1. Introduction
Gastrodia elata (
G. elata), is a medicine food homology product in China, whose roots were traditionally used as a traditional Chinese medicine (TCM) with treatment of headaches, neuralgia, dizziness, hypertension and other related neuralgic disorders [
1,
2,
3]. Now it is also used as one sub-material in food and Chinese Patent Medicines (CPM) widely. As the wild
G. elata is not sufficient enough for commercial large-scale exploitation, its artificial cultivation medicine has become essential to meet the increasing requirement of markers. The herb is mainly planted in the central and southwestern regions in China [
4], such as Yichang (Hubei), Hanzhong (Shanxi), Dabieshan (Anhui) and Zhaotong (Yunnan). Many studies have indicated that the efficacy and quality of herbal medicines are somewhat different depending on cultivation soil and climate, based on geographic origin, even when coming from the same species [
5,
6].
Currently, the approaches for detecting the origin of
G. elata are mainly sensory analysis and analytical methods. The former depends basically on the experience and personal emotion of
G. elata, which is not objective and lacks scientific understanding; the latter is restricted to the detection of a few active components using methods such as HPLC(LC-MS), GC(GC-MS) and capillary electrophoresis [
7,
8,
9], which are often labor-intensive, expensive, time-consuming and require large amounts of organic solvent. In the end, the quality value of the tested sample is determined as “qualified” or “unqualified” according to the presenting acceptance criteria [
10]. Considering these potential quality risks and to ensure the safety and uniformity for pharmaceutical companies and consumers, there is a significant interest in establishing a rapid and effective method for authenticating the origin of
G. elata.
Near infrared spectrum (NIRS) is widely used as alternatives to wet chemistry procedures for qualitative and quantitative analysis, which can be a rapid and effective instrument to classify different origin of
G. elata coupled with chemometrics; since it is fast, easy to handle and non-destructive, it has been applied to classify coffee varieties, milk power and Chinese herbs [
11,
12,
13]. However, few studies explore the identification of the cultivation origins of
G. elata. Some studies have proved that NIR spectroscopy coupled with supervised pattern recognition can be applied to provide qualitative assessment, while to choose the most appropriate method still remains our consideration. In this study, we applied principal component analysis (PCA) to discuss the possibility of
G. elata samples discrimination with NIR spectroscopy and other pattern recognition as discriminant analysis (DA) was applied and compared.
Furthermore, phytochemical studies of
G. elata have validated that the presence of phenolic compounds in
G. elata, for example,
p-hydroxybenzyl alcohol (HA), gastrodin (GA) and parishins compounds have been given rise to more attentions because of their pharmacological features [
14,
15], it is inappropriate to choose only several specific components as essential criteria. Although many efforts have been endeavored to the quality evaluation of
G. elata [
16,
17,
18], further exploration is still required. Numerous studies have revealed that the phenolic compounds content (PCC) in
G. elata is an important quality index when evaluating its medicinal efficacy [
15,
17,
19]. For this application, the correlation between the phenolic compounds content in
G. elata and NIRS was also developed, we explore synergy interval partial least squares (Si-PLS) to provide predictive models of PCC by selecting different subintervals.
Therefore, the objective of this study was to build a new method for quality evaluation of G. elata based on NIRS coupled with multivariate analysis, which concludes: (1) the traditional HPLC method was developed to simultaneously determine four phenolic compounds content in G. elata samples, which provided basic evaluation of their geographical origin and the numerical value in order to build the NIRS model; (2) to develop the classification models using PCA and DA for qualitative analysis, and compare the performance of two methods; and (3) the Si-PLS algorithm was used to choose the most efficient sub-interval spectrums for quantitative analysis of PCC in G. elata; in the end, the evaluation between HPLC and NIRS method were simply compared.
2. Experimental
2.1. Material and Reagents
All
G. elata in our lab were supplied by local farmers or manufactures from four main-cultivated regions of China, namely: (1) the Yangtse Gorges and shennongjia area, (2) the Southwest provinces area, (3) the Dabieshan area, and (4) the Northeast area. Since the chemical components are generally similar among the samples from the same province [
20], samples were acquired from two different provinces in each area during the 2017 and 2018 harvest seasons as shown in
Table 1. So a total of 16 batches of
G. elata were collected in this study and authenticated by Professor Wei Shenhua from Guiyang College of Traditional Chinese Medicine.
Reference standards (gastrodin, p-hydroxybenzyl alcohol, parishin A and parishin B) were supplied by China Food and Drug Testing Institute with the purity (≥98%) determined by HPLC-MS. Methanol and acetonitrile were purchased from Kemmo chemical reagent Co., Ltd. (Tianjin, China). Purified water comes from a Milli-Q water purification device (Millipore, Burlington, MA, USA). Other chemical reagents were all analytical grade.
2.2. Sample Preparation and Standard Solution for HPLC
Upon arrival, after washed cleanly the soil dust from G. eleta surface, the samples were put into an electric steamer (Midea Group, Foshan, China) immediately and heated about 15 min to imitate the steaming process used by manufactures, then spread out and dried in a force-draught oven (Dongguan Ke-chang Machine Co., Dongguan, Guangdong, China) at 50 °C for about 10 h. At last, the samples were ground into fine pieces and screened through a 20 mm sieve that were used for further analysis. All procedures in this study were strictly controlled to lower the risk of uncertain parameters during sample collection, transportation and pre-treatment.
To prepare the further experiment, about 0.5 g raw G. elata powder sample was weighed to be extracted with 25 mL of 50% alcohol then be ultrasounded for 30 min. Weighed again and the lost 50% alcohol was added then the solution was filtered on qualitative filter paper twice. The filtrate was collected and pipetted 10 mL of the subsequent filtrate to concentrated to dry, using the mixed solution of acetonitrile-water (3:97) to resolve residue and fixed the volume to a 10 mL volumetric flask. The extraction solution was filtered with 0.22 μm organic phase filtration membrane before traditional HPLC analysis. The chromatographic analysis was carried on an Agilent 1260 HPLC System comprising a diode-array detector.
The standard stock solutions used here were prepared in advance by dissolving the four reference standards in 50% methanol to a final concentration of 0.0591 mg/mL for gastrodin, 0.0055 mg/mL for p-hydroxybenzyl alcohol, 0.0315 mg/mL for parishin A, 0.0025 mg/mL for parishin B.
2.3. Sample Preparation and Measurement for NIRS
For each G. elata powder sample, about 0.5 g was weighed and densely packed into the sample cup then compressed it. The samples’ particle size was controlled below 0.25 mm. In this study, infrared spectra was collected on an Antaris II FT-NIR spectrophotometer (Thermo Scientific Co., Waltham, MA, USA) with an InGaAs detector (Thermo Scientific Co., USA). The sample container is designed specifically by Thermo Scientific Co. to hold samples. The NIRS measurements were performed within the region from 4000–10,000 cm−1, and the automatic sampler with the reflection mode was used to gain the spectroscopy automatically. Each sample spectrum was collected three times by an average of 64 scans with a resolution of 8 cm−1, all spectra were recorded as log(1/R), where R was the relative reflectance, the mean of three spectra was used in the following analyze step. To lower the risk of uncertain parameter and ensure a representative data, the temperature was controlled at 25 °C and the humidity remained at a steady level in our laboratory.
Before establishing the models, the spectral data should be transformed for an optimal performance. The data preprocessing methods on pattern recognition had been studied including Savitzky-Golay (SG) smoothing, multiplicative scatter correction (MSC), first and second derivative (F/SD) of raw spectrum, and standard normal variate (SNV) which can be used to optimize the model. For example, the SG method was applied to decrease noise, MSC and SNV can be used to correct the light scatter and reduce the path length change of light.
2.4. Chemometric for Geographical Origin Determination of G. elata
The aim of our study is to identify the geographical origin of G. elata by newly-built NIR spectrum method with suitable pattern recognition model. In this study, PCA and DA were explored in the classification of geographical origin, and Si-PLS method was used for prediction of PCC in G. elata.
2.4.1. PCA
Principal Component Analysis (PCA) is a well-known method for variable reduction and transforming the original independent variables into new axes [
21]. By plotting the PCAs, one can detect the possible spectral outliers and discriminate the samples based on the geographical origins. The first principal component (PC1) accounts for the maximum of the total variance, the second (PC2) is uncorrelated with the first and represented to the maximum of the residual variance. The PCA score plots can be used to observe the data distribution and structure of the data.
2.4.2. DA
Discriminant Analysis (DA) is a supervised pattern recognition method where the number of categories and the samples that belong to each category are previously defined. In this study, DA was explored to classify G. elata from different origins based on predefined classes. Discrimination of the groups according the calculated Mahalanobis distance of a sample from their centers of gravity of the considered groups, so the greater the Mahalanobis distance between two given groups, the greater the spectral differences between them. Since the first 10 PCs cover the most variation contained in the spectral data, the DA was performed on the first 10 PCs resulting from the PCA applied on NIR spectral data recorded on G. elata samples.
2.4.3. Si-PLS Model for Predicting PCC
The Si-PLS method proposed by Norgaard is a variable selection algorithm [
22,
23], which is an all-possible-subinterval-combination procedure test that contained relevant information related to the target parameter. The basic principle of Si-PLS is as follows: first, the full-spectrum region was spilt into different numbers of equal subintervals; second, the PLS regression model was constructed with different numbers and spectral subintervals; and lastly the combination of subinterval spectrums which has the lowest root mean square error of the cross validation (RMSECV) showed better performance [
24].
In this study, the full spectrum (4000–10,000 cm−1) of G. elata samples was divided into 11, 12…, 25 intervals combined with two, three or four subintervals. The optimal combination of intervals and the number of PCs were optimized by leave-one-out cross validation and determined according to the lowest RMSECV. The final Si-PLS model’s performance was evaluated by the calibration set samples and tested by the prediction set samples.
Relationship between PCC and NIRS was also explored using a Si-PLS model. To establish a robust and high-performance regression model, it is important to ensure that the samples represent all types of variability as much as possible. The parameters as the root mean square error of the cross validation (RMSECV), root mean square error of the prediction (RMSEP), the coefficients of determination in the calibration data set (RC2) and the prediction data set (RP2) were all taken into account.
2.5. Software
In this study, a nonparametric Kruskal-Wallis test followed by Dun’s multiple comparison test was performed to analyze the significant difference among samples for the HPLC quantitative of four phenolic compounds content from different regions. These results were conducted using Graphpad Prism 7.0 (GraphPad Software, San Diego, CA, USA), the significance levels were set as p < 0.05. The clustering analysis was calculated using SPSS 18.0 (SPSS Inc., Chicago, IL, USA).
To establish the pattern recognition and prediction models, the PCA was carried out using SIMCA-P software (Umetrics AB, Umeå, Sweden), while the DA and the Si-PLS were carried out using TQ analyst software (Thermo Scientific Co.)
3. Results and Discussion
3.1. Optimization of Sample Extraction and Chromatogram for HPLC Method
To acquire more active ingredients and get better fingerprints of the samples, variables included in the extraction condition such as the solvent, methods and time were optimized by the orthogonal experiment shown in
Table 2. We compared water, methanol and 50% alcohol as different extraction solvents, the results showed that diluted 50% alcohol had higher extraction efficiency and better fingerprints than others. Then we compared reflux, ultrasonic extraction and soak at room temperature as different methods, the results showed that there was no significance between two methods. However, ultrasonic extraction is chosen due to its time saving and easy operation. We next tried the sample extraction by ultrasonic treatment in diluted alcohol at 15 min, 30 min and 40 min, the results showed that effective extraction was get between 30 and 40 min. Therefore, the optimal extraction conditions for
G. elata are presented in
Section 2.2.
In this study, four phenolic compounds (GA, HA, PB and PA) were marked as active ingredients to discriminate origins in
G. elata samples. To ensure good separation performance within a short analysis time, the optimization of chromatographic conditions were also investigated and discussed as following: all sample analysis were carried on a Diamonsil C
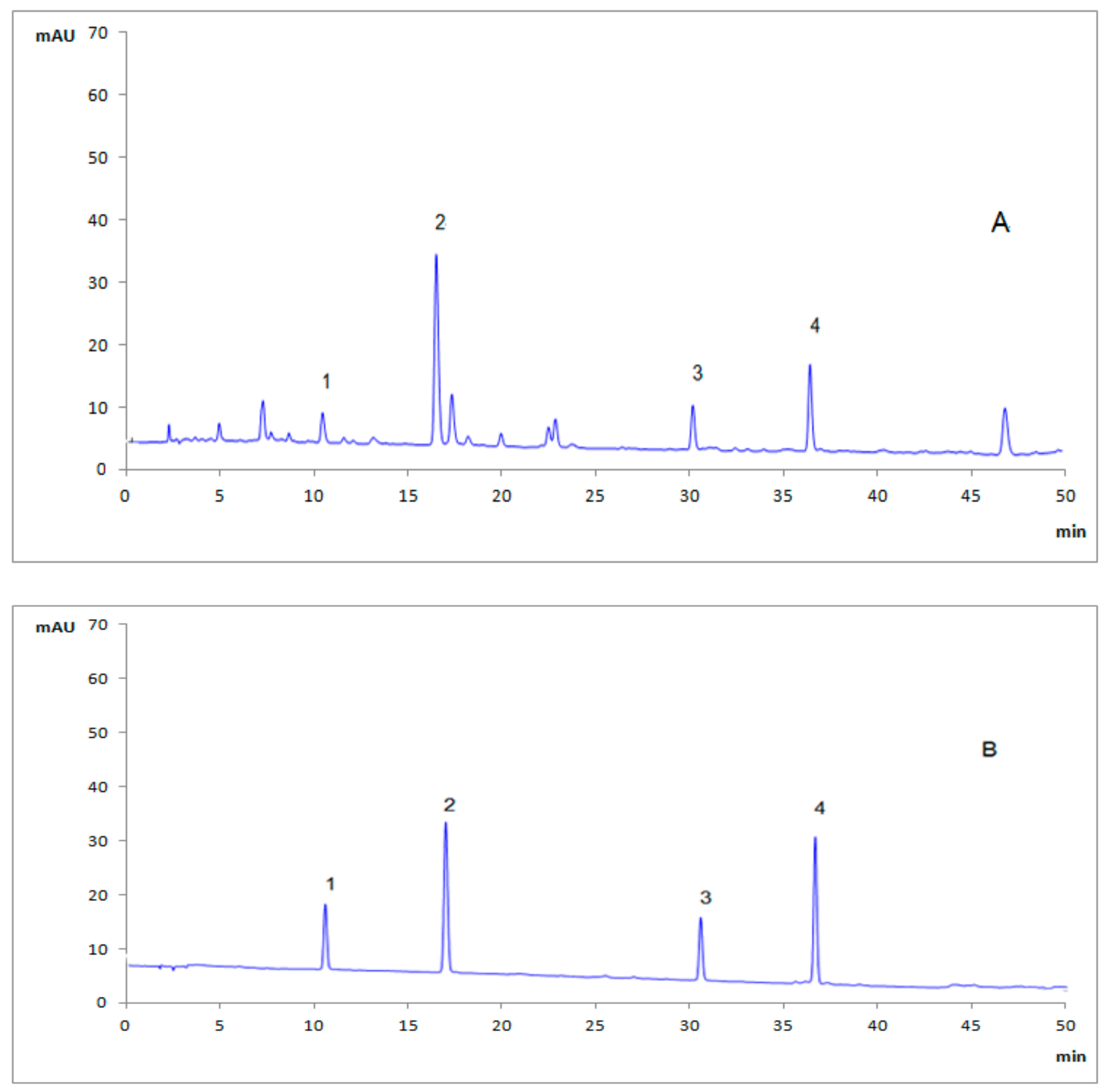
18 column (250 mm × 4.6 mm, 5 μm) at 30 °C, the mobile phase in this study consisted of 0.05% phosphoric acid solution (A) and acetonitrile (B) at a flow rate of 1.0 mL/min. A gradient program was set as: 0–10 min, 3–8%B; 10–18 min, 8–12%B; 18–40 min, 12–25%B; 40–50 min, 25–40%B. The detection wavelength was set at 220 nm, and the injection volume was defined to 10 μL. The typical HPLC chromatogram of
G. elata sample was shown in
Figure 1.
3.2. Quantitative Analysis of four PCC in G. elata Samples
The above-mentioned HPLC method was used to determine four PCC in
G. elata samples from different origins simultaneously, and the results were shown in
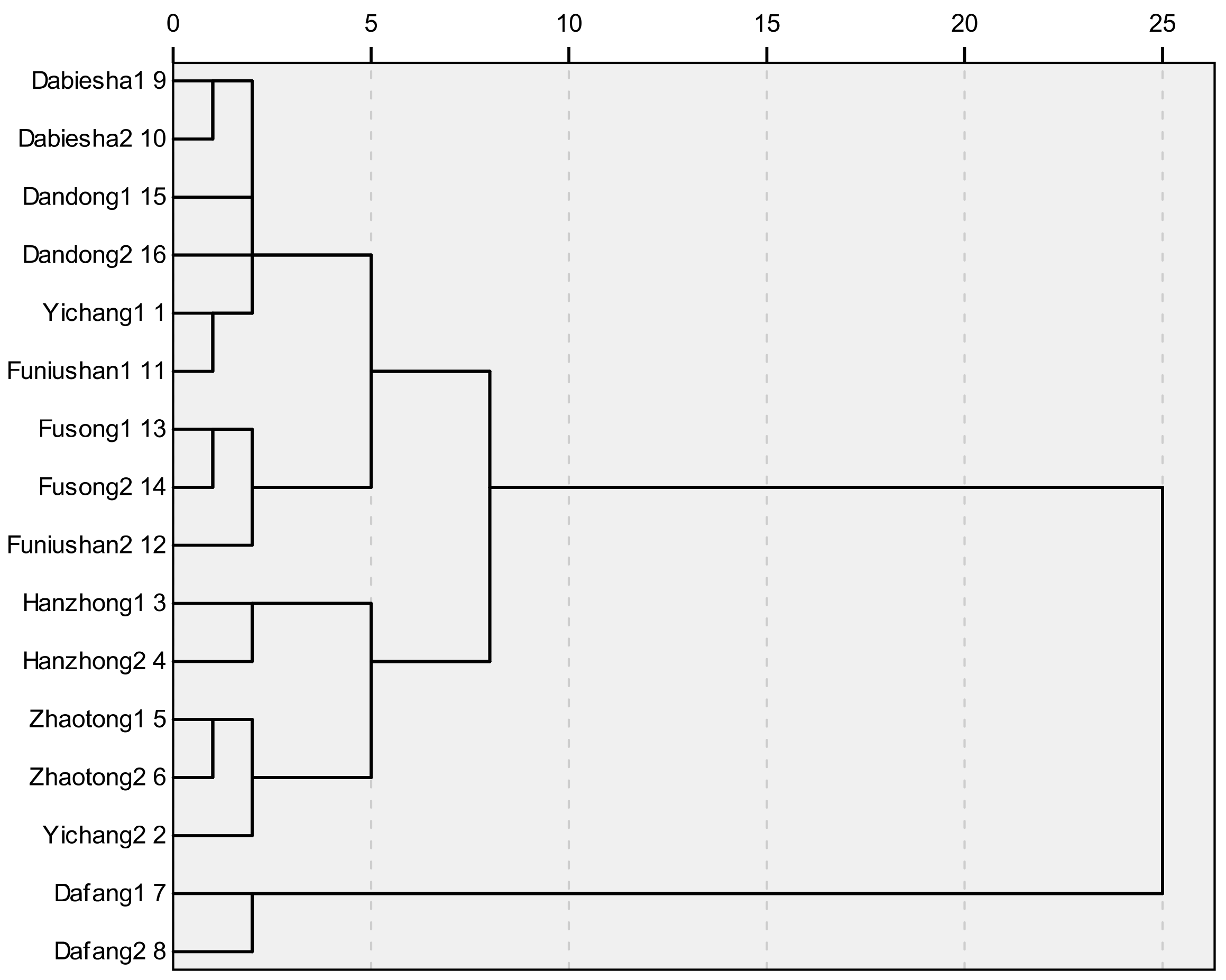
Table 3. It could be concluded that the contents of four analytes varied greatly with the sum of contents of GA and HA ranging from 3.37 mg/g to 7.43 mg/g, and the sum of contents of parishins (PA and PB) ranging from 3.89 mg/g to 9.32 mg/g. The results of statistical analysis showed that the four PCC was remarkably higher in samples from Dafang (Guizhou) compared to other origins. However, no clear separation was concluded among samples in terms of all different geographical origins. The clustering analysis of
G. elata samples from 16 different origins was also calculated, which depended on the four phenolic compounds content in each sample using Euclidean distance in
Figure 2. It could be concluded that the four main-cultivation areas could be discriminated separately mainly due to the difference came from chemical ingredients, while this marked-ingredients method was not able to reveal the chemical difference efficiently among samples from eight different cities, a newly-built NIRS method which was fast, non-destructive and more efficient was developed.
3.3. The NIR Spectra Analysis and Chemical Properties
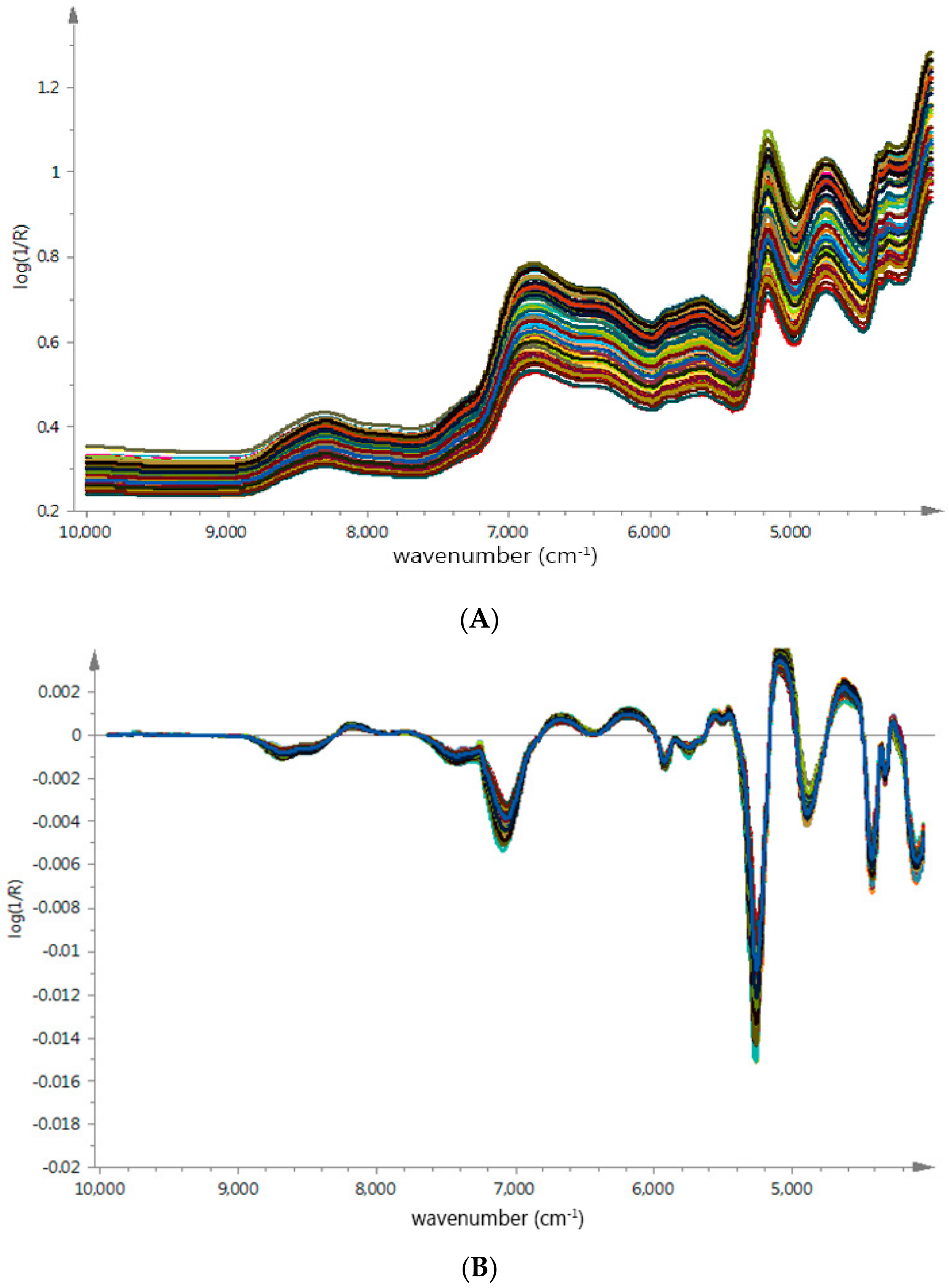
The average raw spectra of
G. elata samples from different origins were shown in
Figure 3A, which presented similar tendencies and indicated that samples contain similar chemical ingredients. We could observe strong absorptions at 4760 cm
−1 to 5210 cm
−1 and 4783 cm
−1 to 4867 cm
−1, related to the combination vibrations of O–H and a stretch and deformation of C–O group [
25,
26], respectively; these bond vibrations were probably corrected with PCC in
G. elata samples [
27,
28]. However, due to physical properties such as particle size effect and numerous chemical ingredients in herb medicines, it was difficult to find specific region based on geographical origin.
In order to remove baseline drift and enhance the absorbance of feature spectral, the SG algorithm and FD were used to make the unique spectra features stand out. As shown in
Figure 3B, the sample spectrum difference were observed in several regions, around 4900 cm
−1 and 8500 cm
−1. Especially the spectra range between 4600 cm
−1 and 5200 cm
−1 related to the interaction between O–H and aromatic ring [
29,
30], these spectrum regions could probably be applied to build identification models more efficiently.
Moreover, further spectral transformations needed to be tried, such as MSC and SNV, to remove slope variation and correct scatter effect, FD/SD and SG to enhance the main absorbance features in spectra. These feature spectra regions will be vital for classification, the detail information was explored in
Section 3.5 in this study.
3.4. Data Processing and Splitting
With different spectra preprocessed, the data were partitioned into a calibration set and validation set, respectively. The former was used to establish the calibration model, the latter was used to test the prediction ability of the model constructed. The Kennard and Stone (K-S) algorithm which can ensure that both sets were well proportioned was adopted and its principle is to cover the space in a uniform manner by maximizing the Euclidean distances between selected and remaining objects. Finally, 10 batches from 16 batches (100 samples) were selected as calibration set, and the remaining (60 samples) were taken as validation set. The mean, range and standard deviation for the parameters measured in both sets are listed in
Table 4, which indicated that these samples were all appropriately distributed.
3.5. Development of Discrimination Models
To compare the performance of different classification models in this study, two pattern recognition method PCA and DA were established using the selected calibration and validation set. PCA was established to extract effective data and main information to examine qualitative difference between two kinds of samples, all the samples’ spectra were used for the PCA model; while DA can be performed to establish a classification model that related to the characteristic sample spectral data by Mahalanobis-distance calculation.
Different transformation methods for spectra such as MSC, SNV, SG and FD/SD were compared and the best data transformation was found in this study.
3.5.1. Optimization of NIRS Transfomation Conditions for PCA Model
The spectra data should be transformed for an optimal performance before the calibration stage. The raw and all transformed spectra methods were compared in this study, which revealed that the MSC and FD was the optimal condition for further analysis. MSC was used to remove physical spectral effect as particle size and noise, FD was used to reduce data dimensions so that PCA could be established based on mainly chemical spectral information.
3.5.2. The Establishment of PCA Model
PCA was used to decrease the data to a small number of PCs, the PCs represent most of the original data that were used to extract useful information from raw spectra data.
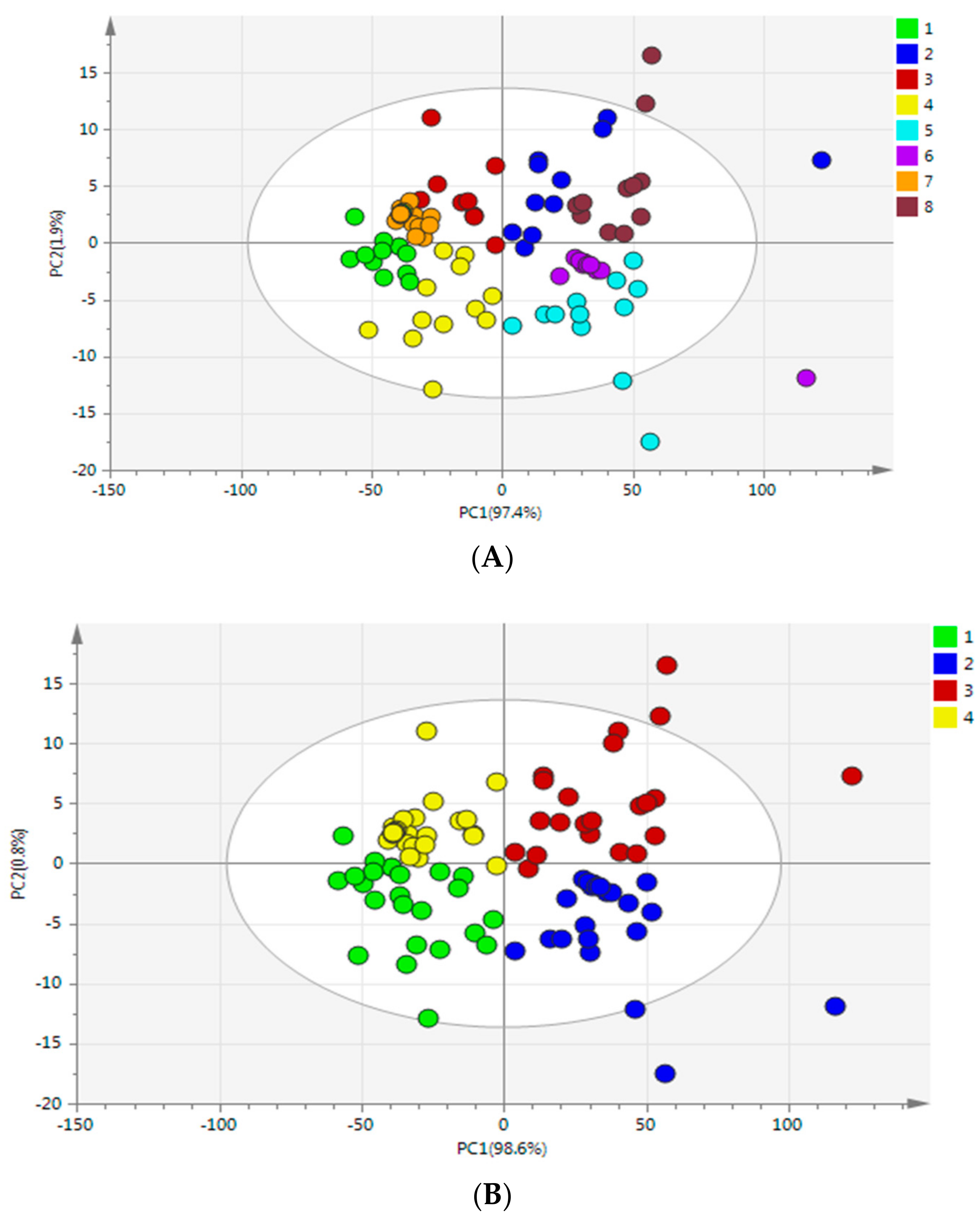
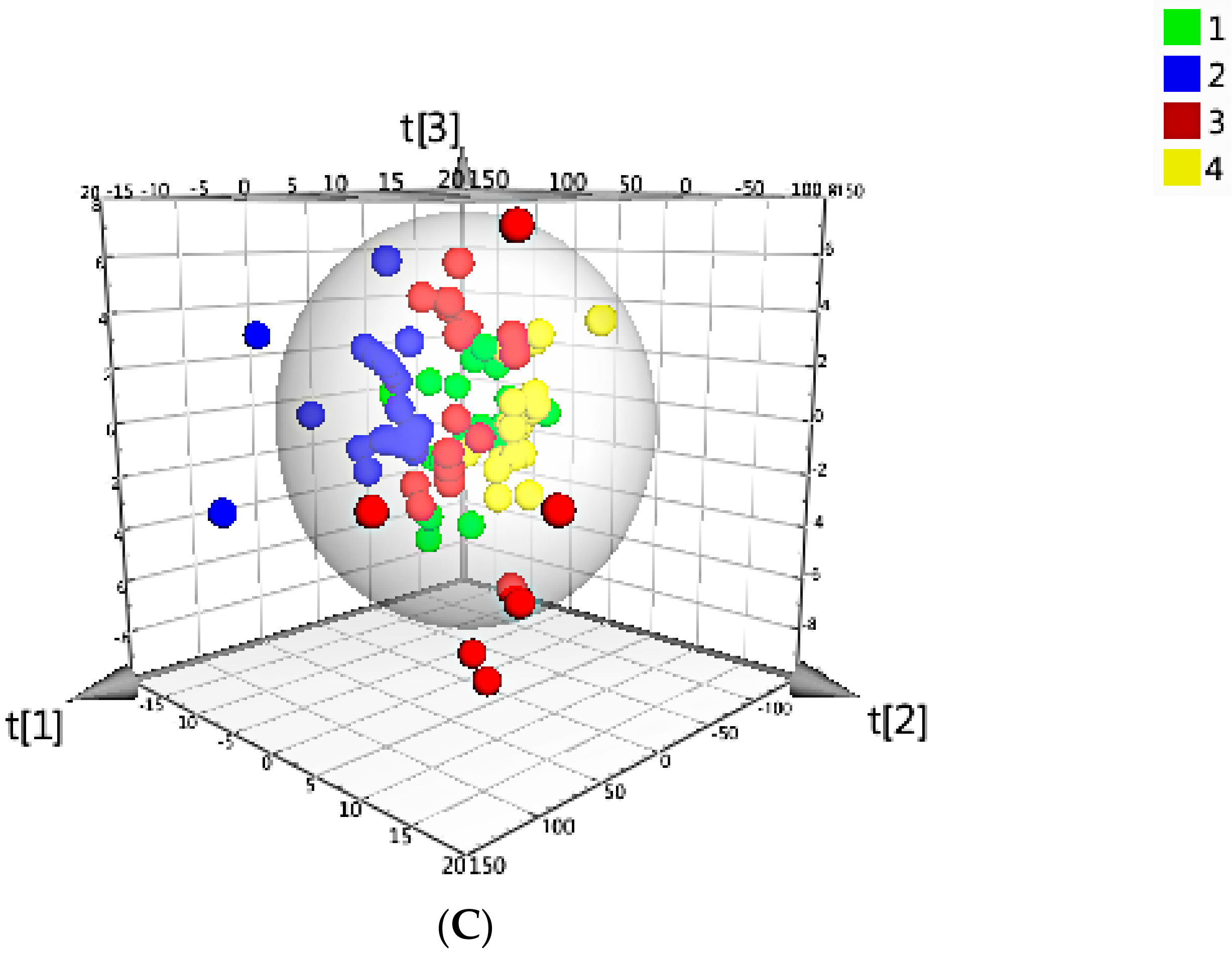
Figure 4A showed the score plot of the first two PCs which account for 99.3% of the variation in the NIR spectra of
G. elata samples from eight different cities. However, the samples’ separation was not all clear and some overlap existed among the groups. As we can see, alone the PC1 (97.4%), the samples were totally separated from different origins, and four areas were also clustered separately, while the samples from Dafang city and Zhaotong city were partly separated along the PC2 (1.9%). It could be concluded that: (1) there were inherent compositional differences among samples from eight different cities though they came from same species; and (2) samples were similar from the same province in terms of their chemical composition, since the environmental conditions such as topography, soil and moisture of different cities in same province are similar. Therefore, further chemometric methods were needed to classify different
G. elata samples.
However,
Figure 4B clearly showed a score plot of
G. elata samples from four different main-cultivate areas represented by PC1 (98.6%) and PC2 (0.8%), the different categories from four main-cultivate areas separated individually indicated that they have unique features probably due to the different cultivated areas with specific growing conditions such as soil and altitude; while the three-dimensional score plot using PC1, PC2, and PC3 for discriminating
G. elata from four main-cultivated areas was shown in
Figure 4C, which performed barely satisfactory than PC1 vs. PC2.
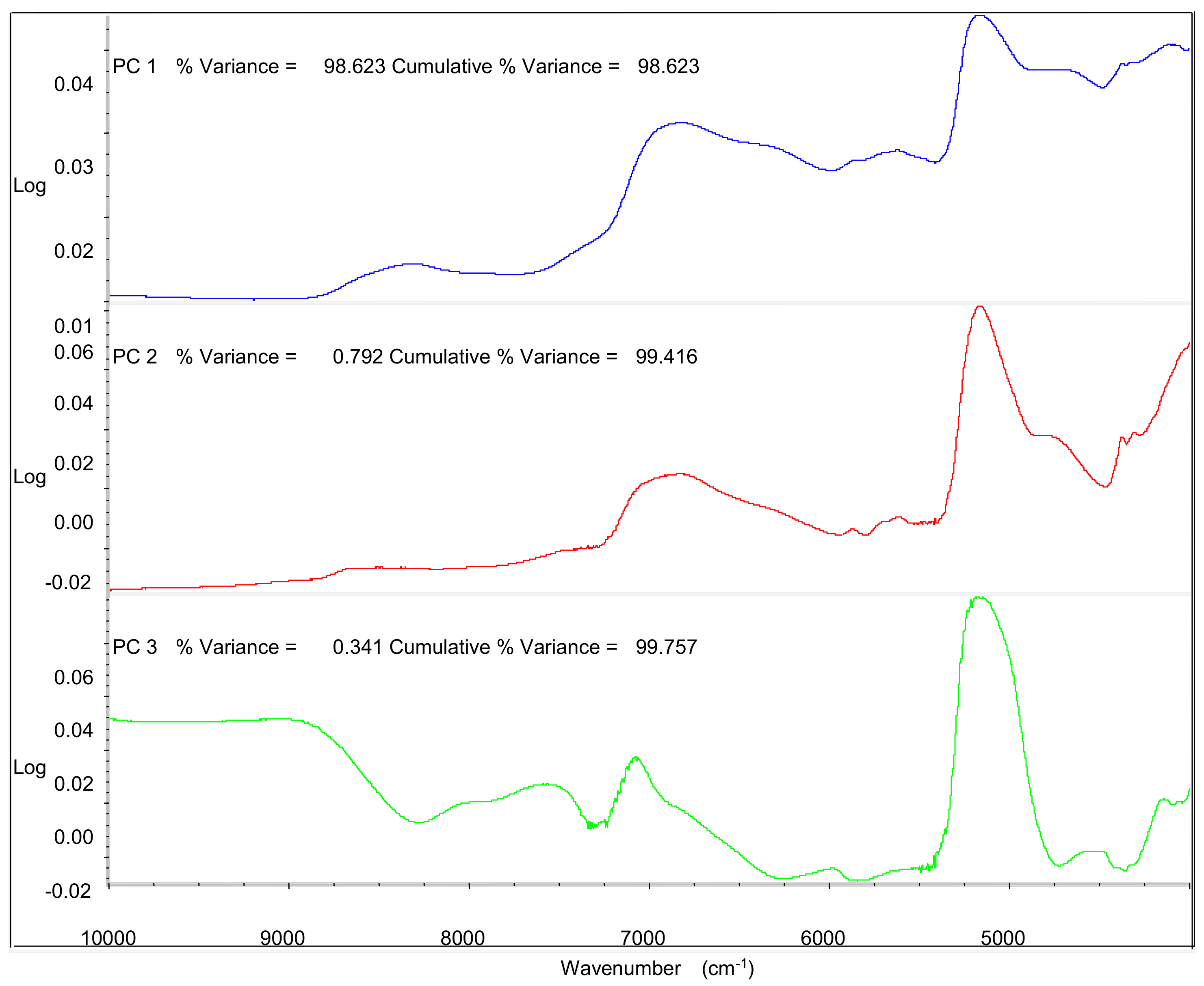
In order to account for the specific regions that used to distinguish the samples from different cultivation areas, the first three PCs that explained 99.8% of the total variance were investigated to discuss in
Figure 5. PC1 had the highest correlation between 4800 cm
−1 and 7200 cm
−1 in spectrum, which related to O–H overtones [
31,
32,
33]; PC2 had the highest correlation between 4200 cm
−1 and 5200 cm
−1 in spectrum, which related to stretch and deformation of C–O group [
34,
35,
36]; PC3 had the highest correlation between 4800 cm
−1 and 5200 cm
−1 in spectrum, which related to interaction between O–H and aromatic ring [
37,
38]; These results could be concluded that the differences of chemical components including the phenolics and water might account for the classification of their geographical origin.
3.5.3. Optimization of NIRS Transformation Conditions for DA Model
Different data transformation was also used for an optimal performance to build DA model. The raw and all transformed spectra were compared in this study in
Table 5, which could be concluded that the MSC and SD was the optimal condition for further analysis, the models discriminate
G. elata samples with the accuracy range from 60% to 98%.
3.5.4. The Establishment of DA Model
DA model was performed on the first 10 PCs of all samples to classify
G. elata samples from eight different cities in
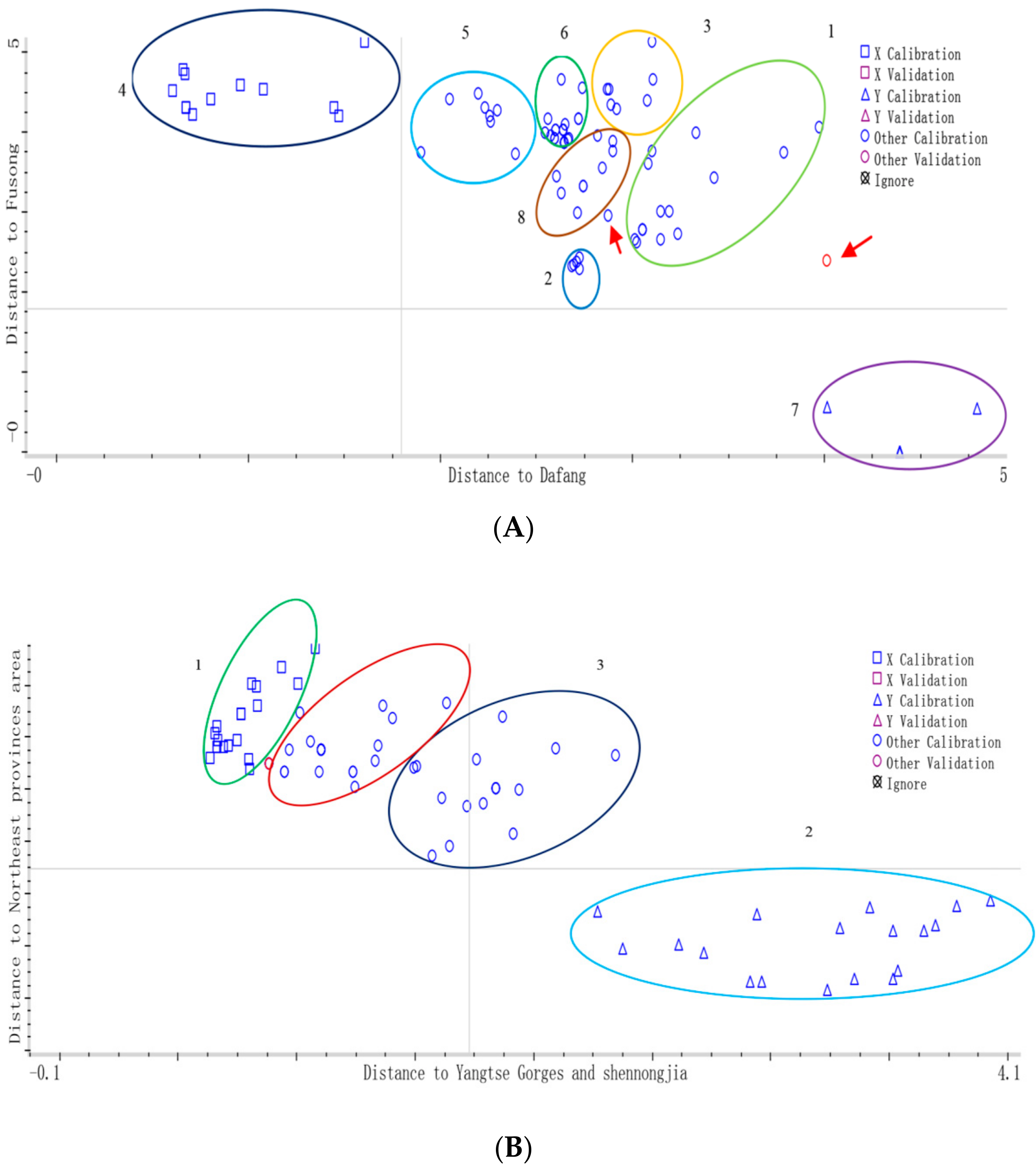
Figure 6A, which accounted for 99.9% variation of the whole spectrum and the total correct classification rate was 98%.
For the calibration set, there was one sample of main-cultivated origin from the “Yangtse Gorges and Shennongjia area”, of which Yichang (Hubei) was identified as “Dandong (Liaoning)”; next is the Northeast area group, where one sample also was wrongly classified; for the other varieties, all the samples were classified correctly. The percentage of correctly identified varieties was 98% for the calibration set. These misclassifications probably due to chemical components varied slightly in samples from different origins; while for validation set, only one sample of “Zhaotong (Yunnan)” was misclassified, others were correctly identified, the percentage of correctly identified varieties was 99% for the validation set.
In a similar way, DA model was performed on the first 9 PCs of all samples to classify
G. elata samples from four different main-cultivate areas, which accounted for 99.9% variation of the whole spectrum and the total correct classification rate was 98% in
Figure 6B. The percentage of correctly identified varieties was 97% and 99% for the calibration and validation set, respectively. In order to compare two established discrimination models PCA and DA for the 16 batches
G. elata samples in this study, the discrimination results (rate%) were shown in
Table 6.
Overall, the geographical origins of G. elata samples could be effectively discriminated by using NIRS combined with the DA method, which may be sufficiently sensitive to be a practical utility.
3.6. Development of PCC Prediction Model
Since the phenolic compounds content (PCC) contained in
G. elata is an important parameter for quality evaluation, the correlation between the PCC and NIRS was also developed. In this study, PCC included four parameters (GA, HA, PA and PB) to be explored. The
G. elata sample parameters (mean, range and standard deviation) of the calibration and validation sets are listed in
Table 2 before. It was indicated that samples were distributed appropriately in the calibration and validation sets.
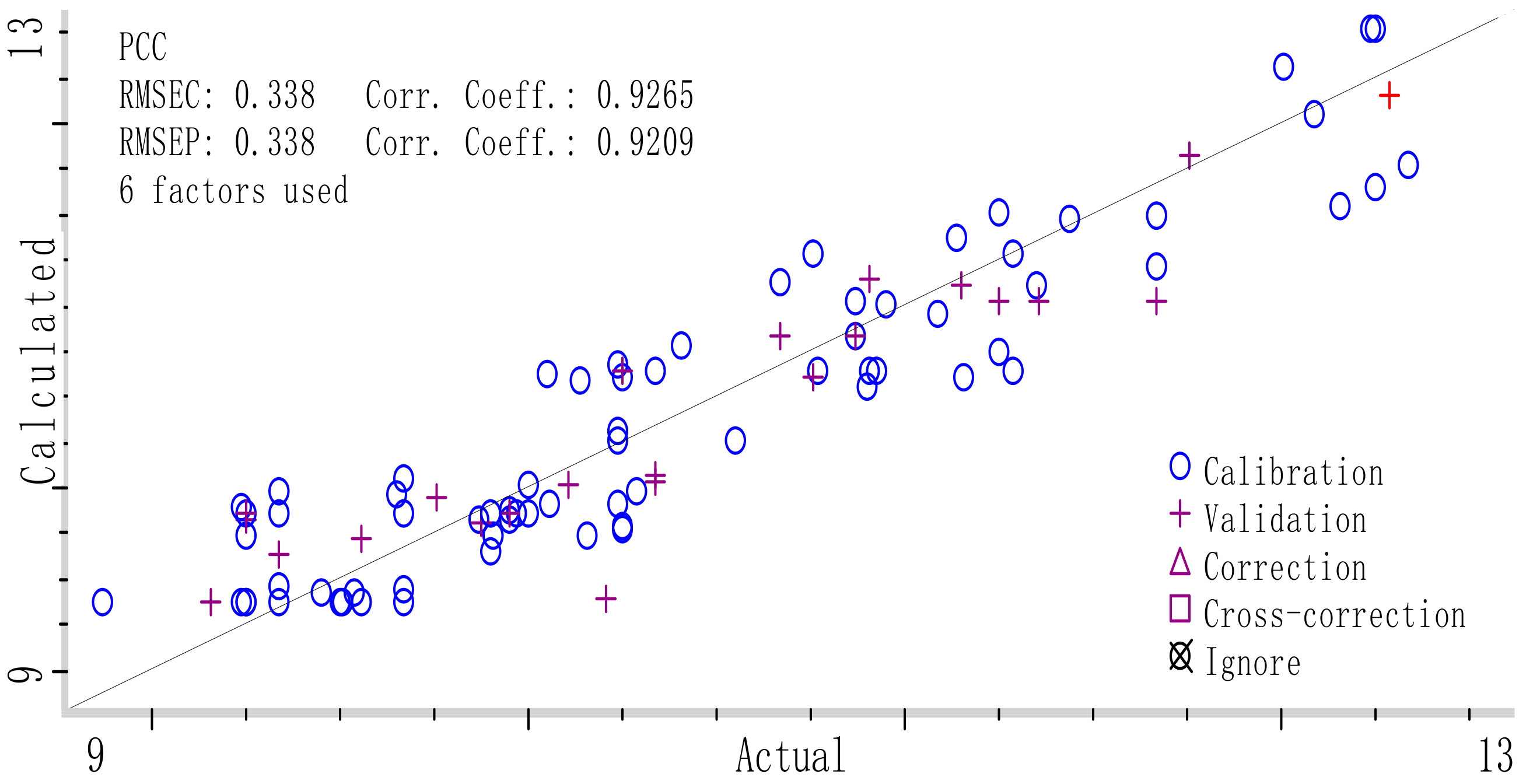
The best PLS calibration model for PCC prediction was obtained with MSC and SD spectrum pre-treatment in the full wavelength range. As we described in
Section 2.4.3, Si-PLS is an efficient variable selection methods which has shown the potential for modeling with good precision used in some research [
39,
40,
41], in this study, the optimal Si-PLS model for PCC prediction from different origins was obtained with the combination of 2 subintervals when the spectrum was divided into 15 subintervals as shown in
Table 7, and the optimal chosen combination subintervals correspond to 4800 cm
−1 to 5200 cm
−1 and 5600 cm
−1 to 6000 cm
−1 in
Figure 7. The parameters for optimal Si-PLS model was as follows: R
C2 was 0.9265 and RMSECV 0.338 in calibration set, the R
P2 was 0.9209 and RMSEP 0.338 in validation set. The established optimal Si-PLS model was shown in
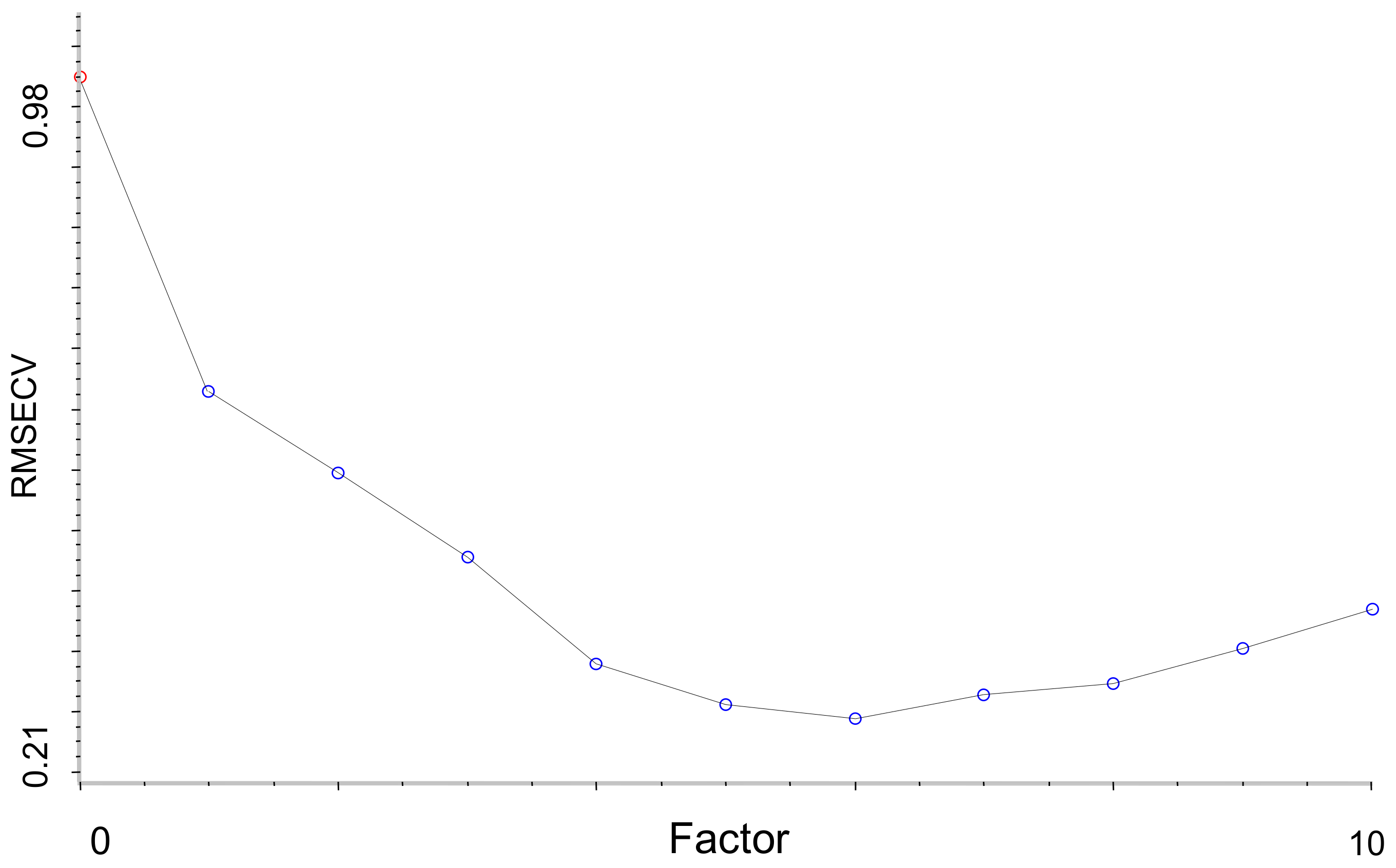
Figure 8 and the plot of RMSECV versus number of factors selected by Si-PLS for PCC was shown in
Figure 9.
It has been known that NIR is based on molecular vibration and often overlapped to find feature spectra origins. One of the selected intervals by Si-PLS 4800 cm
−1 to 5200 cm
−1 was relate to the aromatic ring absorptions of in basic phenolic compound structure [
29,
30,
31,
32] and thus indicated that NIR could be a potential quality-evaluation tool for PCC prediction in
G. elata samples.
3.7. Comparison between HPLC and NIR
To evaluate the TCM quality, numerous methods, such as HPLC, have been applied to establish and prove the quality of medicine. Both HPLC and NIR methods are important due to their individual characteristics. However, compared to target-marker HPLC analysis of G. elata samples from different origins, our study showed that the NIR method developed was more effective and reliable for the classification of G. elata samples. The fast, low cost and non-destructive NIR method coupled with pattern recognition and quantitative PCC evaluation could be helpful in the discrimination of G. elata from different geographic origins. Nevertheless, it was just a first step and more representative samples should be collected to make the models robust.
From the above results, it could be concluded that the NIR method developed, combined with multivariate analysis, is practical for the quality evaluation of G. elata and may also be applicable to other herbal medicines similarly.