Abstract
Natural flavonoids and xanthone glycosides display several biological activities, with the glycoside moiety playing an important role in the mechanism of action of these metabolites. Herein, to give further insights into the inhibitory activity on cell growth of these classes of compounds, the synthesis of four flavonoids (5, 6, 9, and 10) and one xanthone (7) containing one or more acetoglycoside moieties was carried out. Acetyl groups were introduced using acetic anhydride and microwave irradiation. The introduction of one or two acetoglycoside moieties in the framework of 3,7-dihydroxyflavone (4) was performed using two synthetic methods: the Michael reaction and the Koenigs-Knorr reaction. The in vitro cell growth inhibitory activity of compounds 5, 6, 7, 9, and 10 was investigated in six human tumor cell lines: A375-C5 (malignant melanoma IL-1 insensitive), MCF-7 (breast adenocarcinoma), NCI-H460 (non-small cell lung cancer), U251 (glioblastoma astrocytoma), U373 (glioblastoma astrocytoma), and U87MG (glioblastoma astrocytoma). The new flavonoid 3-hydroxy-7-(2,3,4,6-tetra-O-acetyl-β-glucopyranosyl) flavone (10) was the most potent compound in all tumor cell lines tested, with GI50 values < 8 μM and a notable degree of selectivity for cancer cells.
1. Introduction
Flavonoid and xanthone derivatives comprise two classes of oxygenated heterocycles associated with a large number of biological and pharmacological activities [1,2]. These compounds also occur in nature as glycosides, which have been shown to have several biological activities, namely, antimicrobial [3,4,5], antioxidant [6,7,8,9], and antitumor [10,11]. Flavonoid glycosides are widely present in vegetables and fruits and can be found with different types of glycosides, while xanthone glycosides are mainly found in the families Gentianaceae and Polygalaceae with a glucose moiety [12]. In particular, acetylated flavonoid and xanthone glycosides have been reported as bioactive compounds with antioxidant [13,14] and antitumor activities [15,16,17,18,19]. The last step in flavonoid biosynthesis is, in some plant species, terminated by acylation, which is known to increase the solubility and stability of glycosylated flavonoids in lipophilic systems [20]. Thus, we were inspired to perform acetylation and glycosylation in natural flavonoids and xanthones. Rutin (1), diosmin (2), and mangiferin (3) were selected for acetylation due to their use in therapy and/or investigation in clinical trials, and 3,4-dihydroxyflavone (4) was selected for glycosylation to study the structure-activity relationship (Figure 1). All these starting materials have previously been shown to display in vitro and in vivo antitumor activity [11,21,22,23,24].
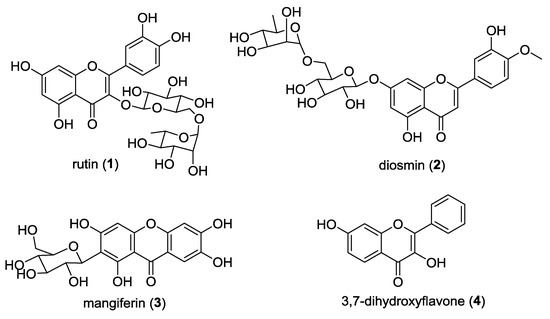
Figure 1.
Structure of the selected precursors 1–4.
2. Results and Discussion
2.1. Synthesis
Rutin peracetate (5) was obtained from rutin (1) according to a classical acetylation method using acetic anhydride under reflux for 4 h in solvent-free conditions (Scheme 1) [25]. Previously, compound 5 was obtained in the presence of pyridine with long reaction times [26]. The synthesis of compound 5 without using pyridine and with short reaction times is reported for the first time.
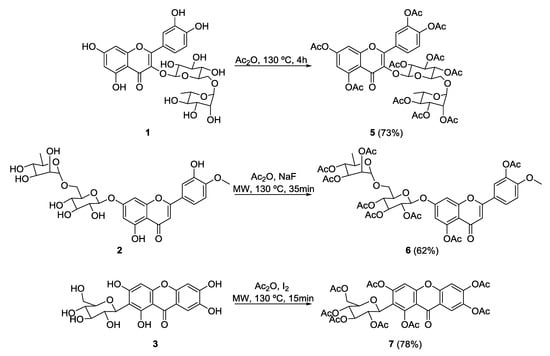
Scheme 1.
Synthesis of rutin peracetate (5), diosmin peracetate (6), and mangiferin peracetate (7). Ac2O—Acetic anhydride; MW—Microwave.
Several products that were difficult to separate were obtained when this acetylation method was applied to diosmin (2) and mangiferin (3). Diosmin peracetate (6) was successfully obtained in only 35 min (62% yield) in the presence of NaF, under microwave (MW) irradiation (Scheme 1) [27]. Peracetylation of mangiferin (3) was achieved in only 15 min (78% yield) under MW irradiation, but only when iodine was used as a catalyst (Scheme 1).
Previous methods described to synthesize compounds 6 and 7 used highly toxic pyridine as solvent and catalyst, which was applied room temperature, leading to long reaction times [28,29,30,31,32]. Herein, the application of MW irradiation without pyridine allowed the synthesis of compounds 6 and 7 in short reaction times.
For glycosylation of flavone 4, 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide (8) was selected as glucose donor and two reaction methods were applied: a Koenigs-Knorr method [33,34,35] and a modified Michael method [36]. Both reactions were carried out at room temperature to decrease the number of side products [37]. Formation of glycosylated products could not be observed using the Koenigs-Knorr approach, even with the addition of 3 equivalents/OH of 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide (8). Using the modified Michael method, with K2CO3 in dry acetone, it was possible to obtain two glycosylated products (Scheme 2), the 3,7-(2,3,4,6-tetra-O-acetyl-β-glucopyranosyl) flavone (9) and the 3-hydroxy-7-(2,3,4,6-tetra-O-acetyl-β-glucopyranosyl) flavone (10), although with low yields (11% and 2%, respectively).
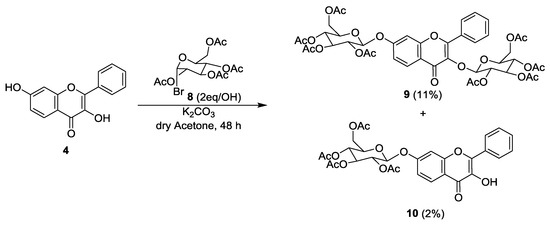
Scheme 2.
Synthesis of 3,7-(2,3,4,6-tetra-O-acetyl-β-glucopyranosyl) flavone (9) and 3-hydroxy-7-(2,3,4,6-tetra-O-acetyl-β-glucopyranosyl) flavone (10).
2.2. Structure Elucidation
The structure of compounds 5–7 was established comparing their 1H and 13C NMR data with the reported in the literature [29,30,38,39], while the structures of the compounds 9 and 10 was elucidated for the first time, using infrared (IR), nuclear magnetic resonance (NMR), and high-resolution mass spectrometry (HRMS) techniques (Figures S1–S4).
For instance, the IR spectrum of compound 9 showed a strong band at 1750 cm−1, which is characteristic of the C=O ester stretching vibration. 1H and 13C NMR spectra of compound 9 indicated the presence of a flavone moiety and of two sugar moieties. 1H and 13C NMR spectra of compound 10 indicated the presence of a flavone moiety and one acetylated sugar moiety. The assignments of the carbon atoms directly bonded to proton atoms were achieved from heteronuclear single quantum correlation (HSQC) experiments, and the chemical shifts of the carbon atoms not directly bonded to proton atoms were deduced from heteronuclear multiple bond correlation (HMBC) correlations. For instance, Figure 2 shows the correlations found for compound 10.
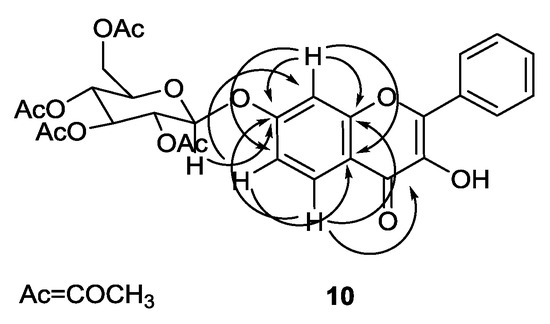
Figure 2.
Main connectivities found in the HMBC spectrum of compound 10.
The position of the sugar moiety in compound 10 was evidenced by the correlation found in the HMBC spectrum between the proton signals of H-1″ (δH 5.16–5.36 ppm) and the carbon signal of C-7 (δC 160.9 ppm).
2.3. Growth Inhibition Activity in Human Tumor Cell Lines
Acetylated flavonosides 5, 6, 9, 10, and acetylated xanthonoside 7, as well as their precursors, compounds 1–4, were evaluated for their in vitro growth inhibitory effect on six human tumor cell lines: A375-C5 (IL-1 insensitive malignant melanoma), MCF-7 (breast adenocarcinoma), NCI-H460 (non-small-cell lung cancer), U251 (glioblastoma astrocytoma), U373 (glioblastoma astrocytoma), and U87MG (glioblastoma astrocytoma). For the tested compounds, a dose–response curve was established, and the results, summarized in Table 1, are expressed as the concentration that was able to cause 50% cell growth inhibition (GI50).

Table 1.
Cell growth inhibitory activity displayed by compounds 1–7, 9, and 10 and their calculated iLogP values *.
Concerning the precursors, flavone 4 was the only precursor that showed an inhibitory effect on the growth of IL-1 insensitive A375-C5, estrogen-dependent MCF-7, and NCI-H460 cell lines.
In contrast to compounds 5, 7, and 10, diosmin peracetate (6) did not show an inhibitory effect on the growth of any of the six human tumor cell lines tested. Rutin peracetate (5) exhibited a good growth inhibitory activity in all the cell lines tested, mangiferin peracetate (7) showed a moderate growth inhibitory activity only in IL-1 insensitive A375-C5, estrogen-dependent MCF-7, and NCI-H460 cell lines, while compound 10 was the most potent compound in all human tumor cell lines tested, with GI50 values < 8 μM.
The presence of the acetyl groups in compounds 5 and 7 was found to be crucial for their cell growth inhibitory activity (comparing with compounds 1 and 3, respectively). Acylation has been a strategy used to increase the penetration of flavonoids through the cell membrane [41,42]. When comparing the peracetylated compounds (5–7, 9), some correlations can be found between lipophilicity and the cell growth inhibitory effect, especially in melanoma cells; compound 5, with the highest calculated logP value, was also associated with the highest inhibitory effect. It is noteworthy that compound 10, the only investigated acetylated glycoside with a free hydroxyl, was the most active derivative, highlighting the importance of both types of substituents. The position of the sugar moiety in flavonoids seems to be critical for the inhibitory activity.
The selectivity index was calculated for compounds 4, 5, 7, 9, 10, and for Doxorubicin (Table 2).

Table 2.
Selectivity index of the compounds 4, 5, 7, 9, 10, and control (Doxorubicin).
As outlined in Table 2, the results clearly reflect a notable degree of selectivity of compound 10 in all cell lines studied. Of note, human breast cancer cell line MCF-7 responded very well by exhibiting a higher selectivity index than the other cancer cell lines. As to the other compounds, some had no selectivity (4 and 7) while the others (5) or (9) exhibited some selectivity regarding the cancer lines in which they were most active. Interestingly, all tested compounds exhibited a better degree of selectivity than Doxorubicin.
3. Materials and Methods
3.1. General Information
Commercially available reagents were purchased from Sigma-Aldrich Co. (Sintra, Portugal) and BioSolve (Dieuze, France). Dichloromethane was dried using the following procedure: pre-drying with CaSO4 and reflux over calcium hydride and distillation onto 4 Å molecular sieves [43]. Extra-dry acetone was purchased from BioSolve (France). Reactions were controlled by thin layer chromatography (TLC) using Merck silica gel 60 (GF254) plates. Compounds were visually detected by absorbance at 254 and/or 365 nm and ferric chloride 10% in methanol (MeOH). Purification of compounds by flash chromatography was carried out using Fluka silica gel 60 (0.04–0.063 mm). MW reactions were performed using a MicroSYNTH 1600 from Milestone (ThermoUnicam, Lisboa, Portugal) synthesizer in open reaction vessels. IR spectra were obtained in KBr microplate in a FTIR spectrometer Nicolet is 10 from Thermo Scientific with Smart OMNI-Transmission accessory (Software OMNIC 8.3, Thermo Scientific, Madison, WI, USA). 1H and 13C NMR spectra were performed in University of Aveiro, Department of Chemistry and were taken in CDCl3 or DMSO-d6, at room temperature, on Bruker Avance 300 instrument (300.13 MHz for 1H and 75.47 MHz for 13C, Bruker Biosciences Corporation, Billerica, MA, USA). 13C NMR assignments were made by 2D HSQC and HMBC experiments (long-range C, H coupling constants were optimized to 7 and 1 Hz). Chemical shifts are expressed in ppm values relative to tetramethylsilane (TMS) as an internal reference. Coupling constants are reported in hertz (Hz). HRMS mass spectra were measured on an APEX III mass spectrometer, recorded as ESI (Electrospray) made in Centro de Apoio Científico e Tecnolóxico á Investigation (CACTI, University of Vigo, Spain). Six human tumor cell lines were used to study antitumor activity: MCF-7 ER (+) (breast adenocarcinoma), NCI-H460 (non-small-cell lung cancer), and A375-C5 (melanoma), U251 (glioblastoma astrocytoma), U373 (glioblastoma astrocytoma) and U87MG (glioblastoma astrocytoma).
3.2. Chemistry
Synthesis of rutin peracetate (5). Rutin (1, 0.3 g, 0.5 mmol) was added to acetic anhydride (12 mL) and the mixture was heated at 130 °C, for 4 h. The solution obtained was poured into ice and extracted with CH2Cl2. The organic layer was extracted with a saturated solution of NaHCO3, dried with anhydrous Na2SO4, filtered, and then evaporated under reduced pressure. The obtained oil was dissolved in ethyl acetate. A solid material was obtained by adding petroleum ether 60–80 °C corresponding to 2-(3,4-di-O-acetylphenyl)-5,7-di-O-acetyl-3-[3,4,5-tri-O-acetyl-α-l-rhamnopyranosyl-(1→6)-3,4,5-tri-O-acetyl-β-d-glucopyranosyloxy]-4H-chromen-4-one (5) (0.43 g, 0.41 mmol, 73% yield); mp 119–120 °C (petroleum ether 60–80 °C).
Synthesis of diosmin peracetate (6). Diosmin (2, 0.05 g, 0.08 mmol) and NaF (0.42 g, 10 mmol) were mixed in acetic anhydride (4 mL) and the mixture was kept under MW irradiation (400 W) at 130 °C, for 35 min. After cooling, the solution obtained was poured into ice and then extracted with CH2Cl2. The organic layer was extracted with NaHCO3 followed by crystallization from MeOH/H2O provided 5,3-O-acetyl-2-(3-O-acetyl-4-methoxyphenyl)-7-[2,3,4-tri-O-acetyl-α-l-rhamnopyranosyl-(1→6)-2,3,4-tri-O-acetyl-β-d-glucopyranosyloxy] oxychromen-4-one (6) as a yellow solid (0.048 g, 0.051 mmol, 62% yield); mp 135–138 °C.
Synthesis of mangiferin peracetate (7). Mangiferin (3, 0.2 g, 0.5 mmol) and iodine (0.009 g, 0.07 mmol) were mixed in acetic anhydride (7 mL) and the reaction was kept under MW irradiation (400 W), at 130 °C, for 15 min. After cooling, a saturated solution of sodium thiosulfate was added to convert iodine (dark yellow) into iodide (yellow). The crude product was extracted with CH2Cl2 and the organic layer was extracted with a saturated solution of NaHCO3 twice, dried with anhydrous Na2SO4, and filtered. The solvent was evaporated under reduced pressure and the oil obtained was dissolved in ethyl acetate. A yellow solid was obtained with petroleum ether 60–80 °C corresponding to 1,3,6,7-tetra-O-acetyl-2-C-(2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl)-9H-xanthen-9-one (7) (0.294 g, 0.39 mmol, 78% yield); mp 143–147 °C (petroleum ether 60–80 °C).
Synthesis of 3,7-(2,3,4,6-tetra-O-acetyl-β-glucopyranosyl) flavone (9) and 3-hydroxy-7-(2,3,4,6-tetra-O-acetyl-β-glucopyranosyl) flavone (10). 3,7-Dihydroxyflavone (4, 0.100 g, 0.4 mmol) and K2CO3 (0.225 g, 1.57 mmol, 2eq/OH) were mixed in dry acetone (10 mL) and the mixture was kept under stirring for 15 min. 2,3,4,6-Tetra-O-acetyl-α-d-glucopyranosyl bromide (8, 0.653 g, 1.57 mmol, 2eq/OH) was added and the mixture was kept under stirring at room temperature, for 48 h. The suspension was filtered to eliminate the K2CO3 and the filtrate was evaporated. The oil obtained was further purified through flash column chromatography (100% CHCl3). The obtained fractions were gathered and crystallized from MeOH to achieve 3,7-(2,3,4,6-tetra-O-acetyl-β-glucopyranosyl) flavone (9, 0.04 g, 0.045 mmol, 11.4% yield) and 3-hydroxy-7-(2,3,4,6-tetra-O-acetyl-β-glucopyranosyl) flavone (10, 0.003 g, 0.004 mmol, 1.2% yield).
3,7-(2,3,4,6-Tetra-O-acetyl-β-glucopyranosyl) flavone (9). Mp 105–108 °C; IR (KBr) υmax: 2962, 2917, 2843, 1750, 1623, 1449, 1381, 1231, 1066, 1040 cm−1; 1H NMR (CDCl3, 300.13 MHz) δ: 8.17 (1H, d, J = 8.8 Hz, H-5), 8.02–7.98 (2H, m, H-2′,6′), 7.50–7.47 (3H, m, H-3′,4′,5′), 7.10 (1H, d, J = 2.2 Hz, H-8), 7.05 (1H, dd, J = 2.3 and 8.9 Hz, H-6), 5.71 (1H, d, J = 7.7 Hz, H-glucose), 5.34–5.04 (7H, m, H-glucose), 4.31–4.19 (2H, m, H-glucose), 3.98–3.91 (3H, m, H-glucose), 3.65–3.60 (1H, m, H-glucose), 2.09 (3H, s, COCH3), 2.08 (3H, s, COCH3), 2.06 (3H, s, COCH3), 2.05 (3H, s, COCH3), 2.04 (3H, s, COCH3), 2.01 (3H, s, COCH3), 2.00 (3H, s, COCH3), 1.90 (3H, s, COCH3) ppm; 13C NMR (CDCl3, 75.47 MHz) δ: 173.4 (C-4), 170.6 (COCH3), 170.5 (COCH3), 170.3 (COCH3), 170.2 (COCH3), 170.0 (COCH3), 169.7 (COCH3), 169.5 (COCH3), 169.4 (COCH3), 160.9 (C-7), 157.4 (C-2), 156.6 (C-9), 136.3 (C-3), 131.0 (C-1′), 130.6 (C-4′), 129.1 (C-3′,5′), 128.3 (C-2′,6′), 127.6 (C-5), 119.9 (C-10), 115.5 (C-6), 104.4 (C-8), 98.9 (C-glucose), 98.5 (C-glucose), 72.9 (C-glucose), 72.6 (C-glucose), 72.6 (C-glucose), 71.8 (C-glucose), 71.8 (C-glucose), 68.4 (C-glucose), 68.2 (C-glucose), 62.1 (C-glucose), 61.5 (C-glucose), 21.0 (COCH3), 20.8 (COCH3), 20.8 (COCH3), 20.7 (COCH3), 20.7 (COCH3) ppm; HRMS (ESI+) m/z calcd for C29H28O13 [M + H] 915.25535, found 915.25410.
3-Hydroxy-7-(2,3,4,6-tetra-O-acetyl-β-glucopyranosyl) flavone (10). Mp 222–223 °C; IR (KBr) υmax: 3442, 2961, 2917, 2847, 1758, 1621, 1572, 1501, 1457, 1235, 1211, 1071 cm−1; 1H NMR (CDCl3, 300.13 MHz) δ: 9.62 (3-OH), 8.24-8.18 (3H, m, H-5,2′,6′), 7.57–7.48 (3H, m, H-3′,4′,5′), 7.13 (1H, d, J = 2.2 Hz, H-8), 7.06 (1H, dd, J = 2.3 and 8.9 Hz, H-6), 5.36–5.16 (4H, m, H-1″,2″,3″,4″), 4.33–4.20 (2H, m, H6″a,b), 4.03–3.97 (1H, m, H-5″), 2.09 (3H, s, COCH3), 2.08 (3H, s, COCH3), 2.07 (3H, s, COCH3), 2.06 (3H, s, COCH3) ppm; 13C NMR (CDCl3, 75.47 MHz) δ: 172.9 (C-4), 170.6 (COCH3), 170.3 (COCH3), 169.6 (COCH3), 169.4 (COCH3), 160.9 (C-7), 156.8 (C-9), 138.4 (C-3), 131.1 (C-1′), 130.4 (C-4′), 128.8 (C-3′,5′), 127.7 (C-2′,6′), 127.4 (C-5), 116.6 (C-10), 115.4 (C-6), 104.3 (C-8), 98.4 (C-1″), 72.6 (C-3″), 72.6 (C-5″), 71.1 (C-2″), 68.3 (C-4″), 62.1 (C-6″), 20.8 (COCH3), 20.8 (COCH3), 20.7 (COCH3), 20.7 (COCH3) ppm; HRMS (ESI+) m/z calcd for C29H28O13 [M + H] 585.16027, found 585.15875.
3.3. Tumor Cell Growth Assay and Selectivity Index
The human tumor cell lines A375-C5 (melanoma), MCF-7 (breast adenocarcinoma), and NCI-H460 (non-small cell lung cancer) were grown in culture medium RPMI-1640 (Biochrom, Berlin, Germany), U251 (glioblastoma astrocytoma), U373 (glioblastoma astrocytoma), and U87MG (glioblastoma astrocytoma) were grown in culture medium DMEM (Biochrom), both supplemented with 5% heat-inactivated fetal bovine serum (FBS, Biochrom). All cell lines were maintained at 37 °C in a 5% CO2 humidified atmosphere (Hera Cell, Heraeus). Cell viability was routinely determined with Trypan Blue (Sigma-Aldrich) exclusion assay and all experiments were performed with exponentially growing cells, showing more than 95% viability.
The effect of compounds on the cell growth was evaluated according to the procedure adopted by the National Cancer Institute (NCI) in the ‘In vitro Anticancer Drug Discovery Screen’, which uses the protein-binding dye sulforhodamine B (SRB) to assess cell growth. Cells were plated in 96-well plates (0.05 × 106 cells/well) in complete culture medium and incubated at 37 °C. Twenty-four hours later, cells were treated with two-fold serial dilutions of the test compounds, ranging from 0 to 150 µM, for 48 h. Control groups received the same amount of sterile Dimethyl Sulfoxide (DMSO, Sigma-Aldrich), used as compounds solvent, up to 0.25% concentration. Then, cells were fixed in situ with 50% (m/v) trichloroacetic acid (Merck Millipore, Darmstadt, Germany), washed with distilled water and stained with Sulforhodamine B (SRB; Sigma-Aldrich) for 30 min at room temperature. The SRB-stained cells were washed 5 times with 1% (v/v) acetic acid (Merck Millipore) and left to dry at room temperature. SRB complexes were solubilized, by adding 10 mM Tris buffer (Sigma-Aldrich) for 30 min. Absorbance was measured at 515 nm in a microplate reader (Biotek Synergy 2,BioTek Instruments, Inc., Winooski, VT, USA). A dose–response curve was obtained for each cell line with each test compound, and the concentration that caused cell growth inhibition of 50% (GI50) was determined.
The degree of selectivity of the compounds was assessed by determining the selectivity index (SI) as the ratio of GI50 in non-tumor Human Pulmonary Alveolar Epithelial Cells (HPAEpiC) over GI50 in cancer cells. An SI value less than 2.0 indicates the general toxicity of the compound [44].
4. Conclusions
In this work, four acetylated flavonosides (5, 6, 9, and 10), and one xanthonoside (7) were synthesized. As far as we know, compounds 9 and 10 are described for the first time. Compound 5 was synthesized without pyridine in a shorter reaction time. Applying MW irradiation to the synthesis of compounds 6 and 7 allowed to shorten the reaction times.
The in vitro growth inhibitory activity of compounds 5–7 and compounds 9 and 10 was investigated. 3-Hydroxy-7-(2,3,4,6-tetra-O-acetyl-β-glucopyranosyl) flavone (10) was the most active compound in all the human tumor cell lines tested with notable selectivity for cancer cells. The mechanism of action of this promising compound deserves to be further explored. The position of the sugar moiety seems to influence the activity of these compounds.
These results are a starting point from which to investigate more stable chemical modifications in order to optimize such molecules and develop detailed structure-activity relationship studies in future.
Supplementary Materials
The following are available online, Figure S1. 1H and 13C NMR of compound 9, Figure S2. 1H and 13C NMR of compound 10, Figure S3. HRMS for compound 9, Figure S4. HRMS for compound 10.
Author Contributions
An.R.N. performed the synthesis, purification and structure elucidation of compounds 5, 6, and 7, and wrote the manuscript; M.C.-d.-S. performed the synthesis, purification and structure elucidation of compounds 9 and 10. M.C.-d.-S., E.S., and M.P. designed the experimental work concerning the synthesis. P.M. A.S. and D.R. performed the inhibition tumor cell growth assays and determined the selectivity index; H.B. designed the experimental work concerning the antitumor screening. M.C.-d.-S., P.M. A.S., D.R., E.S., Hassan Bousbaa, and Madalena Pinto revised the~manuscript.
Funding
This work was supported through national funds provided by Foundation for Science and Technology from the Minister of Science, Technology and Higher Education (FCT/MCTES-PIDDAC) and European Regional Development Fund (ERDF) through the COMPETE—Programa Operacional Factores de Competitividade (POFC) (POCI-01-0145-FEDER-016790), PPCDT—Promover a Produção Científica e Desenvolvimento Tecnológico e a Constituição de Redes Temáticas (Project 3599), under the project PTDC/MAR-BIO/4694/2014 in the framework of the programme PT2020 and INNOVMAR—Innovation and Sustainability in the Management and Exploitation of Marine Resources, reference NORTE-01-0145-FEDER-000035, Research Line NOVELMAR in the framework of North Portugal Regional Operational Programme (NORTE 2020).
Acknowledgments
Marta Correia-da-Silva thanks FCT for the postdoctoral fellowship SFRH/BPD/81878/2011 and Ana R. Neves and Patrícia M.A. Silva for the Ph.D. fellowships SFRH/BD/114856/2016 and SFRH/BD/90744/2012, respectively.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Pinto, M.M.M.; Sousa, M.E.; Nascimento, M.S.J. Xanthone derivatives: New insights in biological activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, V.; Ningnuek, N.; Pohmakotr, M.; Yoosook, C.; Napaswad, C.; Kasisit, J.; Santisuk, T.; Tuchinda, P. Anti HIV-1 flavonoid glycosides from ochna integerrima. Planta Med. 2007, 73, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.N.A.; Tako, M.; Hanashiro, I.; Tamaki, H. Antioxidant flavonoid glycosides from the leaves of Ficus pumila L. Food Chem. 2008, 109, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ahmad, A.; Rawat, P.; Khan, M.F.; Rasheed, N.; Gupta, P.; Sathiamoorthy, B.; Bhatia, G.; Palit, G.; Maurya, R. Antioxidant flavonoid glycosides from evolvulus alsinoides. Fitoterapia 2010, 81, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhao, Y.; Jiang, Y.; Yu, L.; Zeng, X.; Yang, J.; Tian, M.; Liu, H.; Yang, B. Identification of a flavonoid c-glycoside as potent antioxidant. Free Radic. Biol. Med. 2017, 110, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Abd El-kader, A.M.; Ahmed, A.S.; Nafady, A.M.; Ibraheim, Z.Z. Xanthone and lignan glycosides from the aerial parts of polygonum bellardii all growing in egypt. Pharmacogn. Mag. 2013, 9, 135–143. [Google Scholar] [PubMed]
- Li, S.; Dong, P.; Wang, J.; Zhang, J.; Gu, J.; Wu, X.; Wu, W.; Fei, X.; Zhang, Z.; Wang, Y.; et al. Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma smmc-7721 cells via a ros/jnk-dependent mitochondrial pathway. Cancer Lett. 2010, 298, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, J.; Yang, B.; Xiang, T.; Yin, X.; Peng, W.; Cheng, W.; Wan, J.; Luo, F.; Li, H.; et al. Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and beta-catenin signaling pathway. Toxicol. Appl. Pharmacol. 2013, 272, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.M.; Kijjoa, A. Naturally-occurring xanthones: Recent developments. Curr. Med. Chem. 2005, 12, 2413–2446. [Google Scholar] [CrossRef] [PubMed]
- Delazar, A.; Celik, S.; Göktürk, R.S.; Unal, O.; Nahar, L.; Sarker, S.D. Two acylated flavonoid glycosides from stachys bombycina, and their free radical scavenging activity. Pharmazie 2005, 60, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Uriarte-Pueyo, I.; Calvo, M.I. Structure–activity relationships of acetylated flavone glycosides from Galeopsis ladanum L. (lamiaceae). Food Chem. 2010, 120, 679–683. [Google Scholar] [CrossRef]
- Browning, A.M.; Walle, U.K.; Walle, T. Flavonoid glycosides inhibit oral cancer cell proliferation-role of cellular uptake and hydrolysis to the aglycones. J. Pharm. Pharmacol. 2005, 57, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Poteet-Smith, C.E.; Xu, Y.; Errington, T.M.; Hecht, S.M.; Lannigan, D.A. Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Res. 2005, 65, 1027–1034. [Google Scholar] [PubMed]
- Kong, C.S.; Kim, Y.A.; Kim, M.M.; Park, J.S.; Kim, J.A.; Kim, S.K.; Lee, B.J.; Nam, T.J.; Seo, Y. Flavonoid glycosides isolated from Salicornia herbacea inhibit matrix metalloproteinase in HT1080 cells. Toxicol. In Vitro 2008, 22, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Deguin, B.; Loizzo, M.R.; Bonesi, M.; Statti, G.A.; Tillequin, F.; Menichini, F. Potential antitumor agents: Flavones and their derivatives from linaria reflexa desf. Bioorg. Med. Chem. Lett. 2005, 15, 4757–4760. [Google Scholar] [CrossRef] [PubMed]
- Aligiannis, N.; Mitaku, S.; Mitrocotsa, D.; Leclerc, S. Flavonoids as cycline-dependent kinase inhibitors: Inhibition of cdc 25 phosphatase activity by flavonoids belonging to the quercetin and kaempferol series. Planta Med. 2001, 67, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Viskupicova, J.; Ondrejovic, M.; Maliar, T. Enzyme-mediated preparation of flavonoid esters and their applications. In Biochemistry; Ekinci, D., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Dixit, S. Anticancer effect of rutin isolated from the methanolic extract of triticum aestivum straw in mice. Med. Sci. 2014, 2, 153–160. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Domínguez, F.; García-Carrancá, A. Rutin exerts antitumor effects on nude mice bearing sw480 tumor. Arch. Med. Res. 2013, 44, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, A.; Urdaci, M.C.; Pinchuk, I.V.; Lopez-Moratalla, N.; Martinez-Irujo, J.J. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr. Cancer 2004, 50, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Antiproliferative activity of flavonoids on several cancer cell lines. Biosci. Biotechnol. Biochem. 1999, 63, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, S.; Nyavanandi, V.K.; Sanjeeva Rao Thunuguntla, S.; Kasu, S.; Pallerla, M.K.; Sai Ram, P.; Rajagopal, S.; Ajaya Kumar, R.; Ramanujam, R.; Moses Babu, J.; et al. Synthesis and structure–activity relationships of andrographolide analogues as novel cytotoxic agents. Bioorg. Med. Chem. Lett. 2004, 14, 4711–4717. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Chu, J.; Wang, H.; Fu, X.; Quan, D.; Ding, H.; Yao, Q.; Yu, P. Regioselective iodination of flavonoids by n-iodosuccinimide under neutral conditions. Tetrahedron Lett. 2013, 54, 6345–6348. [Google Scholar] [CrossRef]
- Mogilaiah, K.; Rani, J.U.; Vidya, K.; Sakram, B. Microwave-promoted rapid and efficient method for acetylation of phenols with acetic anhydride using naf as catalyst under solvent-free conditions. Orient. J. Chem. 2009, 25, 187–190. [Google Scholar]
- Dar, A.; Faizi, S.; Naqvi, S.; Roome, T.; Zikr-ur-Rehman, S.; Ali, M.; Firdous, S.; Moin, S.T. Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship. Biol. Pharm. Bull. 2005, 28, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liang, D.; Ning, M.; Wang, Q.; Meng, X.; Li, Z. Semi-synthesis of neomangiferin from mangiferin. Tetrahedron Lett. 2014, 55, 3083–3086. [Google Scholar] [CrossRef]
- Faizi, S.; Zikr-ur-Rehman, S.; Ali, M.; Naz, A. Temperature and solvent dependent nmr studies on mangiferin and complete nmr spectral assignments of its acyl and methyl derivatives. Magn. Reson. Chem. 2006, 44, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Lewin, G.; Maciuk, A.; Moncomble, A.; Cornard, J.-P. Enhancement of the water solubility of flavone glycosides by disruption of molecular planarity of the aglycone moiety. J. Nat. Prod. 2013, 76, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Quintin, J.; Roullier, C.; Thoret, S.; Lewin, G. Synthesis and anti-tubulin evaluation of chromone-based analogues of combretastatins. Tetrahedron 2006, 62, 4038–4051. [Google Scholar] [CrossRef]
- Mei, Q.; Wang, C.; Zhao, Z.; Yuan, W.; Zhang, G. Synthesis of icariin from kaempferol through regioselective methylation and para-claisen–cope rearrangement. Beilstein J. Org. Chem. 2015, 11, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Needs, P.W.; Williamson, G. Syntheses of daidzein-7-yl β-d-glucopyranosiduronic acid and daidzein-4′,7-yl di-β-d-glucopyranosiduronic acid. Carbohydr. Res. 2001, 330, 511–515. [Google Scholar] [CrossRef]
- Smith, J.A.; Maloney, D.J.; Clark, D.E.; Xu, Y.; Hecht, S.M.; Lannigan, D.A. Influence of rhamnose substituents on the potency of SL0101, an inhibitor of the Ser/Thr kinase, RSK. Bioorg. Med. Chem. Lett. 2006, 14, 6034–6042. [Google Scholar] [CrossRef] [PubMed]
- Ngameni, B.; Patnam, R.; Sonna, P.; Ngadjui, B.T.; Roy, R.; Abegaz, B.M. Hemisynthesis and spectroscopic characterization of three glycosylated 4-hydrocylonchocarpins from dorstenia barteri bureau. ARKIVOC 2008, 2008, 152–159. [Google Scholar]
- Christensen, H.M.; Oscarson, S.; Jensen, H.H. Common side reactions of the glycosyl donor in chemical glycosylation. Carbohydr. Res. 2015, 408, 51–95. [Google Scholar] [CrossRef] [PubMed]
- Velandia, J.R.; de Carvalho, M.G.; Braz-Filho, R.; Werle, A.A. Biflavonoids and a glucopyranoside derivative from ouratea semiserrata. Phytochem. Anal. 2002, 13, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Mabry, T.; Markham, K.R.; Thomas, M.B. The NMR spectra of flavonoids. In The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970; pp. 274–343. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. Ilogp: A simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the gb/sa approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef] [PubMed]
- Kodelia, G.; Athanasiou, K.; Kolisis, F.N. Enzymatic synthesis of butyryl-rutin ester in organic solvents and its cytogenetic effects in mammalian cells in culture. Appl. Biochem. Biotechnol. 1994, 44, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Suda, I.; Oki, T.; Masuda, M.; Nishiba, Y.; Furuta, S.; Matsugano, K.; Sugita, K.; Terahara, N. Direct absorption of acylated anthocyanin in purple-fleshed sweet potato into rats. J. Agric. Food Chem. 2002, 50, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.; Lygo, B.; Procter, G. Advanced and Practical Organic Chemistry, 3rd ed.; Taylor and Francis Group, LLC: Boca Raton, FL, USA, 2013. [Google Scholar]
- Badisa, R.B.; Ayuk-Takem, L.T.; Ikediobi, C.O.; Walker, E.H. Selective anticancer activity of pure licamichauxiioic-b acid in cultured cell lines. Pharm. Biol. 2006, 44, 141–145. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 5, 6, 7 and 9 are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).