Culture Medium Development for Microbial-Derived Surfactants Production—An Overview
Abstract
1. Introduction
2. General Culture Medium Development for Cultivation Process
3. Metabolic Pathways of Biosurfactant Production
4. Media Components for Biosurfactant Production
4.1. Carbon Sources
4.2. Nitrogen Sources
4.3. C/N Ratio
4.4. Minerals
4.5. Vitamins
4.6. Metabolic Regulators
4.6.1. Inhibitor
4.6.2. Inducer
4.7. Salinity Level
4.8. Water
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saharan, B.S.; Sahu, R.K.; Sharma, D. A review on biosurfactants: Fermentation, current developments and perspectives. Genet. Eng. Biotechnol. J. 2012, 2011, 1–14. [Google Scholar]
- Zana, R. Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: A review. Adv. Colloid Interface Sci. 2002, 97, 205–253. [Google Scholar] [CrossRef]
- Zheng, O.; Zhao, J.X. Solubilization of pyrene in aqueous micellar solutions of gemini surfactants C12-s-C12.2Br. J. Colloid Interface Sci. 2006, 300, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Biosurfactants in cosmetic formulations: Trends and challenges. Crit. Rev. Biotechnol. 2017, 37, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.d.C.F.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int. J. Mol. Sci. 2014, 15, 12523–12542. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.N.R.L.; Farias, C.B.B.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Glycerol as substrate for the production of biosurfactant by Pseudomonas aeruginosa UCP0992. Colloids Surfaces B Biointerfaces 2010, 79, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, R.; Sivakumaar, P.K. Effect of Different Carbon Sources on the Production of Biosurfactant by Pseudomonas fluorescens Isolated from Mangrove Forests (Pichavaram), Tamil Nadu, India. Glob. J. Environ. Res. 2009, 3, 99–101. [Google Scholar]
- Priya, T.; Usharani, G. Comparative study for biosurfactant production by using Bacillus subtilis and Pseudomonas aeruginosa. Bot. Res. Int. 2009, 2, 284–287. [Google Scholar]
- Campos, J.M.; Stamford, T.L.M.; Sarubbo, L.A.; Luna, J.M.; Rufino, R.D.; Banat, I.M. Microbial biosurfactants as additives for food industries. Biotechnol. Prog. 2013, 29, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Das, P.; Sen, R. Towards commercial production of microbial surfactants. Trends Biotechnol. 2006, 24, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.S.; Rockne, K.J. Comparison of synthetic surfactants and biosurfactants in enhancing biodegradation of polycyclic aromatic hydrocarbons. Environ. Toxicol. Chem. 2003, 22, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Gharaei-Fathabad, E. Biosurfactactants in Pharmaceutical Industry: A Mini-Review. Am. J. Drug Discov. Dev. 2011, 1, 58–69. [Google Scholar] [CrossRef]
- Ranasalva, N.; Sunil, R.; Poovarasan, G. Importance of Biosurfactant in Food Industry. IOSR J. Agric. Vet. Sci. 2014, 7, 6–9. [Google Scholar] [CrossRef]
- Randhawa, K.K.S.; Rahman, P.K.S.M. Rhamnolipid biosurfactants-past, present, and future scenario of global market. Front. Microbiol. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Syldatk, C.; Hausmann, R. Microbial biosurfactants. Eur. J. Lipid Sci. Technol. 2010, 112, 615–616. [Google Scholar] [CrossRef]
- George, S.; Jayachandran, K. Production and characterization of rhamnolipid biosurfactant from waste frying coconut oil using a novel Pseudomonas aeruginosa D. J. Appl. Microbiol. 2013, 114, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Amodu, O.S.; Ntwampe, S.K.O.; Ojumu, T.V. Optimization of biosurfactant production by Bacillus licheniformis STK 01 grown exclusively on Beta vulgaris waste using response surface methodology. BioResources 2014, 9, 5045–5065. [Google Scholar] [CrossRef]
- Cavalcante Fai, A.E.; Resende Simiqueli, A.P.; de Andrade, C.J.; Ghiselli, G.; Pastore, G.M. Optimized production of biosurfactant from Pseudozyma tsukubaensis using cassava wastewater and consecutive production of galactooligosaccharides: An integrated process. Biocatal. Agric. Biotechnol. 2015, 4, 535–542. [Google Scholar] [CrossRef]
- Mariano, A.P.; Bonotto, D.M.; Angelis, D.F.; Pirôllo, M.P.S.; Contiero, J. Use of weathered diesel oil as a low-cost raw material for biosurfactant production. Brazilian J. Chem. Eng. 2008, 25, 269–274. [Google Scholar] [CrossRef]
- Willenbacher, J.; Yeremchuk, W.; Mohr, T.; Syldatk, C.; Hausmann, R. Enhancement of surfactin yield by improving the medium composition and fermentation process. AMB Express 2015, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, G.; Swetha, S.; Madhavan, K.N.; Sukumaran, R.K.; Ashok, P. Response surface methodology for the optimization of alpha amylase production by Bacillus amyloliquefaciens. Bioresour. Technol. 2008, 99, 4597–4602. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, P.; Sivapathasekaran, C.; Sen, R. Enhanced production of biosurfactant by a marine bacterium on statistical screening of nutritional parameters. Biochem. Eng. J. 2008, 42, 254–260. [Google Scholar] [CrossRef]
- Bisen, P.S.; Sharma, A. Fermentation. In Introduction to Instrumentation in Life Sciences; CRC Press: London, UK, 2012; p. 258. [Google Scholar]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Media for Industrial Fermentations. In Principles of Fermentation Technology; Butterworth-Heinemann: Oxford, UK, 2016; p. 213. [Google Scholar]
- Takahashi, M.; Morita, T.; Wada, K.; Hirose, N.; Fukuoka, T.; Imura, T.; Kitamoto, D. Production of sophorolipid glycolipid biosurfactants from sugarcane molasses using Starmerella bombicola NBRC 10243. J. Oleo Sci. 2011, 60, 267–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gudiña, E.J.; Fernandes, E.C.; Rodrigues, A.I.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Front. Microbiol. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Itoh, S.; Honda, H.; Tomita, F.; Suzuki, T. Rhamnolipids produced by Pseudomonas aeruginosa grown on n-paraffin (Mixture of C12, C13 and C14 fractions). J. Antibiot. (Tokyo). 1971, 24, 855–859. [Google Scholar] [CrossRef]
- Benincasa, M.; Abalos, A.; Oliveira, I.; Manresa, A. Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie Van Leeuwenhoek 2004, 85, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; Ron, E.Z. Biosurfactants. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 281–294. [Google Scholar]
- Price, N.P.J.; Ray, K.J.; Vermillion, K.; Kuo, T.M. MALDI-TOF mass spectrometry of naturally occurring mixtures of monorhamnolipids and dirhamnolipids. Carbohydr. Res. 2009, 344, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar] [PubMed]
- Weber, L.; Döge, C.; Haufe, G.; Hommel, R.; Kleber, H.P. Oxygenation of hexadecane in the biosynthesis of cyclic glycolipids in Torulopsis apicola. Biocatal. Biotransform. 1992, 5, 267–272. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lipid, O.F.S.; Candida, B.Y. Regulation of sophorose lipid production by Candida (Torulopsis) apicola. Biotechnol. Lett. 1993, 15, 853–858. [Google Scholar]
- Taylor, P.; Korla, K.; Mitra, C.K. Modelling the Krebs cycle and oxidative phosphorylation. J. Biomol. Struct. Dyn. 2013, 32, 242–256. [Google Scholar] [CrossRef]
- Giraud, M.-F.; Naismith, J.H. The rhamnose pathway. Curr. Opin. Struct. Biol. 2000, 10, 687–696. [Google Scholar] [CrossRef]
- Mouafo, T.H.; Mbawala, A.; Ndjouenkeu, R. Effect of different carbon sources on biosurfactants’ production by three Strains of Lactobacillus spp. Biomed Res. Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M. Recent advances in engineering the central carbon metabolism of industrially important bacteria. Microb. Cell Fact. 2012, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Nie, Y.; Tang, Y.Q.; Wu, G.; Wu, X.L. N-Alkane Chain Length Alters Dietzia sp. strain DQ12-45-1b biosurfactant production and cell surface activity. Appl. Environ. Microbiol. 2013, 79, 400–402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perry, J.J. Microbial metabolism of cyclic alkanes. In Petroleum Microbiology; Atlas, R.M., Ed.; Macmilan: New York, NY, USA, 1984; pp. 61–98. [Google Scholar]
- Throne-Holst, M.; Wentzel, A.; Ellingsen, T.E.; Kotlar, H.K.; Zotchev, S.B. Identification of novel genes involved in long-chain n-alkane degradation by Acinetobacter sp. strain DSM 17874. Appl. Environ. Microbiol. 2007, 73, 3327–3332. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.M.; Glaser, L.; Burton, R.M. The enzymatic synthesis of a rhamnose-containing glycolipid by extracts of Pseudomonas aeruginosa. J. Biol. Chem. Chem. 1963, 238, 2595–2602. [Google Scholar]
- Das, P.; Mukherjee, S.; Sen, R. Genetic regulations of the biosynthesis of microbial surfactants: An overview. Biotechnol. Genet. Eng. Rev. 2008, 25, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Roongsawang, N.; Washio, K.; Morikawa, M. Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants. Int. J. Mol. Sci. 2011, 12, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A. Strategies for Fermentation Medium Optimization: An In-Depth Review. Front. Microbiol. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Price, N.P.J.; Ray, K.J.; Kuo, T.M. Production of sophorolipid biosurfactants by multiple species of the Starmerella (Candida) bombicola yeast clade. FEMS Microbiol. Lett. 2010, 311, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ghribi, D.; Ellouze-Chaabouni, S. Enhancement of Bacillus subtilis lipopeptide biosurfactants production through optimization of medium composition and adequate control of aeration. Biotechnol. Res. Int. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.-F.M.; Azemi, M.-A.F.M.; Amirul, A.-A.A.; Bhubalan, M.E.A.W.K. Simultaneous Production of Biopolymer and Biosurfactant by Genetically Modified Pseudomonas aeruginosa UMTKB-5. Int. Proc. Chem. Biol. Environ. Eng. 2015, 90, 16–21. [Google Scholar]
- Tomar, G.S.; Srinikethan, G. Studies on production of biosurfactant from Pseudomonas aeruginosa (MTCC7815) & its application in microbial enhanced oil recovery. Res. J. Chem. Environ. Sci. 2016, 4, 84–91. [Google Scholar]
- Fooladi, T.; Bin, A.; Hamid, A.; Mohtar, W.; Yusoff, W. Production of Biosurfactant by Indigenous Isolated Bacteria in Fermentation System. AIP Conf. Proc. 2013, 197, 197–201. [Google Scholar]
- Yeh, M.; Wei, Y.; Chang, J. Enhanced Production of Surfactin from Bacillus subtilis by addition of solid carriers. Biotechnol. Prog. 2005, 21, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Willenbacher, J.; Rau, J.T.; Rogalla, J.; Syldatk, C.; Hausmann, R. Foam-free production of Surfactin via anaerobic fermentation of Bacillus subtilis DSM 10T. AMB Express 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moussa, T.A.A.; Mohamed, M.S.; Samak, N. Production and characterization of di-rhamnolipid produced by Pseudomonas aeruginosa TMN. Brazilian J. Chem. Eng. 2014, 31, 867–880. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Gudiña, E.J.; Costa, R.; Vitorino, R.; Teixeira, J.A.; Coutinho, J.A.P.; Rodrigues, L.R. Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel 2013, 111, 259–268. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S. Effects of Various Nutritional Supplements on Biosurfactant Production by a Strain of Bacillus subtilis at 45 °C. J. Surfactants Deterg. 2002, 5, 11–17. [Google Scholar] [CrossRef]
- Kanna, R.; Gummadi, S.N.; Kumar, G.S. Production and characterization of biosurfactant by Pseudomonas putida MTCC 2467. J. Biol. Sci. 2014, 14, 436–445. [Google Scholar] [CrossRef]
- Liu, X.; Ren, B.; Gao, H.; Liu, M.; Dai, H.; Song, F.; Yu, Z.; Wang, S.; Hu, J.; Kokare, C.R.; et al. Optimization for the production of surfactin with a new synergistic antifungal activity. PLoS One 2012, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.M.; Mody, K.; Joshi, N.; Mishra, A.; Jha, B. Production and structural characterization of biosurfactant produced by an alkaliphilic bacterium, Klebsiella sp.: Evaluation of different carbon sources. Colloids Surfaces B Biointerfaces 2013, 108, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Lowor, S.T.; Agyeute-Badu, C.K. Mineral and proximate composition of cashew apple (Anarcadium occidentale L.) juice from northern savannah, forest and coastal savannah regions in Ghana. Am. J. Food Technol. 2009, 4, 154–161. [Google Scholar] [CrossRef][Green Version]
- Giro, M.E.A.; Martins, J.J.L.; Rocha, M.V.P.; Melo, V.M.M.; Gonçalves, L.R.B. Clarified cashew apple juice as alternative raw material for biosurfactant production by Bacillus subtilis in a batch bioreactor. Biotechnol. J. 2009, 4, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Wittgens, A.; Tiso, T.; Arndt, T.T.; Wenk, P.; Hemmerich, J.; Müller, C.; Wichmann, R.; Küpper, B.; Zwick, M.; Wilhelm, S.; et al. Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440. Microb. Cell Fact. 2011, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Pendse, A.; Aruna, K. Studies on optimization of biosurfactant production by Pseudomonas aeruginosa F23 isolated from oil contaminated soil sample. Int. J. Curr. Biotechnol. 2014, 2, 20–30. [Google Scholar]
- Asri, N.P.; Sari, D.A.P.; Poedjojono, B. Suprapto Pre-treatment of waste frying oils for biodiesel production. Mod. Appl. Sci. 2015, 9, 99–106. [Google Scholar] [CrossRef]
- Gusmao, C.A.B.de; Rufino, R.D.; Sarubbo, L.A. Laboratory production and characterization of a new biosurfactant from Candida glabrata UCP1002 cultivated in vegetable fat waste applied to the removal of hydrophobic contaminant. World J. Microbiol. Biotechnol. 2010, 26, 1683–1692. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Salgueiro, A.A.; Sarubbo, L.A. Synthesis and evaluation of biosurfactant produced by Candida lipolytica using animal fat and corn steep liquor. J. Pet. Sci. Eng. 2013, 105, 43–50. [Google Scholar] [CrossRef]
- Pepi, M.; Focardi, S.; Lobianco, A.; Angelini, D.L.; Borghini, F.; Focardi, S.E. Degradation of fatty acids and production of biosurfactant as an added value, by a bacterial strain Pseudomonas aeruginosa DG2a isolated from aquaculture wastewaters. Water. Air. Soil Pollut. 2013, 224, 1–11. [Google Scholar] [CrossRef]
- Nazren Radzuan, M.; Banat, I.; Winterburn, J. Production and characterization of rhamnolipid using palm oil agricultural refinery waste. Bioresour. Technol. 2017, 225, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Jeon, J.W.; Kim, B.H.; Ahn, C.Y.; Oh, H.M.; Yoon, B.D. Extracellular production of a glycolipid biosurfactant, mannosylerythritol lipid, by Candida sp. SY16 using fed-batch fermentation. Appl. Microbiol. Biotechnol. 2006, 70, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Cameotra, S.S.; Singh, P. Synthesis of rhamnolipid biosurfactant and mode of hexadecane uptake by Pseudomonas species. Microb. Cell Fact. 2009, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, N.K.; Konwar, B.K. Bacterial biosurfactant in enhancing solubility and metabolism of petroleum hydrocarbons. J. Hazard. Mater. 2009, 170, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Priya, J. Biodegradation of Diesel by Aeromonashydrophila. Int. J. Pharm. Sci. Invent. 2013, 2, 24–36. [Google Scholar] [CrossRef]
- Suryanti, V.; Marliyana, S.D.; Handayani, D.S.; Ratnaningrum, D. Production and characterization of biosurfactant by Pseudomonas fluorescens using cassava flour wastewater as media. Indones. J. Chem. 2013, 13, 229–235. [Google Scholar] [CrossRef]
- Rocha, M.V.P.; Oliveira, A.H.S.; Souza, M.C.M.; Gonçalves, L.R.B. Natural cashew apple juice as fermentation medium for biosurfactant production by Acinetobacter calcoaceticus. World J. Microbiol. Biotechnol. 2006, 22, 1295–1299. [Google Scholar] [CrossRef]
- Leal, M.; Leite, J.; Chagas, M.; da Maia, R.; Cortez, L. Feasibility assessment of converting sugar mills to bioenergy production in Africa. Agriculture 2016, 6, 45. [Google Scholar] [CrossRef]
- Patel, R.M.; Desai, A.J. Biosurfactant production by Pseudomonas aeruginosa GS3 from molasses. Lett. Appl. Microbiol. 1997, 25, 91–94. [Google Scholar] [CrossRef]
- Nitschke, M.; Pastore, G.M. Biosurfactant production by Bacillus subtilis using cassava-processing effluent. Appl. Biochem. Biotechnol.—Part A Enzym. Eng. Biotechnol. 2004, 112, 163–172. [Google Scholar] [CrossRef]
- Sharma, D.; Ansari, M.J.; Gupta, S.; Al Ghamdi, A.; Pruthi, P.; Pruthi, V. Structural characterization and antimicrobial activity of a biosurfactant obtained from Bacillus pumilus DSVP18 grown on potato peels. Jundishapur J. Microbiol. 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lotfabad, T.B.; Ebadipour, N.; Roostaazad, R. Evaluation of a recycling bioreactor for biosurfactant production by Pseudomonas aeruginosa MR01 using soybean oil waste. J. Chem. Technol. Biotechnol. 2016, 91, 1368–1377. [Google Scholar] [CrossRef]
- Raza, Z.A.; Rehman, A.; Khan, M.S.; Khalid, Z.M. Improved production of biosurfactant by a Pseudomonas aeruginosa mutant using vegetable oil refinery wastes. Biodegradation 2007, 18, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Zhang, J.; Chen, D.; Chen, X.; Tucker, M.; Liang, Y. Sweet sorghum bagasse and corn stover serving as substrates for producing sophorolipids. J. Ind. Microbiol. Biotechnol. 2017, 44, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Zhang, J.; Chen, D.; Liang, Y. Sophorolipid production from biomass hydrolysates. Appl. Biochem. Biotechnol. 2014, 175, 2246–2257. [Google Scholar] [CrossRef] [PubMed]
- Onbasli, D.; Aslim, B. Determination of rhamnolipid biosurfactant production in molasses by some Pseudomonas spp. New Biotechnol. 2009, 25, 161–163. [Google Scholar] [CrossRef]
- Chooklin, C.S.; Maneerat, S.; Saimmai, A. Utilization of banana peel as a novel substrate for biosurfactant production by Halobacteriaceae archaeon AS65. Appl. Biochem. Biotechnol. 2014, 173, 624–645. [Google Scholar] [CrossRef] [PubMed]
- Rivera, O.M.P.; Moldes, A.B.; Torrado, A.M.; Domínguez, J.M. Lactic acid and biosurfactants production from hydrolyzed distilled grape marc. Process Biochem. 2007, 42, 1010–1020. [Google Scholar] [CrossRef]
- George, S.; Jayachandran, K. Analysis of rhamnolipid biosurfactants produced through submerged fermentation using orange fruit peelings as sole carbon source. Appl. Biochem. Biotechnol. 2009, 158, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.P.; Janardhan, A.; Viswanath, B.; Monika, K.; Jung, J.Y.; Narasimha, G. Evaluation of orange peel for biosurfactant production by Bacillus licheniformis and their ability to degrade naphthalene and crude oil. 3 Biotech 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oje, O.A.; Okpashi, V.E.; Uzor, J.C.; Uma, U.O.; Irogbolu, A.O.; Onwurah, I.N.E. Effect of acid and alkaline pretreatment on the production of biosurfactant from rice husk using Mucor indicus. Res. J. Environ. Toxicol. 2016, 10, 60–67. [Google Scholar] [CrossRef][Green Version]
- Dikit, P.; Riansa-Ngawong, W.; Chookaew, T.; Maneerat, S.; Hwanhlem, N.; Kamcharoen, A.; Saimmai, A. Production and characterization of biosurfactant produced by Ochrobactrum anthropi 2/3 using durian seed powder as a novel substrate. In Proceedings of the The 16th TSAE National Conference and the 8th TSAE International Conference, Sukhumvit, Bangkok, Thailand, 17–19 March 2015; pp. 215–222. [Google Scholar]
- Noparat, P.; Maneerat, S.; Saimmai, A. Utilization of palm oil decanter cake as a novel substrate for biosurfactant production from a new and promising strain of Ochrobactrum anthropi 2/3. World J. Microbiol. Biotechnol. 2014, 30, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Chooklin, C.S.; Petmeaun, S.; Maneerat, S.; Saimmai, A. Isolation and characterization of a biosurfactant from Deinococcus caeni PO5 using jackfruit seed powder as a substrate. Ann. Microbiol. 2014, 64, 1007–1020. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Cameotra, S.S.; Chopra, H.K. Biosurfactant from Lysinibacillus chungkukjangi from rice bran oil sludge and potential applications. J. Surfactants Deterg. 2016, 19, 957–965. [Google Scholar] [CrossRef]
- Saikia, D.; Deka, S.C. Cereals: From staple food to nutraceuticals. Int. Food Res. J. 2011, 18, 21–30. [Google Scholar]
- Vecino, X.; Rodríguez-López, L.; Gudiña, E.J.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Vineyard pruning waste as an alternative carbon source to produce novel biosurfactants by Lactobacillus paracasei. J. Ind. Eng. Chem. 2017, 55, 40–49. [Google Scholar] [CrossRef]
- Desai, A.J.; Patel, R.M.; Desai, J.D. Advances in production of biosurfactant and their commercial applications. J. Sci. Ind. Res. 1994, 53, 619–629. [Google Scholar]
- Deepika, K.V.; Nagaraju, G.P.; Bramhachari, P.V. Optimization of cultural conditions for marine microbial biosurfactant production: Future prospects from untapped marine resources. In Marine Pollution and Microbial Remediation; Naik, M.M., Dubey, S.K., Eds.; Springer Science & Business Media: Singapore, 2016; pp. 105–128. [Google Scholar]
- Mulligan, C.N.; Gibbs, B.F. Correlation of nitrogen metabolism with biosurfactant production by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 1989, 55, 3016–3019. [Google Scholar] [PubMed]
- Gao, P.; Tian, H.; Li, G.; Sun, H.; Ma, T. Microbial diversity and abundance in the Xinjiang Luliang long-term water-flooding petroleum reservoir. Microbiol. Open 2015, 4, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant-Producing Lactobacilli: Screening, Production Profiles, and Effect of Medium Composition. Appl. Environ. Soil Sci. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Amézcua-Vega, C.; Poggi-Varaldo, H.M.; Esparza-García, F.; Ríos-Leal, E.; Rodríguez-Vázquez, R. Effect of culture conditions on fatty acids composition of a biosurfactant produced by Candida ingens and changes of surface tension of culture media. Bioresour. Technol. 2007, 98, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.G.; Paddock, D.A. Production of a biosurfactant from Torulopsis bombicola. Appl. Environ. Microbiol. 1984, 47, 173–176. [Google Scholar] [PubMed]
- Adamczak, M.; Bednarski, W. Influence of medium composition and aeration on the synthesis of biosurfactants produced by Candida antarctica. Biotechnol. Lett. 2000, 22, 313–316. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Farias, C.B.B.; Campos-Takaki, G.M. Co-utilization of canola oil and glucose on the production of a surfactant by Candida lipolytica. Curr. Microbiol. 2007, 54, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Cherif, N.; Tifrit, A.; Larbi Daouadji, K.; Mezouari, S.; Chama, Z.; Abbouni, B. Effect of carbon and nitrogen source on the microbial production of biosurfactants by Pseudomonas aeruginosa. Der Pharm. Lett. 2015, 7, 42–48. [Google Scholar]
- Jayanthi, C.; Revathi, K. Optimization of biosurfactant production from hydrocarbonoclastic bacteria Pseudomonas putida. Int. J. Curr. Res. Biol. Med. 2016, 1, 22–27. [Google Scholar]
- Agarry, S.E.; Salam, K.K.; Arinkoola, A.; Aremu, M.O. Biosurfactant production by indigeneous Pseudomonas and Bacillus species isolated from auto-mechanic soil environment toward microbial enhanced oil recovery. Eur. J. Eng. Technol. 2015, 3, 27–39. [Google Scholar]
- Zhang, J.; Xue, Q.; Gao, H.; Lai, H.; Wang, P. Production of lipopeptide biosurfactants by Bacillus atrophaeus 5-2a and their potential use in microbial enhanced oil recovery. Microb. Cell Fact. 2016, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, R. Studies in Grease and Lubricant Wastes as Substrates for Growth of Metabolites. In 15th Lubricating Grease Conference; National Lubricating Grease Institute: Jaipur, India, 2014; pp. 1–5. [Google Scholar]
- Benincasa, M.; Contiero, J.; Manresa, M.A.; Moreaes, I.O. Rhampolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. J. Food Eng. 2002, 54, 283–288. [Google Scholar] [CrossRef]
- Onwosi, C.O.; Odibo, F.J.C. Effects of carbon and nitrogen sources on rhamnolipid biosurfactant production by Pseudomonas nitroreducens isolated from soil. World J. Microbiol. Biotechnol. 2012, 28, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Thaniyavarn, J.; Chongchin, A.; Wanitsuksombut, N.; Thaniyavarn, S.; Pinphanichakarn, P.; Leepipatpiboon, N.; Morikawa, M.; Kanaya, S. Biosurfactant production by Pseudomonas aeruginosa A41 using palm oil as carbon source. J. Gen. Appl. Microbiol. 2006, 52, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.D.; de Luna, J.M.; de Campos Takaki, G.M.; Sarubbo, L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2014, 17, 34–38. [Google Scholar] [CrossRef]
- Joshi, P.A.; Shekhawat, D.B. Effect of carbon and nitrogen source on biosurfactant production by biosurfactant producing bacteria isolated from petroleum contaminated site. Adv. Appl. Sci. Res. 2014, 5, 159–164. [Google Scholar]
- Thavasi, R.; Jayalakshmi, S.; Balasubramanian, T.; Banat, I.M. Production and characterization of a glycolipid biosurfactant from Bacillus megaterium using economically cheaper sources. World J. Microbiol. Biotechnol. 2008, 24, 917–925. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M.N. Pyrene biodegradation enhancement potential of lipopeptide biosurfactant produced by Paenibacillus dendritiformis CN5 strain. J. Hazard. Mater. 2017, 321, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, M.; Gurkok, S.; Ozdal, O.G. Optimization of rhamnolipid production by Pseudomonas aeruginosa OG1 using waste frying oil and chicken feather peptone. 3 Biotech 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ebadipour, N.; Lotfabad, T.B.; Yaghmaei, S.; RoostAazad, R. Optimization of low-cost biosurfactant production from agricultural residues through response surface methodology. Prep. Biochem. Biotechnol. 2016, 46, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Mironescu, M.; Mironescu, I.D.; Georgescu, C. Investigations on using wastewater from corn processing as substrate for probiotics. J. Hyg. Eng. Des. 2016, 15, 66–71. [Google Scholar]
- Wu, J.Y.; Yeh, K.L.; Lu, W.B.; Lin, C.L.; Chang, J.S. Rhamnolipid production with indigenous Pseudomonas aeruginosa EM1 isolated from oil-contaminated site. Bioresour. Technol. 2008, 99, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Syldatk, C.; Lang, S.; Wagner, F.; Wray, V.; Witte, L. Chemical and physical characterization of four interfacial-active rhamnolipids from Pseudomonas sp. DSM 2874 grown on n-alkanes. Z. Naturforsch. 1985, 40, 51–60. [Google Scholar]
- Rashedi, H.; Jamshidi, E.; Mazaheri, M.; Bonakdarpour, B. Isolation and production of biosurfactant from Pseudomonas aeruginosa isolated from Iranian southern wells oil. Int. J. Environ. Sci. Technol. 2005, 2, 121–127. [Google Scholar]
- Prieto, L.M.; Michelon, M.; Burkert, J.F.M.; Kalil, S.J.; Burkert, C.A.V. The production of rhamnolipid by a Pseudomonas aeruginosa strain isolated from a southern coastal zone in Brazil. Chemosphere 2008, 71, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Rekha, R.; Hemen, S.; Debahuti, D. Achieving the Best Yield in Glycolipid Biosurfactant Preparation by Selecting the Proper Carbon/Nitrogen Ratio. J. Surfactant Deterg. 2014, 17, 563–571. [Google Scholar] [CrossRef]
- Abouseoud, M.; Maachi, R.; Amrane, A.; Boudergua, S.; Nabi, A. Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination 2008, 223, 143–151. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lu, W.B.; Wei, Y.H.; Chen, W.M.; Chang, J.S. Improved production of biosurfactant with newly isolated Pseudomonas aeruginosa S2. Biotechnol. Prog. 2007, 23, 661–666. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, C.F.C.; Michelon, M.; Burkert, J.F.M.; Kalil, S.J.; Burkert, C.A.V. Production of a rhamnolipid-type biosurfactant by Pseudomonas aeruginosa LBM10 grown on glycerol. African J. Biotechnol. 2010, 9, 9012–9017. [Google Scholar] [CrossRef]
- Saikia, R.R.; Deka, S.; Deka, M.; Banat, I.M. Isolation of biosurfactant-producing Pseudomonas aeruginosa RS29 from oil-contaminated soil and evaluation of different nitrogen sources in biosurfactant production. Ann. Microbiol. 2012, 62, 753–763. [Google Scholar] [CrossRef]
- Abouseoud, M.; Maachi, R.; Amrane, A. Biosurfactant production from olive oil by Pseudomonas fluorescens. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 340–347. [Google Scholar]
- Hamzah, A.; Sabturani, N.; Radiman, S. Screening and optimization of biosurfactant production by the hydrocarbon-degrading bacteria. Sains Malaysiana 2013, 42, 615–623. [Google Scholar]
- Fonseca, R.R.; Silva, A.J.R.; De Franca, F.P.; Cardoso, V.L.; Servulo, E.F.C. Optimizing carbon/nitrogen ratio for biosurfactant production by a Bacillus subtilis strain. Appl. Biochem. Biotechnol. 2007, 136, 471–486. [Google Scholar]
- Heryani, H.; Putra, M.D. Kinetic study and modeling of biosurfactant production using Bacillus sp. Electron. J. Biotechnol. 2017, 27, 49–54. [Google Scholar] [CrossRef]
- Fontes, G.C.; Fonseca Amaral, P.F.; Nele, M.; Zarur Coelho, M.A. Factorial Design to Optimize Biosurfactant Production by Yarrowia lipolytica. J. Biomed. Biotechnol. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Luepongpattana, S.; Jindamarakot, S.; Thaniyavarn, S.; Thaniyavarn, J. Screening of biosurfactant production yeast and yeast-like fungi isolated from the coastal areas of Koh Si Chang. In Proceedings of the 26th Annual Meeting of the Thai Society for Biotechnology and International Conference, Mae Fah Luang University, Chiang Rai, Thailand, 26–29 November 2014; pp. 468–477. [Google Scholar]
- Elazzazy, A.M.; Abdelmoneim, T.S.; Almaghrabi, O.A. Isolation and characterization of biosurfactant production under extreme environmental conditions by alkali-halo-thermophilic bacteria from Saudi Arabia. Saudi J. Biol. Sci. 2015, 22, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Schobert, B. The Binding of a Second Divalent Metal Ion Is Necessary for the Activation of ATP Hydrolysis and Its Inhibition by Tightly Bound ADP in the ATPase from Halobacterium saccharouorum. J. Biol. Chem. 1992, 267, 10252–10257. [Google Scholar] [PubMed]
- Epstein, W. The Roles and Regulation of Potassium in Bacteria. Prog. Nucleic Acid Res. Mol. Biol. 2003, 75, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Diaz, C.; Pons, R.; Teruel, J.A.; Aranda, F.J.; Ortiz, A.; Manresa, A.; Marques, A.M. The production and physicochemical properties of a biosurfactant mixture obtained from Sphingobacterium detergens. J. Colloid Interface Sci. 2013, 394, 368–379. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tempest, D.W.; Dicks, J.W.; Hunter, J.R. The interrelationship between Potassium, Magnesium and Phosphorus in Potassium-limited Chemostat Cultures of Aerobacter aerogenes. J. Gen. Microbiol. 1966, 45, 135–146. [Google Scholar] [CrossRef][Green Version]
- Dominguez, D.C. Calcium signalling in bacteria. Mol. Microbiol. 2004, 54, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Činátl, J. Inorganic-organic multimolecular complexes of salt solutions, culture media and biological fluids and their possible significance for the origin of life. J. Theor. Biol. 1969, 23, 1–10. [Google Scholar] [CrossRef]
- Gout, E.; Rebeille, F.; Douce, R.; Bligny, R. Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: Unravelling the role of Mg2+ in cell respiration. Proc. Natl. Acad. Sci. 2014, 111, 4560–4567. [Google Scholar] [CrossRef] [PubMed]
- Thavasi, R.; Subramanyam Nambaru, V.R.M.; Jayalakshmi, S.; Balasubramian, T.; Banat, I.M. Biosurfactant Production by Pseudomonas aeruginosa from Renewable Resources. Indian J. Microbiol. 2011, 51, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.A.; Singh, N.; Shekhawat, D.B. Effect of metal ions on growth and biosurfactant production by Halophilic bacteria. Adv. Appl. Sci. Res. 2015, 6, 152–156. [Google Scholar]
- Putri, M.; Hertadi, R. Effect of glycerol as carbon source for biosurfactant production by halophilic bacteria Pseudomonas stutzeri BK-AB12. Procedia Chem. 2015, 16, 321–327. [Google Scholar] [CrossRef]
- Guerra-Santos, L.; Kappeli, O.; Fiechter, A. Dependence of Pseudomonas aeruginosa continous culture biosurfactant production on nutritional and environmental factors. Appl. Microbiol. Biotechnol. 1986, 24, 443–448. [Google Scholar] [CrossRef]
- Kiran, G.S.; Hema, T.A.; Gandhimathi, R.; Selvin, J.; Thomas, T.A.; Rajeetha Ravji, T.; Natarajaseenivasan, K. Optimization and production of a biosurfactant from the sponge-associated marine fungus Aspergillus ustus MSF3. Colloids Surf. B Biointerfaces 2009, 73, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Tahzibi, A.; Kamal, F.; Assadi, M.M. Improved Production of Rhamnolipids by Pseudomonas aeruginosa Mutant. Iran. Biomed. J. 2004, 8, 25–31. [Google Scholar]
- Usman, M.M.; Arezoo, D.; Tzin, L.K.; Fahim, M.A.; Salmah, I. Application of biosurfactants in environmental biotechnology; remediation of oil and heavy metal. Bioengineering 2016, 3, 289–304. [Google Scholar] [CrossRef]
- Qazi, M.A.; Malik, Z.A.; Qureshi, G.D.; Hameed, A.; Ahmed, S. Yeast Extract as the Most Preferable Substrate for Optimized Biosurfactant Production by rhlB Gene Positive Pseudomonas putida SOL-10 Isolate. Bioremediat. Biodegrad. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Holland, B.; Widdowson, E.M.; Unwin, I.; Buss, D. Vegetables, Herbs and Spices: Fifth Supplement to McCance and Widdowson’s The Composition of Foods, 4th ed.; Royal Society of Chemistry: Cambridge, UK, 1991. [Google Scholar]
- Al-Ajlani, M.; Sheikh, M.; Ahmad, Z.; Hasnain, S. Production of surfactin from Bacillus subtilis MZ-7 grown on pharmamedia commercial medium. Microb. Cell Fact. 2007, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, R.A.; Haroun, B.M.; Ghazal, E.A.; Maher, Y.A. Structural Analysis and Characteristics of Biosurfactants Produced by Some Crude Oil Utilizing Bacterial Strains. Aust. J. Basic Appl. Sci. 2010, 4, 3484–3498. [Google Scholar]
- Busscher, H.J.; van Hoogmoed, C.G.; Geertsema-doornbusch, S.I.; van der Kuijl-booij, M.; van der Mei, H.C. Streptococcus thermophilus and Its Biosurfactants Inhibit Adhesion by Candida spp. on Silicone Rubber. Appl. Environ. Microbiol. 1997, 63, 3810–3817. [Google Scholar] [PubMed]
- Bicca, F.C.; Fleck, L.C.; Ayub, M.A.Z. Production of biosurfactant by hydrocarbon degrading Rhodococcus ruber and Rhodococcus erythroplis. Rev. Microbiol. 1999, 30, 231–236. [Google Scholar] [CrossRef]
- Horne, D.; Tomasz, A. Release of lipoteichoic acid from Streptococcus sanguis: Stimulation of release during penicillin treatment. J. Bacteriol. 1979, 137, 1180–1184. [Google Scholar] [PubMed]
- Volbrecht, E.; Heckmann, R.; Wray, V.; Nimtz, M.; lang, S. Production and structure elucidation of di-and oligosaccharide lipids (biosurfactans) from Tsukamurella sp. nov. Appl. Microbiol. Biotechnol. 1998, 50, 530–537. [Google Scholar] [CrossRef]
- Hua, Z.; Song, R.; Du, G.; Li, H.; Chen, J. Effects of EDTA and Tween60 on biodegradation of n -hexadecane with two strains of Pseudomonas aeruginosa. Biochem. Eng. J. 2006, 36, 66–71. [Google Scholar] [CrossRef]
- Salam, J.A.; Das, N. Induced biosurfactant production and degradation of lindane by soil Basidiomycetes Yeast, Rhodotorula sp. VITJzN03. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 664–670. [Google Scholar]
- Meneses, D.P.; Gudiña, E.J.; Fernandes, F.; Gonçalves, L.R.B.; Rodrigues, L.R.; Rodrigues, S. The yeast-like fungus Aureobasidium thailandense LB01 produces a new biosurfactant using olive oil mill wastewater as an inducer. Microbiol. Res. 2017, 204, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Bento, F.M.; Gaylarde, C.C. The production of interfacial emulsions by bacterial isolates from diesel fuels. Int. Biodeterior. Biodegrad. 1996, 38, 31–33. [Google Scholar] [CrossRef]
- Pal, M.P.; Vaidya, B.K.; Desai, K.M.; Joshi, R.M.; Nene, S.N.; Kulkarni, B.D. Media optimization for biosurfactant production by Rhodococcus erythropolis MTCC 2794: Artificial intelligence versus a statistical approach. J. Ind. Microbiol. Biotechnol. 2009, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Rismani, E.; Fooladi, J.; Ebrahimi Por, G.H. Biosurfactant production in batch culture by a Bacillus licheniformis isolated from the Persian Gulf. Pak. J. Biol. Sci. 2006, 9, 2498–2502. [Google Scholar] [CrossRef]
- Chandran, P.; Das, N. Biosurfactant production and diesel oil degradation by yeast species Trichosporon asahii isolated from petroleum hydrocarbon contaminated soil. Int. J. Eng. Sci. Technol. 2010, 2, 6942–6953. [Google Scholar]
- Ilori, M.O.; Amobi, C.J.; Odocha, A.C. Factors affecting biosurfactant production by oil degrading Aeromonas spp. isolated from a tropical environment. Chemosphere 2005, 61, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, A.; Assadi, M.M.; Noohi, A.A.; Sajadian, V.A. Isolation of biosurfactant producing bacteria from oil reservoirs. Iran. J. Environ. Heal. Sci. Eng. 2005, 2, 6–12. [Google Scholar]
- Mahdy, H.M.; Fareid, M.A.; Hamdan, M.N. Production of biosurfactant from certain Candida strains under special conditions. Researcher 2012, 4, 39–55. [Google Scholar]
- Saikia, R.R.; Deka, S.; Deka, M.; Sarma, H. Optimization of environmental factors for improved production of rhamnolipid biosurfactant by Pseudomonas aeruginosa RS29 on glycerol. J. Basic Microbiol. 2012, 52, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Timmis, K.N.; Wray, V.; Fredrickson, H.L. Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl. Environ. Microbiol. 1995, 61, 1706–1713. [Google Scholar] [PubMed]
- Peter, J.K.; Singh, D.P. Characterization of emulsification activity of partially purified rhamnolipids from Pseudomonas fluorescens. Int. J. Innov. Sci. Res. 2014, 3, 88–100. [Google Scholar]
- Shoeb, E.; Ahmed, N.; Akhter, J.; Badar, U.; Siddiqui, K.; Ansari, F.A.; Waqar, M.; Imtiaz, S.; Akhtar, N.; Shaikh, Q.U.A.; et al. Screening and characterization of biosurfactant-producing bacteria isolated from the Arabian Sea coast of Karachi. Turkish J. Biol. 2015, 39, 210–216. [Google Scholar] [CrossRef]
- Taran, M.; Mohamadian, E.; Asadi, S.; Bakhtiyari, S. Surface active agent production from olive oil in high salt conditions and its process optimization. Polish J. Chem. Technol. 2012, 14, 30–34. [Google Scholar] [CrossRef]
- Sharp, K.A. Water: Structure and Properties. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd: Chichester, UK, 2001; pp. 1–7. [Google Scholar]
- Liu, J.; Chen, Y.; Xu, R.; Jia, Y. Screening and evaluation of biosurfactant-producing strains isolated from oilfield wastewater. Indian J. Microbiol. 2013, 53, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.C.; Vessoni-Penna, T.C.; Arni, S.A.; Domínguez, J.M.; Converti, A.; Oliveira, R.P.D.S. Influence of toluene and salinity on biosurfactant production by Bacillus sp.: Scale up from flasks to a bench-scale bioreactor. Braz. J. Chem. Eng. 2017, 34, 395–405. [Google Scholar] [CrossRef]
- Ong, C.; Ibrahim, S.; Sen Gupta, B. A Survey of Tap Water Quality in Kuala Lumpur. Urban Water J. 2007, 4, 1–13. [Google Scholar] [CrossRef]
- Singh, V. Biosurfactant—Isolation, Production, Purification & Significance. Int. J. Sci. Res. Publ. 2012, 2, 2250–3153. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
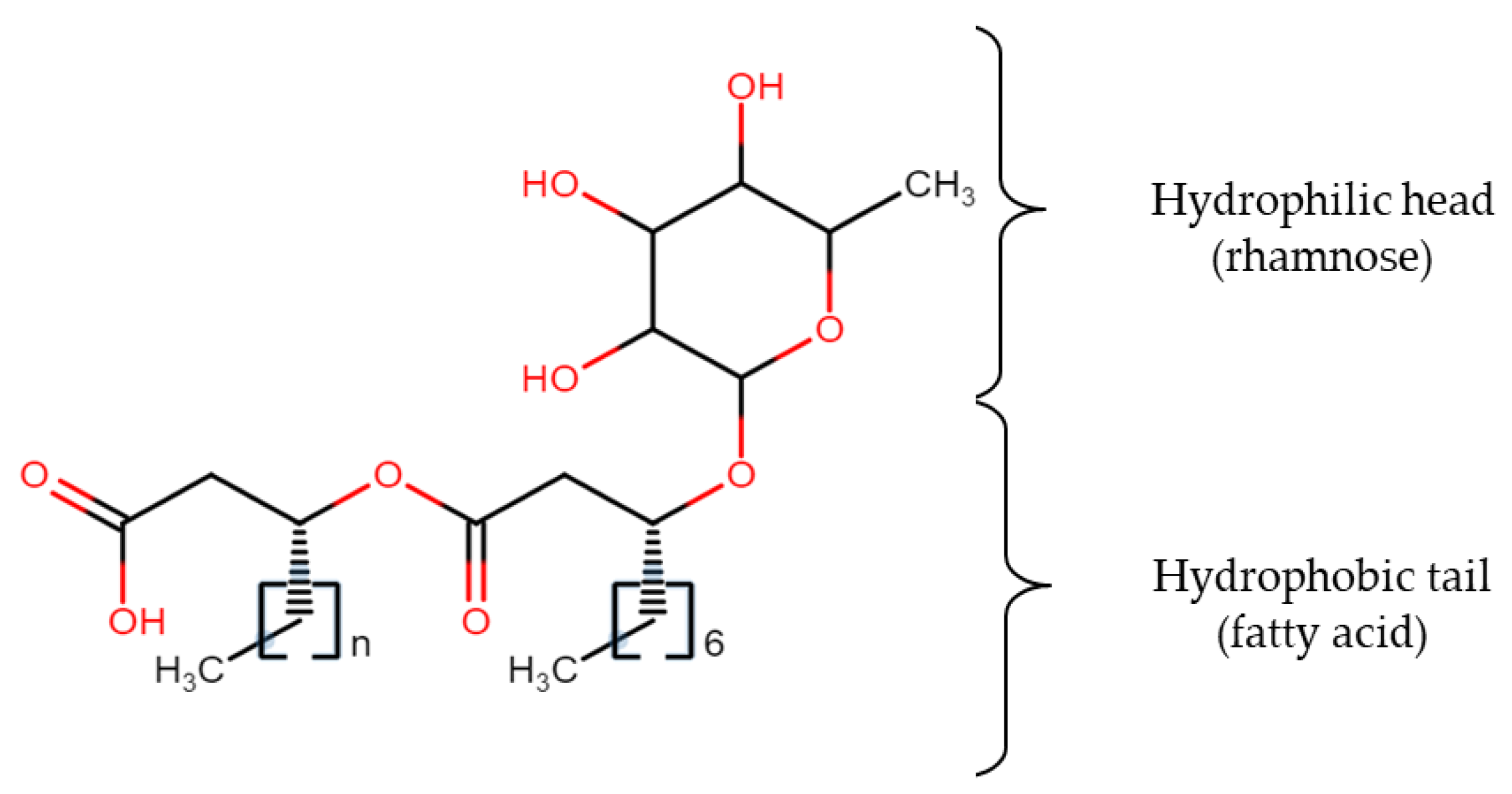
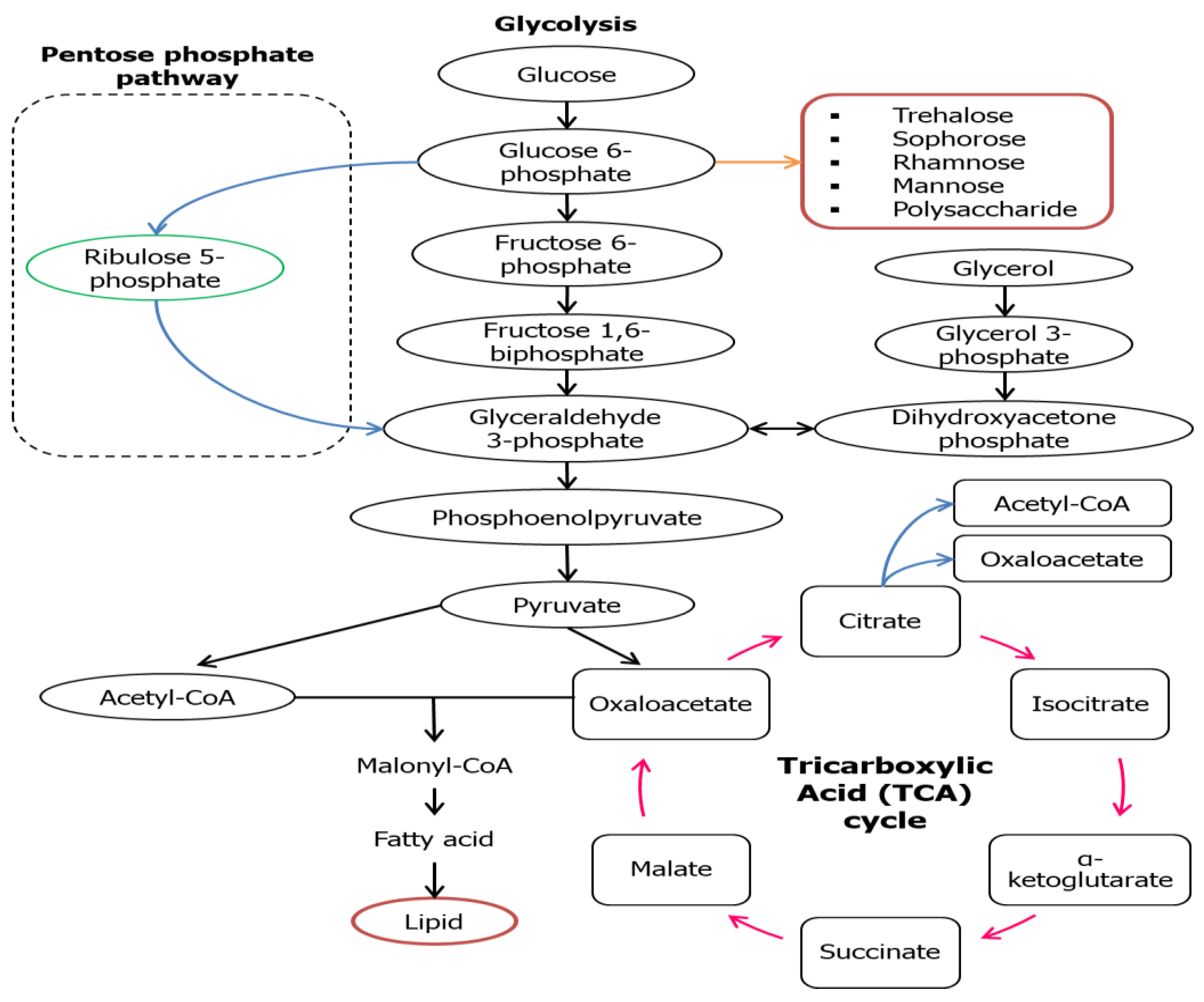
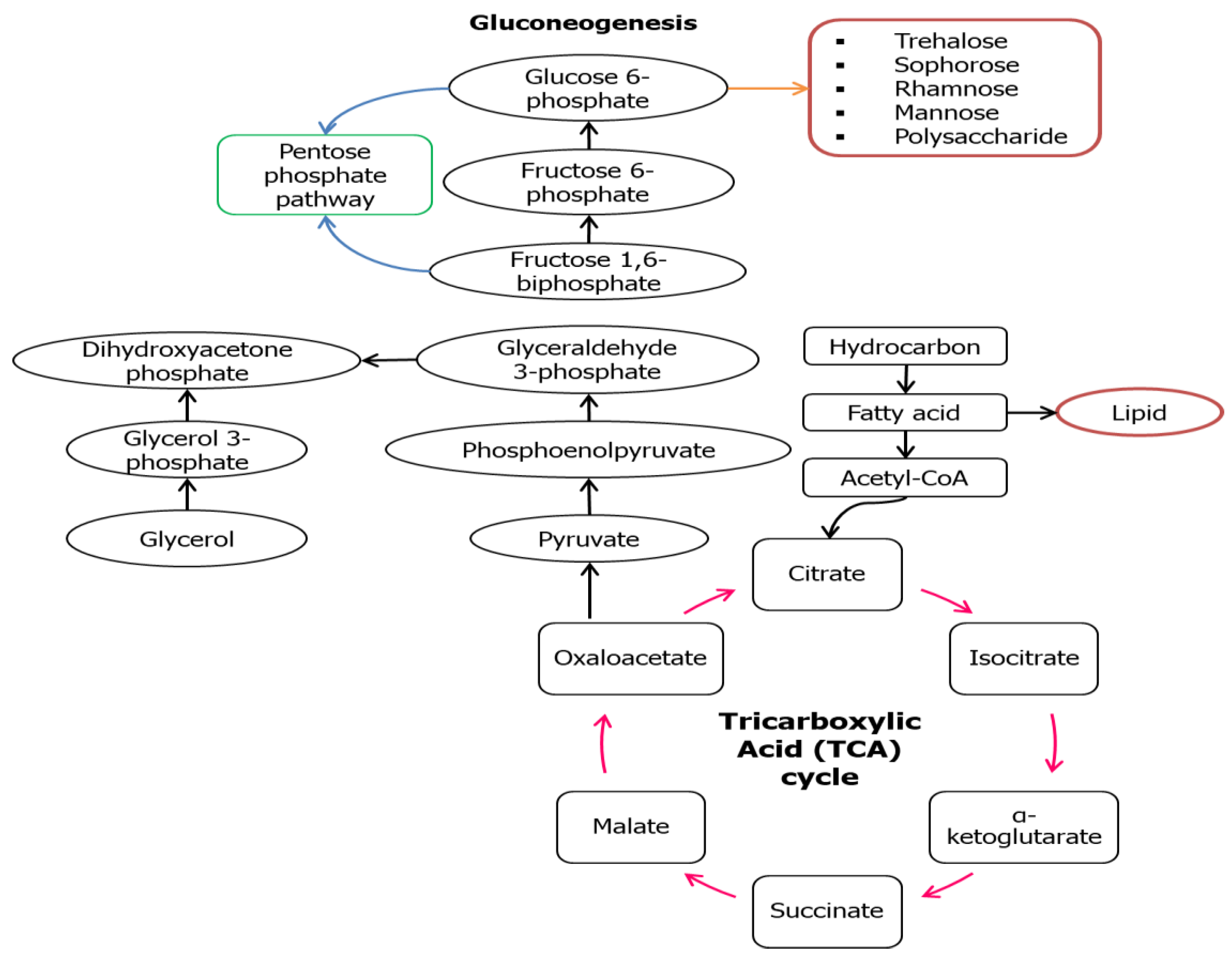
| Carbon Source | Conc. (g/L) | Microorganisms | Biosurfactant (g/L) | Biomass (g/L) | Ref. |
|---|---|---|---|---|---|
| Glucose | 8 | B. subtilis | 1.1 | 2.2 | [21] |
| 150 | Candida bombicola NRRL Y-17069 | 95.4 | - | [48] | |
| 40 | B. subtilis SPB1 strain | 0.72 | - | [49] | |
| 20 | P. aeruginosa UMTKB-5 | 2.72 | 1.4 | [50] | |
| 20 | P. aeruginosa MTCC 7815 | 3.88 | 5.67 | [51] | |
| 30 | B. pumilus 2IR | 0.72 | 2.75 | [52] | |
| 40 | B. subtilis | 3.6 | - | [53] | |
| 10 | B. subtilis DSM 10T | 0.16 | 0.59 | [54] | |
| 40 | P. aeruginosa TMN | 0.3 | 2.9 | [55] | |
| Sucrose | 10 | B. subtilis strains #573 | 2.16 | 1.03 | [56] |
| 20 | B. subtilis | 0.8 | 2.5 | [57] | |
| 20 | P. putida MTCC 2467 | 1.3 | 2.3 | [58] | |
| 20 | B. amyloliquefaciens MB199 | 1.34 | - | [59] | |
| Starch | 30 | Klebsiella sp. RJ-03 | 10.1 | - | [60] |
| Waste (Carbon Source) | Conc. (g/L) | Microorganisms | Biosurfactant (g/L) | Biomass (g/L) | Ref. |
|---|---|---|---|---|---|
| Molasses | 70 | P. aeruginosa GS3 | 0.24 | 0.8 | [77] |
| Corn steep liquor | 100 | B. subtilis #573 | 4.47 | - | [27] |
| Cassava processing effluent | - | B. subtilis LB5a | 3.0 | - | [78] |
| Potato peels | 20 | B. pumilus DSVP18 | 3.2 | - | [79] |
| Soybean oil waste | 80 | P. aeruginosa MR01 | 25.5 | 5.15 | [80] |
| Canola oil refinery wastes | 20 | P. aeruginosa EBN-8 mutant | 8.5 | 4.5 | [81] |
| Corn stover hydrolysate + yellow grease | 10 | C. bombicola | 52.1 | 8.5 | [82] |
| Baggase + soybean oil | 100 | 84.6 | 7.7 | [83] | |
| Sugar beet molasses | 50 | P. luteola B17 | 0.53 | - | [84] |
| P. putida B12 | 0.52 | - | |||
| Banana peel | 250 | Halobacteriaceae archaeon AS65 | 5.3 | 4.8 | [85] |
| Hydrolyzed distilled grape marc | - | L. pentosus | 0.005 | - | [86] |
| Orange peel | 30 | P. aeruginosa MTCC 2297 | 9.18 | - | [87] |
| 40 | B. licheniformis | 1.796 | - | [88] | |
| Rice husk | 125 | Mucor indicus | 0.078 | - | [89] |
| Durian seed powder | 45 | Ochrobactrum anthropi 2/3 | 4.10 | 4.84 | [90] |
| Palm oil decanter cake | 250 | 4.52 | - | [91] |
| Nitrogen | Carbon | Microbe | Scale | Biosurfactant (g/L) | Biomass (g/L) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Source | Conc. (g/L) | Source | Conc. (g/L) | |||||
| Yeast extract | 2.0 | Corn oil | 20 | C. ingens CB-216 | 500 mL | 5.6 | 24.0 | [101] |
| 5.0 | Safflower oil | 100 | Torulopsis bombicola ATCC22214 | 7 L | 18.0 | 12.4 | [102] | |
| 1.0 | Soybean oil | 80 | C. antarctica ATCC20509 | - | 46.0 | 28.4 | [103] | |
| 2.0 | Canola oil | 100 | C. lipolytica UCP0988 | 250 mL | 8.0 | - | [104] | |
| 5.0 | Glycerol | 30 | P. aeruginosa | 250 mL | 2.7 | 1.9 | [105] | |
| 4.0 | Corn oil | 10 | P. putida | 250 mL | 3.5 | - | [106] | |
| 3.0 | Glucose | 1 | Bacillus isolate | - | 2.56 | 3.20 | [107] | |
| Urea | 3.0 | Brown sugar | 10 | B. atrophaeus 5-2a | 600 mL | 0.78 | 0.99 | [108] |
| 1.5 | Metalworking fluid oil | 50.6 | P. aeruginosa ATCC 9027 | - | 4.4 | - | [109] | |
| Peptone | 1.0 | Soybean oil | 100 | Candida sp. SY 16 | 5 L | 37.0 | 10.0 | [70] |
| Nitrogen | Carbon | Microbe | Scale | Biosurfactant (g/L) | Biomass (g/L) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Source | Conc. (g/L) | Nitrogen Count (g/L) | Source | Conc. (g/L) | |||||
| Ammonium nitrate, NH4NO3 | 4.0 | 1.40 | Palm oil | 20 | P. aeruginosa A41 | - | 6.58 | - | [112] |
| 10.0 | 3.50 | Soybean oil residue & glutamic acid | 60 & 10 | C. lipolytica UCP 0988 | - | 8.0 | 11.0 | [113] | |
| 1.0 | 0.35 | Sodium acetate | 20 | Bacillus sp. | - | 2.4 | 2.0 | [114] | |
| Sodium nitrate, NaNO3 | 2.0 | 0.33 | Glucose | 20 | P. nitroreducens | 250 mL | 5.46 | - | [111] |
| 6.0 | 0.99 | Glycerol | 30 | P. aeruginosa UCP0992 | 500 mL | 5.5 | 4.0 | [6] | |
| 14.0 | 2.30 | Crude oil | 20 | B. megaterium | 500 mL | 3.58 | 1.4 | [115] | |
| 3.0 | 0.49 | Glucose | 1 | Pseudomonas isolate | - | 2.20 | 2.40 | [107] | |
| Ammonium sulfate, (NH4)2SO4 | 3.0 | 0.63 | Sucrose | 20 | B. subtilis | 1 L | 0.20 | 0.8 | [57] |
| 1.0 | 0.21 | Glucose & fructose from cashew apple juice | 10 & 8.7 | B. subtilis | 250 mL | 0.123 | - | [62] | |
| 0.4 | 0.09 | Pyrene | 0.1 | Paenibacillus dendritiformis CN5 | 250 mL | 6.0 | - | [116] | |
| Potassium nitrate, KNO3 | 3.0 | 0.42 | Glucose | 30 | B. pumilus 2 IR | 1 L | 0.72 | 3.46 | [52] |
| Microorganisms | Trace Elements (g/L) | Biosurfactant (g/L) | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Zn | Cu | Mo | B | Mn | |||
| Bacillus sp. | 2.32 | 1.0 | 0.39 | 0.56 | 1.78 | 2.0 | [114] |
| P. nitroreducens | 0.005 | 0.071 | 0.015 | 0.015 | 0.2 | 6.0 | [111] |
| P. aeruginosa PTCC1637 | 0.29 | 0.25 | - | - | 0.17 | 12.5 | [148] |
| P. aeruginosa RS29 | 0.7 | 0.50 | 0.06 | 0.26 | 0.50 | 0.80 | [124] |
| B. megaterium | 0.7 | 0.50 | 0.06 | 0.26 | 0.50 | 7.8 | [115] |
| V. salarius | 0.29 | 0.25 | - | - | 0.17 | 2.8 | [135] |
| Microorganisms | Substrate | Type of Vitamins | Concentration (g/L) | Surface Tension (mN/m) |
|---|---|---|---|---|
| P. illinoisensis-21 | Crude oil | Folic acid | 0.2 | 39 |
| B. subtilis-27 | Thiamine HCl | 0.2 | 40 | |
| Bordetella hinizi-DAFI | Folic acid | 0.2 | 42 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurfarahin, A.H.; Mohamed, M.S.; Phang, L.Y. Culture Medium Development for Microbial-Derived Surfactants Production—An Overview. Molecules 2018, 23, 1049. https://doi.org/10.3390/molecules23051049
Nurfarahin AH, Mohamed MS, Phang LY. Culture Medium Development for Microbial-Derived Surfactants Production—An Overview. Molecules. 2018; 23(5):1049. https://doi.org/10.3390/molecules23051049
Chicago/Turabian StyleNurfarahin, Abdul Hamid, Mohd Shamzi Mohamed, and Lai Yee Phang. 2018. "Culture Medium Development for Microbial-Derived Surfactants Production—An Overview" Molecules 23, no. 5: 1049. https://doi.org/10.3390/molecules23051049
APA StyleNurfarahin, A. H., Mohamed, M. S., & Phang, L. Y. (2018). Culture Medium Development for Microbial-Derived Surfactants Production—An Overview. Molecules, 23(5), 1049. https://doi.org/10.3390/molecules23051049





