J. Agric. Food Chem. 2013, 61, 10960–10970. [Google Scholar] [CrossRef] [PubMed]
- Seong, G.-U.; Hwang, I.-W.; Chung, S.-K. Antioxidant capacities and polyphenolics of Chinese cabbage (Brassica rapa L. ssp. Pekinensis) leaves. Food Chem. 2016, 199, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Mageney, V.; Neugart, S.; Albach, D. A Guide to the variability of flavonoids in Brassica oleracea. Molecules 2017, 22, 252. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhang, Y.; Xu, S.; Xu, W.; Chu, K.; Xu, W.; Zhao, H.; Lu, J. Identification and quantification of phenolic compounds in Vitex negundo L. var. cannabifolia (Siebold et Zucc.) Hand.-Mazz. using liquid chromatography combined with quadrupole time-of-flight and triple quadrupole mass spectrometers. J. Pharm. Biomed. Anal. 2015, 108, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mouradov, A.; Spangenberg, G. Flavonoids: A metabolic network mediating plants adaptation to their real estate. Front. Plant Sci. 2014, 5, 620. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar]
- Kim, J.K.; Chu, S.M.; Kim, S.J.; Lee, D.J.; Lee, S.Y.; Lim, S.H.; Ha, S.-H.; Kweon, S.J.; Cho, H.S. Variation of glucosinolates in vegetable crops of Brassica rapa L. ssp. pekinensis. Food Chem. 2010, 119, 423–428. [Google Scholar] [CrossRef]
- Vicas, S.I.; Teusdea, A.C.; Carbunar, M.; Socaci, S.A.; Socaciu, C. Glucosinolates profile and antioxidant capacity of Romanian Brassica vegetables obtained by organic and conventional agricultural practices. Plant Foods Hum. Nutr. 2013, 68, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18 Pt B, 757–781. [Google Scholar] [CrossRef]
- Isabelle, M.; Lee, B.L.; Lim, M.T.; Koh, W.P.; Huang, D.; Ong, C.N. Antioxidant activity and profiles of common vegetables in Singapore. Food Chem. 2010, 120, 993–1003. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Isabelle, M.; Lee, B.L.; Ong, C.N.; Liu, X.; Huang, D. Peroxyl radical scavenging capacity, polyphenolics, and lipophilic antioxidant profiles of mulberry fruits cultivated in southern China. J. Agric. Food Chem. 2008, 56, 9410–9416. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Class | Constituent | Pakchoi | Choysum | Chinese Cabbage | Kailan | Brussels Sprout | Cabbage | Cauliflower | Broccoli | Rocket Salad | Red Cherry Radish | Daikon Radish | Water-Cress |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxy- cinnamic acids and derivatives | Ferulic acid | 1.81 ± 0.21 | 2.37 ± 0.64 | 4.58 ± 1.55 | 4.68 ± 1.68 | 0.72 ± 0.33 | 1.43 ± 0.70 | 1.46 ± 0.72 | 1.95 ± 0.46 | 0.89 ± 0.09 | 0.28 ± 0.02 | 0.26 ± 0.07 | 7.24 ± 3.08 |
| Sinapic acid | 2.94 ± 0.63 | 5.78 ± 0.51 | 5.69 ± 2.44 | 8.06 ± 4.21 | 4.69 ± 1.73 | 9.20 ± 2.25 | 15.16 ± 5.68 | 6.66 ± 1.28 | 45.44 ± 16.22 | ND | ND | 1.90 ± 0.43 | |
| Iso-sinapic acid | ND | ND | 1.93 ± 0.59 | ND | 0.49 ± 0.50 | 1.50 ± 0.83 | ND | ND | ND | 0.40 ± 0.03 | ND | 0.33 ± 0.26 | |
| Caffeic acid | 1.56 ± 0.28 | 2.58 ± 0.02 | 0.69 ± 0.37 | 1.79 ± 0.29 | 2.13 ± 0.85 | 2.00 ± 0.74 | 0.73 ± 0.40 | 0.59 ± 0.04 | 0.79 ± 0.53 | 0.54 ± 0.23 | 0.08 ± 0.09 | 1.78 ± 1.00 | |
| p-Coumaric acid | 0.15 ± 0.06 | 2.33 ± 1.24 | 0.34 ± 0.36 | 0.25 ± 0.18 | 0.69 ± 0.61 | 0.73 ± 0.51 | 0.59 ± 0.31 | 1.38 ± 0.50 | 0.05 ± 0.11 | 0.01 ± 0.04 | 0.19 ± 0.30 | 14.17 ± 5.20 | |
| 1,2-Diferuloyl gentiobiose | 0.63 ± 0.11 | 2.42 ± 1.52 | 2.16 ± 0.44 | 118.55 ± 9.69 | 13.45 ± 11.25 | 2.65 ± 0.67 | 1.70 ± 1.56 | 125.75 ± 39.35 | ND | ND | ND | 2.11 ± 1.28 | |
| 1-Sinapoyl-2-2′diferuloyl gentiobiose | 0.14 ± 0.02 | 0.20 ± 0.08 | 0.30 ± 0.10 | 11.33 ± 0.79 | 1.99 ± 1.68 | 1.13 ± 0.37 | 0.34 ± 0.27 | 7.31 ± 2.17 | ND | ND | ND | ND | |
| 1,2,2′-Trisinapoyl gentiobiose | 58.61 ± 8.88 | 157.71 ± 16.19 | 138.72 ± 33.18 | 413.88 ± 21.06 | 1101.56 ± 157.39 | 876.00 ± 166.76 | 237.21 ± 48.15 | 648.78 ± 46.04 | 263.33 ± 70.93 | ND | ND | 0.06 ± 0.09 | |
| 1,2′-Disinapoyl-2-feruloyl gentiobiose | 19.24 ± 5.04 | 27.67 ± 6.05 | 34.34 ± 9.23 | 509.36 ± 31.26 | 312.67 ± 149.90 | 184.51 ± 37.16 | 30.56 ± 16.66 | 305.62 ± 50.75 | 0.69 ± 0.16 | ND | ND | ND | |
| 1,2-Disinapoyl gentiobiose | 1741.74 ± 417.34 | 3318.31 ± 383.26 | 7617.68 ± 3501.44 | 8610.26 ± 1248.64 | 29,214.88 ± 1147.33 | 40,030.20 ± 16,038.61 | 5847.86 ± 1884.12 | 17,839.37 ± 5576.89 | 8919.02 ± 3174.88 | ND | ND | 6315.94 ± 3250.87 | |
| 1-Sinapoyl-2-feruloyl gentiobiose | 546.28 ± 113.26 | 615.91 ± 106.82 | 1548.59 ± 894.68 | 11,811.37 ± 1230.59 | 7266.49 ± 2408.39 | 4786.59 ± 1807.72 | 844.80 ± 604.89 | 15,724.93 ± 5151.71 | 21.18 ± 5.59 | ND | ND | 433.82 ± 115.63 | |
| 4 or 5-Feruloyl quinic acid | 0.65 ± 0.20 | 2.21 ± 0.18 | 5.06 ± 2.62 | 13.18 ± 1.08 | 1.50 ± 0.57 | 0.84 ± 0.43 | 0.11 ± 0.03 | 1.22 ± 0.89 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.00 | 0.03 ± 0.00 | |
| 3-Caffeoyl quinic acid | 4.08 ± 1.31 | 14.30 ± 4.11 | 23.60 ± 18.16 | 64.04 ± 4.61 | 9.01 ± 5.20 | 2.61 ± 2.22 | 2.87 ± 2.81 | 42.36 ± 10.52 | 1.15 ± 1.09 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.12 ± 0.02 | |
| 4-Caffeoyl quinic acid | 9.27 ± 2.87 | 29.43 ± 3.47 | 30.86 ± 7.62 | 150.42 ± 13.57 | 114.65 ± 56.30 | 25.15 ± 6.19 | 5.43 ± 1.30 | 23.56 ± 1.03 | 0.19 ± 0.06 | 008 ± 0.01 | 0.10 ± 0.00 | 0.07 ± 0.01 | |
| 3-Feruloyl quinic acid | 7.22 ± 1.85 | 21.87 ± 3.48 | 44.47 ± 9.71 | 128.84 ± 6.66 | 24.40 ± 0.62 | 3.61 ± 2.66 | 1.12 ± 0.29 | 11.16 ± 3.77 | 0.46 ± 0.06 | 0.16 ± 0.02 | 0.16 ± 0.01 | ND | |
| 5-Caffeoyl quinic acid | 77.68 ± 34.49 | 174.53 ± 39.31 | 149.08 ± 72.37 | 745.86 ± 77.58 | 418.52 ± 157.09 | 93.46 ± 30.74 | 100.98 ± 58.86 | 206.43 ± 66.65 | 0.39 ± 0.16 | 0.16 ± 0.01 | 0.17 ± 0.01 | 0.16 ± 0.01 | |
| Total hydroxycinnamic acids and derivatives | 2472.00 ± 433.94 | 4377.62 ± 400.24 | 9608.09 ± 3614.89 | 22,591.87 ± 1755.35 | 38,487.84 ± 2681.78 | 46,021.61 ± 16,141.09 | 7090.92 ± 1980.38 | 34,947.07 ± 7592.93 | 9253.61 ± 3175.72 | 1.68 ± 0.24 | 1.02 ± 0.32 | 6777.73 ± 3252.94 | |
| Flavonoids and derivatives | Kaempferol-triglucoside | 224.70 ± 13.88 | 216.77 ± 26.66 | 5.06 ± 0.43 | 94.11 ± 18.46 | 16.98 ± 4.57 | 6.12 ± 3.42 | 1.07 ± 0.88 | 3.42 ± 1.88 | 0.80 ± 0.62 | ND | ND | 38.68 ± 10.81 |
| Kaempferol-diglucoside | 9.19 ± 1.11 | 6.34 ± 3.69 | 0.31 ± 0.03 | 2.94 ± 0.90 | ND | ND | ND | 0.46 ± 0.24 | ND | ND | ND | 0.10 ± 0.09 | |
| Kaempferol-triglucoside | 10.04 ± 3.13 | 9.72 ± 2.60 | ND | 4.56 ± 1.86 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Kaempferol-glucoside | 5.38 ± 1.22 | 4.33 ± 1.37 | ND | 2.17 ± 1.10 | ND | ND | ND | 1.27 ± 0.43 | 1.81 ± 1.06 | ND | ND | 0.79 ± 0.65 | |
| Kaempferol-glucoside | 3.53 ± 1.35 | 2.51 ± 1.01 | ND | 17.94 ± 1.82 | 0.84 ± 0.76 | 1.35 ± 0.61 | 0.41 ± 0.47 | 5.89 ± 2.60 | 9.13 ± 1.88 | ND | ND | ND | |
| Kaempferol-diglucoside | 2.29 ± 0.30 | 3.01 ± 0.21 | 0.19 ± 0.44 | 16.02 ± 1.37 | ND | ND | 0.87 ± 0.88 | 4.46 ± 1.20 | 57.27 ± 2.96 | ND | ND | ND | |
| Kaempferol-diglucoside | 12.55 ± 2.30 | 15.61 ± 0.68 | 0.84 ± 0.74 | 12.58 ± 1.45 | 0.44 ± 0.58 | 0.01 ± 0.21 | 0.38 ± 0.57 | ND | ND | ND | ND | 32.89 ± 4.90 | |
| Quercetin-3-O-glucoside | 2.36 ± 0.28 | 1.97 ± 0.71 | 0.27 ± 0.14 | 0.64 ± 0.24 | ND | ND | ND | 0.38 ± 0.40 | 24.79 ± 12.36 | ND | ND | 42.83 ± 7.13 | |
| Quercetin-triglucoside | 0.56 ± 0.31 | 0.36 ± 0.20 | ND | 0.26 ± 0.08 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Quercetin-triglucoside | ND | ND | ND | ND | ND | ND | ND | ND | 0.39 ± 0.05 | ND | ND | ND | |
| Quercetin-triglucoside | 1.22 ± 0.34 | 1.11 ± 0.43 | ND | 0.90 ± 0.28 | 0.05 ± 0.03 | ND | ND | ND | ND | ND | ND | 6.59 ± 1.10 | |
| Quercetin-triglucoside | ND | ND | ND | 0.92 ± 0.17 | ND | ND | ND | 0.58 ± 0.59 | 2.04 ± 0.12 | ND | ND | ND | |
| Quercetin-triglucoside | ND | ND | ND | ND | ND | ND | ND | ND | 2.36 ± 0.13 | ND | ND | ND | |
| Quercetin-diglucoside | 1.74 ± 0.66 | 1.00 ± 0.43 | 0.01 ± 0.05 | 0.03 ± 0.05 | ND | ND | ND | 0.05 ± 0.16 | 1.80 ± 0.62 | ND | ND | 0.09 ± 0.19 | |
| Quercetin-diglucoside | ND | ND | ND | ND | ND | ND | ND | ND | 36.17 ± 6.06 | ND | ND | ND | |
| Quercetin-diglucoside | 0.13 ± 0.02 | 0.32 ± 0.04 | 0.07 ± 0.07 | 6.00 ± 1.05 | 0.26 ± 0.16 | 0.07 ± 0.06 | 0.31 ± 0.44 | 0.91 ± 0.73 | 48.53 ± 9.68 | ND | ND | 1.42 ± 0.16 | |
| Isohamnetin-3-O-rutinoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 2.20 ± 0.52 | |
| Isorhamnetin-glucoside | 0.24 ± 0.05 | 0.06 ± 0.10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Isorhamnetin-diglucoside | ND | ND | ND | ND | ND | ND | ND | ND | 3.55 ± 0.52 | ND | ND | ND | |
| Isorhamnetin-triglucoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.88 ± 0.36 | |
| Isorhamnetin-triglucoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.39 ± 0.13 | |
| Isorhamnetin-triglucoside | 0.60 ± 0.18 | 0.10 ± 0.14 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.27 ± 0.21 | |
| Isorhamnetin-diglucoside | 226.92 ± 57.15 | 112.07 ± 68.68 | 0.63 ± 0.61 | ND | ND | ND | ND | 0.32 ± 0.41 | 4.60 ± 2.18 | ND | ND | 0.58 ± 0.08 | |
| Isorhamnetin-triglucoside | 0.32 ± 0.12 | 0.27 ± 0.16 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Isorhamnetin-diglucoside | 0.27 ± 0.08 | 0.09 ± 0.10 | ND | ND | ND | ND | ND | 0.88 ± 0.93 | 14.32 ± 5.88 | ND | ND | ND | |
| Isorhamnetin-diglucoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 119.05 ± 29.96 | |
| Rutin | ND | ND | ND | ND | ND | ND | ND | ND | 0.82 ± 0.94 | ND | ND | 126.57 ± 2.05 | |
| Nicotiflorin (kaempferol-3-O-rutinoside) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 14.41 ± 3.33 | |
| Quercetin | 41.44 ± 14.56 | 92.23 ± 26.62 | 43.85 ± 12.40 | 53.83 ± 14.18 | 50.14 ± 18.80 | 50.98 ± 17.88 | 169.31 ± 36.21 | 128.76 ± 36.60 | 86.33 ± 27.25 | 65.83 ± 19.58 | 13.44 ± 7.36 | 87.24 ± 23.97 | |
| Isorhamnetin | 0.44 ± 0.19 | 1.61 ± 0.92 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Kaempferol-3-O-caffeoyl diglucoside-7-O-diglucoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.66 ± 0.49 | |
| Kaempferol-3-O-caffeoyl diglucoside-7-O-diglucoside | 1.10 ± 0.42 | 0.15 ± 0.23 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Kaempferol-3-O-feruloyl triglucoside-7-O-glucoside | ND | ND | ND | 7.26 ± 1.92 | ND | ND | ND | 0.10 ± 0.17 | ND | ND | ND | ND | |
| Kaempferol-3-O-sinapoyl diglucoside-7-O-glucoside | 26.25 ± 2.71 | 44.68 ± 7.12 | 0.18 ± 0.05 | 21.88 ± 5.56 | 1.51 ± 0.59 | ND | ND | 0.06 ± 0.19 | ND | ND | ND | 0.69 ± 0.14 | |
| Kaempferol-3-O-feruloyl diglucoside-7-O-glucoside | 31.88 ± 2.64 | 35.02 ± 5.37 | ND | 48.14 ± 11.44 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Kaempferol-3-O-p-coumaroyl diglucoside-7-O-glucoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.13 ± 0.23 | |
| Kaempferol-3-O-feruloyl triglucoside-7-O-glucoside | ND | 0.02 ± 0.12 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Kaempferol-3-O-caffeoyl diglucoside-7-O-diglucoside | 1.88 ± 0.70 | ND | 0.36 ± 0.37 | 44.00 ± 12.04 | 0.62 ± 0.46 | ND | 0.24 ± 0.45 | 5.10 ± 2.76 | ND | ND | ND | ND | |
| Kaemperol-3-O-sinapoyl triglucoside-7-O-diglucoside | ND | ND | ND | 0.19 ± 0.17 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Kaempferol-3-O-caffeoyl diglucoside-7-O-glucoside | 598.05 ± 159.49 | 628.11 ± 178.38 | 0.34 ± 0.38 | 261.36 ± 66.57 | 1.66 ± 0.58 | ND | 3.15 ± 2.46 | 0.73 ± 0.45 | 3.35 ± 3.79 | ND | ND | ND | |
| Kaempferol-3-O-caffeoyl diglucoside-7-O-glucoside | ND | ND | ND | 8.72 ± 4.47 | 0.92 ± 1.04 | ND | ND | 3.07 ± 3.07 | ND | ND | ND | ND | |
| Kaemperol-3-O-sinapoyl triglucoside-7-O-diglucoside | ND | ND | ND | 22.07 ± 4.89 | 2.18 ± 1.64 | ND | ND | 0.92 ± 0.85 | ND | ND | ND | ND | |
| Kaemperol-3-O-sinapoyl diglucoside-7-O-diglucoside | 1.70 ± 0.55 | ND | 1.82 ± 1.38 | 52.63 ± 4.01 | 5.09 ± 2.18 | 4.44 ± 1.00 | 0.38 ± 0.48 | 5.28 ± 2.21 | ND | ND | ND | ND | |
| Kaempferol-3-O-p-coumaroyl diglucoside-7-O-glucoside | 96.58 ± 20.96 | 108.00 ± 20.50 | ND | 51.84 ± 18.65 | ND | ND | 0.44 ± 0.62 | ND | 0.05 ± 0.50 | ND | ND | ND | |
| Kaempferol-3-O-caffeoyl diglucoside-7-O-diglucoside | 1.78 ± 0.69 | 1.99 ± 0.93 | ND | 1.34 ± 0.36 | ND | ND | ND | 1.63 ± 1.29 | ND | ND | ND | ND | |
| Kaempferol-3-O-caffeoyl diglucoside-7-O-diglucoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 140.19 ± 30.20 | |
| Kaempferol-3-O-feruloyl diglucoside-7-O-glucoside | ND | ND | ND | 0.16 ± 0.22 | ND | ND | ND | 2.96 ± 1.70 | ND | ND | ND | ND | |
| Kaempferol-3-O-feruloyl diglucoside-7-O-glucoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 40.29 ± 24.09 | |
| Kaempferol-3-O-sinapoyl diglucoside-7-O-glucoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 54.08 ± 27.52 | |
| Kaempferol-3-O-sinapoyl diglucoside | 5.96 ± 1.28 | 10.64 ± 0.56 | ND | 10.78 ± 3.27 | ND | ND | 0.48 ± 0.70 | 0.07 ± 0.42 | ND | ND | ND | 6.09 ± 1.13 | |
| Kaempferol-3-O-feruloyl diglucoside | 0.16 ± 0.10 | 0.22 ± 0.17 | ND | 0.79 ± 0.65 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Quercetin-3-O-feruloyl diglucoside-7-O-diglucoside | ND | ND | ND | 2.12 ± 0.68 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Quercetin-3-O-caffeoyl diglucoside-7-O-glucoside | 18.15 ± 2.32 | 15.10 ± 6.93 | ND | 3.00 ± 0.75 | 0.09 ± 0.08 | ND | 0.04 ± 0.17 | ND | ND | ND | ND | 0.13 ± 0.02 | |
| Quercetin-3-O-feruloyl diglucoside-7-O-glucoside | 62.82 ± 18.68 | 91.35 ± 26.44 | 0.06 ± 0.09 | 29.24 ± 11.25 | ND | 0.04 ± 0.16 | ND | ND | 0.23 ± 0.38 | ND | ND | ND | |
| Quercetin-3-O-feruloyl diglucoside-7-O-glucoside | 9.90 ± 2.89 | 10.67 ± 4.75 | ND | 1.04 ± 0.31 | ND | ND | ND | ND | ND | ND | ND | 0.05 ± 0.02 | |
| Quercetin-3-O-sinapoyl diglucoside | 0.05 ± 0.03 | 0.28 ± 0.11 | ND | 1.07 ± 0.25 | ND | ND | 0.05 ± 0.20 | ND | ND | ND | ND | 19.91 ± 5.43 | |
| Quercetin-3-O-sinapoyl diglucoside | ND | ND | ND | ND | ND | ND | ND | ND | 0.55 ± 0.21 | ND | ND | ND | |
| Quercetin-3-O-feruloyl diglucoside-7-O-diglucoside | 0.07 ± 0.06 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Total flavonoids and derivatives | 1400.25 ± 173.05 | 1415.71 ± 198.12 | 53.99 ± 12.54 | 780.53 ± 76.45 | 80.78 ± 19.61 | 63.01 ± 18.24 | 177.13 ± 36.34 | 167.30 ± 37.17 | 298.89 ± 33.09 | 65.83 ± 19.58 | 13.44 ± 7.36 | 739.20 ± 66.66 | |
| Total | 5279.75 ± 673.23 | 8910.55 ± 992.69 | 11,157.73 ± 3638.45 | 25,275.21 ± 1819.35 | 40,270.79 ± 2754.00 | 47,835.56 ± 16,152.09 | 12,972.52 ± 2318.92 | 39,571.96 ± 7690.26 | 12,450.21 ± 3303.01 | 2282.31 ± 652.32 | 484.73 ± 244.95 | 10,449.67 ± 3350.06 | |
| Vegetable | Scientific Name | DPPH (μmol TE/g FW) | ORAC (μmol TE/g FW) | TPC (mg GAE/g FW) |
|---|---|---|---|---|
| Pakchoi | B. rapa var. chinensis | 4.22 ± 0.41 c | 13.51 ± 2.35 bcd | 0.78 ± 0.16 cd |
| Choysum | B. rapa var. parachinensis | 3.84 ± 1.03 c | 11.97 ± 5.79 cd | 0.68 ± 0.20 cde |
| Chinese cabbage | B. rapa var. pekinensis | 1.32 ± 0.05 d | 3.45 ± 0.25 d | 0.21 ± 0.03 ef |
| Kailan | B. oleracea var. alboglabra | 6.83 ± 1.23 b | 23.73 ± 4.89 abc | 1.28 ± 0.19 b |
| Brussels sprout | B. oleracea var. gemmifera | 9.54 ± 0.77 a | 26.67 ±10.48 ab | 1.92 ± 0.24 a |
| Cabbage | B. oleracea var. capitata | 1.64 ± 0.24 d | 7.05 ± 1.55 d | 0.35 ± 0.03 def |
| Cauliflower | B. oleracea var. botrytis | 2.71 ± 0.75 cd | 9.53 ± 3.56 cd | 0.57 ± 0.06 cdef |
| Broccoli | B. oleracea var. italica | 3.85 ± 0.58 c | 23.09 ± 4.16 abc | 1.06 ± 0.12 bc |
| Rocket salad | E. sativa | 8.18 ± 1.20 ab | 32.08 ± 7.52 a | 1.93 ± 0.35 a |
| Red cherry radish | R. sativus | 2.70 ± 0.29 cd | 22.08 ± 3.47 abc | 0.68 ± 0.07 cde |
| Daikon radish | R. sativus | 1.11 ± 0.23 d | 5.34 ± 2.50 d | 0.16 ± 0.04 f |
| Watercress | N. officinale | 7.76 ± 0.46 ab | 32.92 ± 1.70 a | 1.44 ± 0.15 ab |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables. Molecules 2018, 23, 1139. https://doi.org/10.3390/molecules23051139
Li Z, Lee HW, Liang X, Liang D, Wang Q, Huang D, Ong CN. Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables. Molecules. 2018; 23(5):1139. https://doi.org/10.3390/molecules23051139
Chicago/Turabian StyleLi, Zhifeng, Hui Wen Lee, Xu Liang, Dong Liang, Qi Wang, Dejian Huang, and Choon Nam Ong. 2018. "Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables" Molecules 23, no. 5: 1139. https://doi.org/10.3390/molecules23051139
APA StyleLi, Z., Lee, H. W., Liang, X., Liang, D., Wang, Q., Huang, D., & Ong, C. N. (2018). Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables. Molecules, 23(5), 1139. https://doi.org/10.3390/molecules23051139




