Improvement of Trehalose Production by Immobilized Trehalose Synthase from Thermus thermophilus HB27
Abstract
1. Introduction
2. Results and Discussion
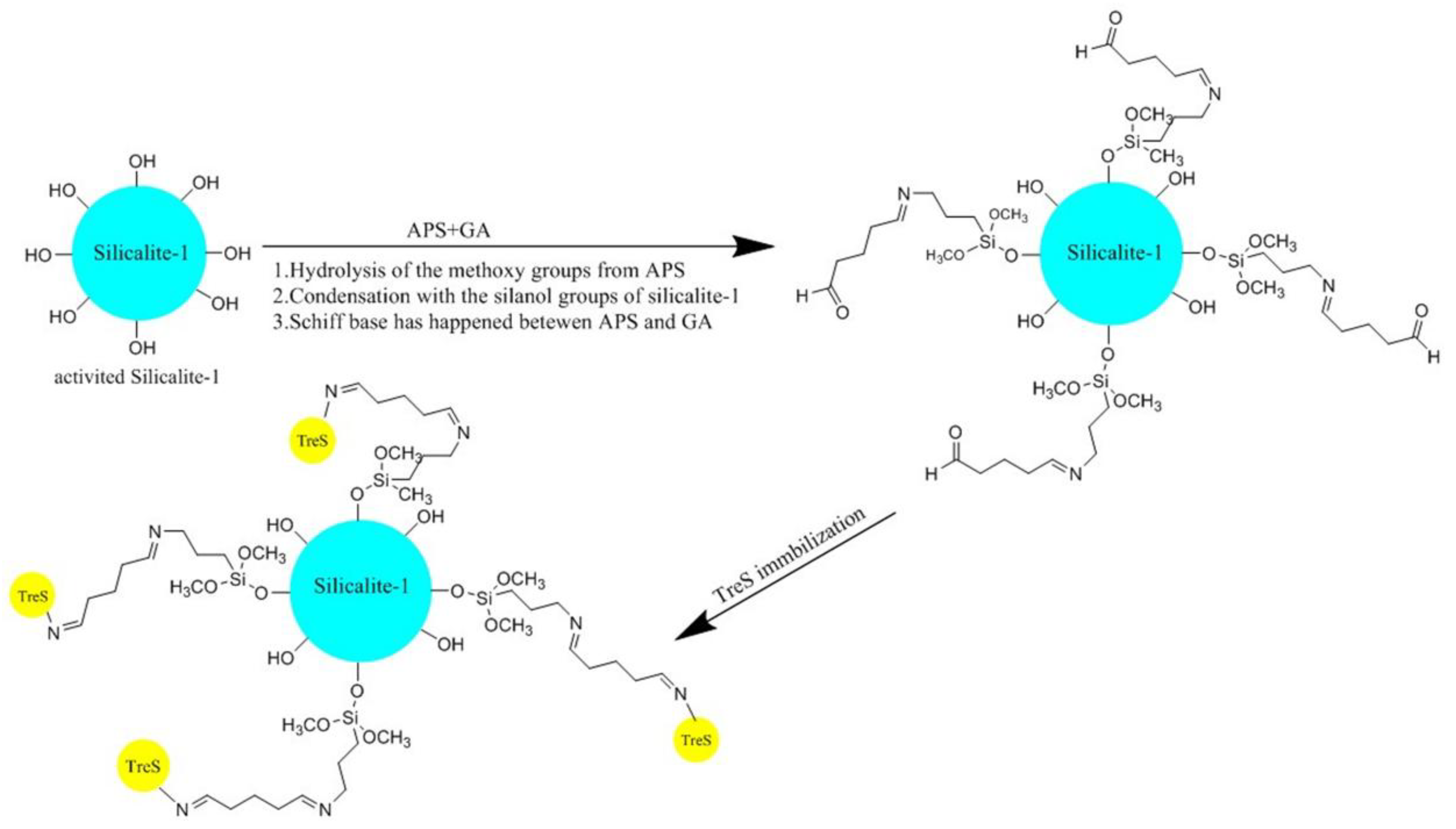
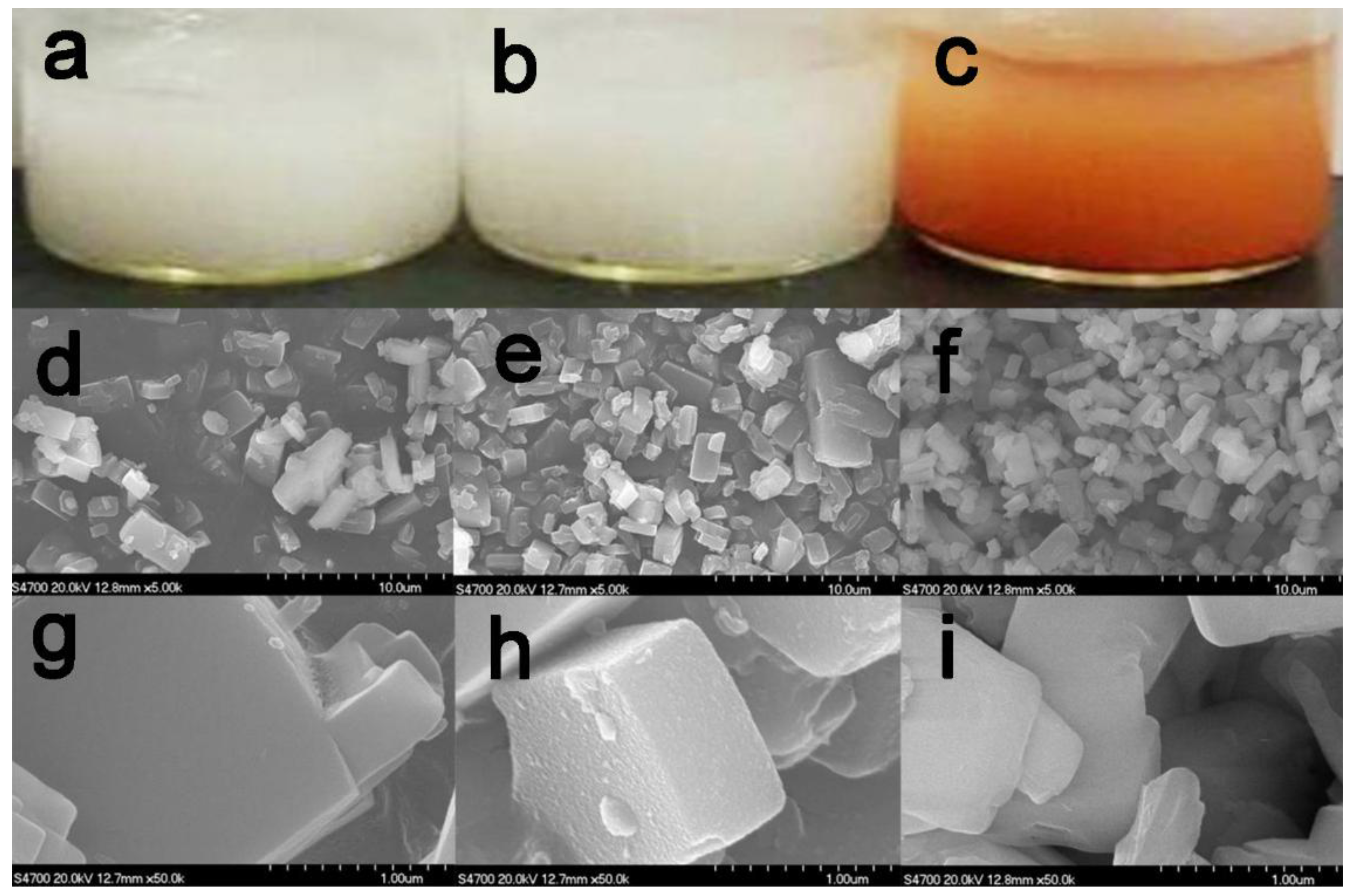
2.1. Immobilization and Characterization of TtTreS on Silicalite-1-Based Support
2.2. Effects of Temperature and pH on the Activity of Free and Immobilized TtTreS
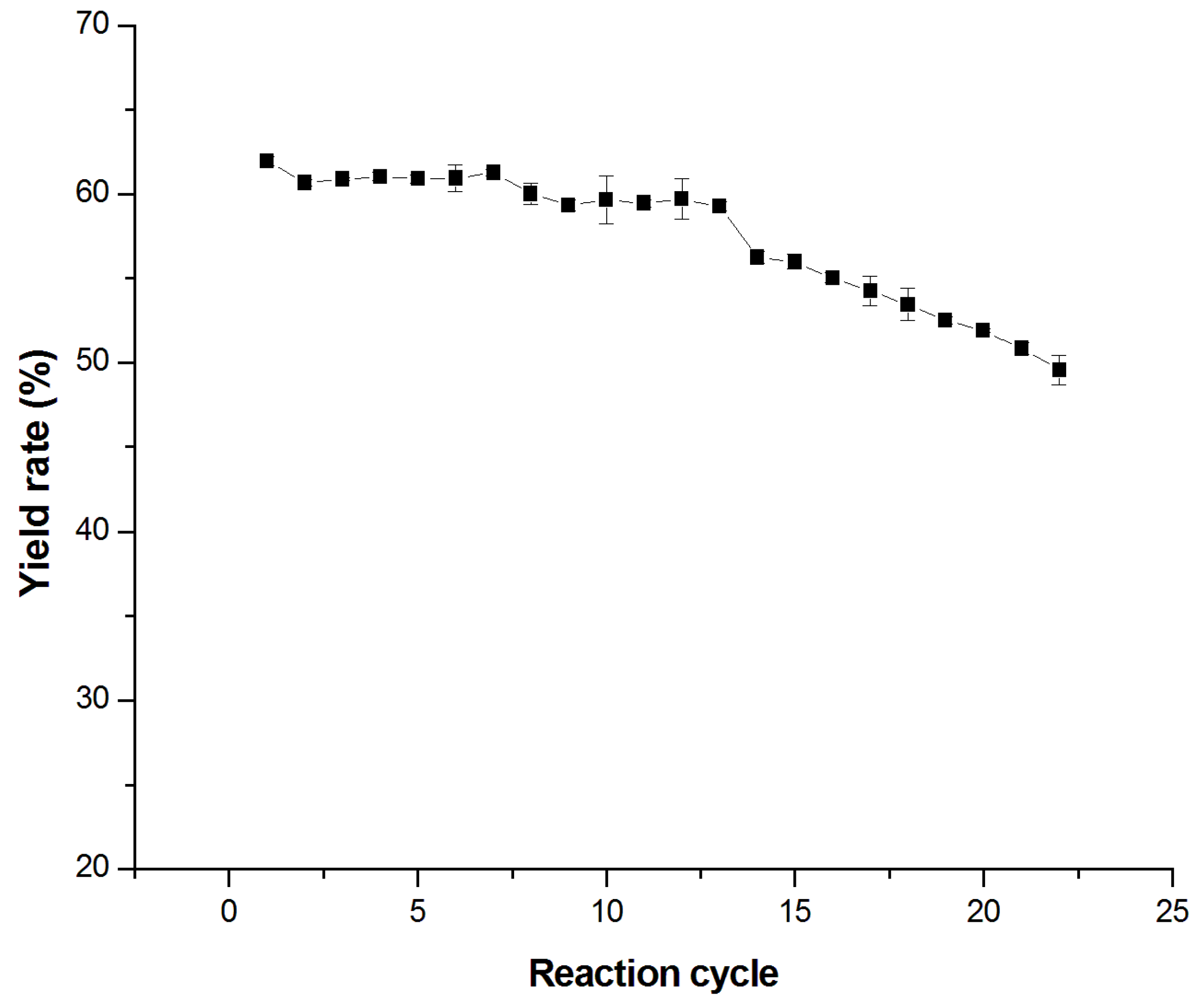
2.3. Reusability of Immobilized TtTreS
3. Materials and Methods
3.1. Bacterial Strain and Medium
3.2. Expression and Prepararion of TtTreS
3.3. Synthesis of Support Materials and TtTreS Immobilization
3.4. Effect of Temperature and pH on the Activity of Free and Immobilized TtTreS
3.5. Determination of Trehalose, Maltose, and Glucose
3.6. Trehalose Production by Immobilized TtTreS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schiraldi, C.; Lernia, I.D.; Rosa, M.D. Trehalose production: exploiting novel approaches. Trends Biotechnol. 2002, 20, 420–425. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Fan, X.; Zhang, J.; Tang, P.; Yuan, Q. Metabolic responses in Candida tropicalis to complex inhibitors during xylitol bioconversion. Fungal Genet. Biol. 2015, 82, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.A.; Lindquist, S. Thermotolerance in Saccharomyces cerevisiae: The Yin and Yang of trehalose. Trends Biotechnol. 1998, 16, 460–468. [Google Scholar] [CrossRef]
- Ohtake, S.; Wang, Y.J. Trehalose: Current use and future applications. J. Pharm. Sci. 2011, 100, 2020–2053. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liang, Y.; Xu, F.; Sun, B.; Wang, Z. Trehalose rescues Alzheimer’s disease phenotypes in APP/PS1 transgenic mice. J. Pharm. Pharmacol. 2013, 65, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Bell, W.; Sun, W.; Hohmann, S.; Wera, S.; Reinders, A.; De Virgilio, C.; Wiemken, A.; Thevelein, J.M. Composition and functional analysis of the Saccharomyces cerevisiae trehalose synthase complex. J. Biol. Chem. 1998, 273, 33311–33319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lin, M.; Zhang, Y.; Li, Y.; Xu, X.; Li, S.; Huang, H. Identification and characterization of a novel trehalose synthase gene derived from saline-alkali soil metagenomes. PLoS ONE 2013, 8, e77437. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, R.; Huang, Y.; Wang, X.; Du, L.; Wei, Y. Cloning, expression, properties, and functional amino acid residues of new trehalose synthase from Thermomonospora curvata DSM 43183. J. Mol. Catal. B Enzym. 2013, 90, 26–32. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Feng, Y.; Yuan, Q. Cloning, expression and activity optimization of trehalose synthase from Thermus thermophilus HB27. Chem. Eng. Sci. 2015, 135, 323–329. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Feng, Y.; Yuan, Q. Improving trehalose synthase activity by adding the C-terminal domain of trehalose synthase from Thermus thermophilus. Bioresour. Technol. 2017, 245, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Manoel, E.A.; Dos Santos, J.C.; Freire, D.M.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzym. Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszynska, D. Immobilization as a strategy for improving enzyme properties-application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Panek, A.; Pietrow, O.; Synowiecki, J.; Filipkowski, P. Immobilization on magnetic nanoparticles of the recombinant trehalose synthase from Deinococcus geothermalis. Food Bioprod. Process. 2013, 91, 632–637. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Park, O.-J.; Shin, H.-J. Immobilization of thermostable trehalose synthase for the production of trehalose. Enzym. Microb. Technol. 2006, 39, 108–113. [Google Scholar] [CrossRef]
- Wu, T.-T.; Lin, S.-C.; Shaw, J.-F. Integrated process for the purification and immobilization of recombinant trehalose synthase for trehalose production. Process Biochem. 2011, 46, 1481–1485. [Google Scholar] [CrossRef]
- Liu, P.; Xing, G.W.; Li, X.W.; Ye, Y.H. Adsorptive immobilization of four proteases on different molecular sieves. Acta Phys.-Chim. Sin. 2010, 26, 1113–1118. [Google Scholar]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Guerrero, C.; Vera, C.; Serna, N.; Illanes, A. Immobilization of Aspergillus oryzae β-galactosidase in an agarose matrix functionalized by four different methods and application to the synthesis of lactulose. Bioresour. Technol. 2017, 232, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Virgen-Ortiz, J.J.; Fernandez-Lafuente, R. Effect of protein load on stability of immobilized enzymes. Enzym. Microb. Technol. 2017, 98, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferro, P.; Tandler, C.; Ramos, A.; Saavedra, J.P.; Brusco, A. Immunofluorescence and glutaraldehyde fixation. A new procedure based on the Schiff-quenching method. J. Neurosci. Methods 1997, 77, 191–197. [Google Scholar] [PubMed]
- Castro, H.F.; Silva, M.L.C.P.; Silva, G.L.J.P. Evaluation of inorganic matrixes as supports for immobilization of microbial lipase. Braz. J. Chem. Eng. 2000, 17, 849–858. [Google Scholar] [CrossRef]
- Villalba, M.; Verdasco-Martin, C.M.; Dos Santos, J.C.; Fernandez-Lafuente, R.; Otero, C. Operational stabilities of different chemical derivatives of Novozym 435 in an alcoholysis reaction. Enzym. Microb. Technol. 2016, 90, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.T.; Zhu, Q.X.; Luo, Z.F.; Lu, F.S.; Chen, F.Z.; Wang, Q.Y.; Huang, K.; Meng, J.Z.; Wang, R.; Huang, R.B. Cloning, expression and identification of a new trehalose synthase gene from Thermobifida fusca genome. Acta Biochim. Et Biophys. Sin. 2004, 36, 477–484. [Google Scholar] [CrossRef][Green Version]
- Wu, C.; Xu, C.; Ni, H.; Yang, Q.; Cai, H.; Xiao, A. Preparation and characterization of tannase immobilized onto carboxyl-functionalized superparamagnetic ferroferric oxide nanoparticles. Bioresour. Technol. 2016, 205, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tang, S.; Jiang, L.; Zhu, L.; Huang, H. Integrated biocatalytic process for trehalose production and separation from maltose. Ind. Eng. Chem. Res. 2016, 55, 10566–10575. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Material | Silicalite-1 | APS-Silicalite-1 | GA-APS-Silicalite-1 |
|---|---|---|---|
| 1st batch | 61.20% | 35.09% | 61.52% |
| 2nd batch | 0.00% | 0.00% | 60.28% |
| Type of Enzyme | Optimal Reaction pH | pH at 0 h | pH at 24 h | ∆pH |
|---|---|---|---|---|
| Immobilized-TtTreS | 8.00 | 8.04 ± 0.12 | 7.30 ± 0.21 | 0.70 ± 0.09 |
| Free-TtTreS | 9.00 | 9.03 ± 0.08 | 6.50 ± 0.15 | 2.50 ± 0.07 |
| Enzyme Type | Km * Value (mM) | Vmax * (µmol/min) |
|---|---|---|
| Immobilized TtTres | 53.33 ± 5.21 | 2.93 ± 0.22 |
| Free TtTres | 14.12 ± 1.84 | 8.72 ± 0.38 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wang, S.; Li, W.; Li, R.; Chen, S.; Ri, H.I.; Kim, T.M.; Kang, M.S.; Sun, L.; Sun, X.; et al. Improvement of Trehalose Production by Immobilized Trehalose Synthase from Thermus thermophilus HB27. Molecules 2018, 23, 1087. https://doi.org/10.3390/molecules23051087
Sun J, Wang S, Li W, Li R, Chen S, Ri HI, Kim TM, Kang MS, Sun L, Sun X, et al. Improvement of Trehalose Production by Immobilized Trehalose Synthase from Thermus thermophilus HB27. Molecules. 2018; 23(5):1087. https://doi.org/10.3390/molecules23051087
Chicago/Turabian StyleSun, Jing, Shizeng Wang, Wenna Li, Ruimin Li, Sheng Chen, Hyon Il Ri, Tae Mun Kim, Myong Su Kang, Lu Sun, Xinxiao Sun, and et al. 2018. "Improvement of Trehalose Production by Immobilized Trehalose Synthase from Thermus thermophilus HB27" Molecules 23, no. 5: 1087. https://doi.org/10.3390/molecules23051087
APA StyleSun, J., Wang, S., Li, W., Li, R., Chen, S., Ri, H. I., Kim, T. M., Kang, M. S., Sun, L., Sun, X., & Yuan, Q. (2018). Improvement of Trehalose Production by Immobilized Trehalose Synthase from Thermus thermophilus HB27. Molecules, 23(5), 1087. https://doi.org/10.3390/molecules23051087




