The Chemical Composition and Metabolic Effects of Attalea phalerata Nut Oil in Hyperlipidemic Rats Induced by a High-Fructose Diet
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield and Composition of the Fatty Acids in A. phalerata Nut Oil (APNO)
2.2. The Effects of A. phalerata Nut Oil on the Parameters of Food Intake and Feces and Urine Excretion
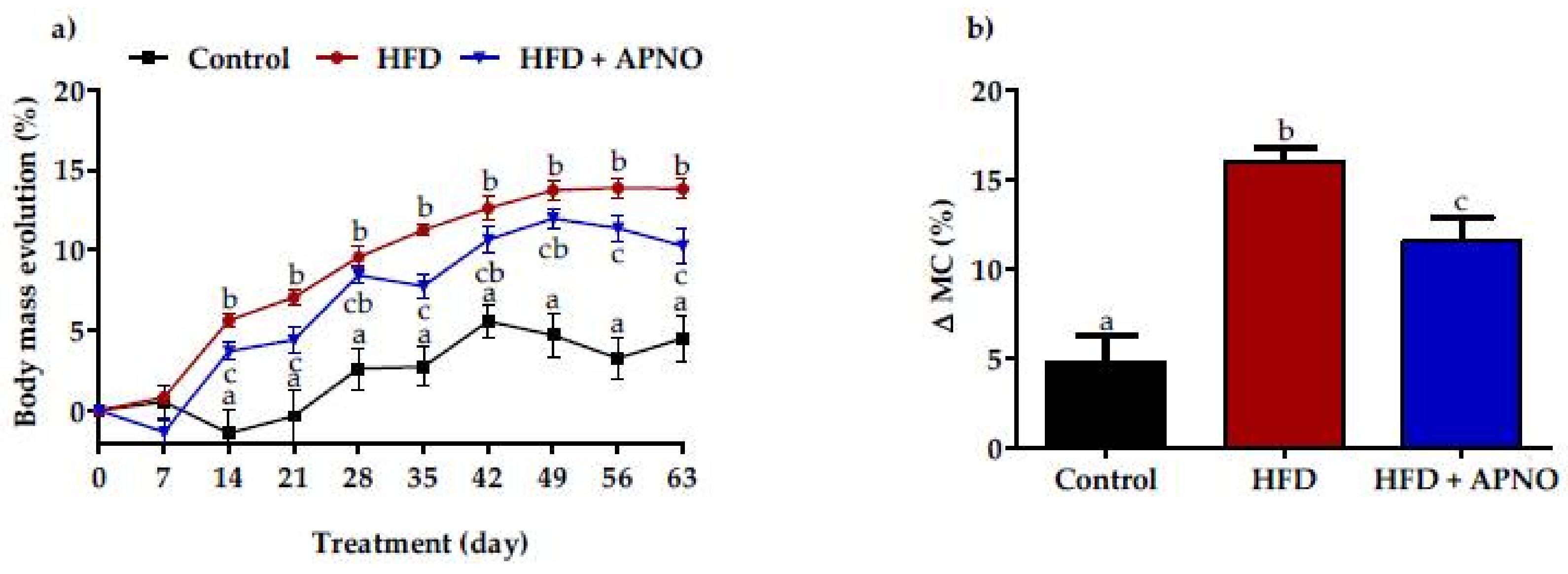
2.3. Effect of A. phalerata Nut Oil on Body Mass, Temperature, and White Adipose Tissue Depots
2.4. Effect of A. phalerata Nut Oil on the Mass of Muscle Tissue and Organs
3. Materials and Methods
3.1. Botanical Material
3.2. Extraction of the Nut Oil from A. phalerata
3.3. Chemical Composition of APNO
3.4. Diet-Induced Hyperlipidemia
3.5. Animals
3.6. Experimental Design
3.6.1. APNO Dose Determination
3.6.2. Hyperlipidemia Induced by the High-Fructose Diet
3.6.3. Evaluation of the Metabolic Parameters in Rats
3.6.4. Collection of Feces, Blood, Organs, and Tissues
3.6.5. Quantification of Lipids in the Liver and Feces
3.6.6. Biochemical Analysis
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- TROPICOS Missouri Botanical Garden. Attalea phalerata Mart. ex Spreng. Available online: http://www.tropicos.org/Name/50065600?tab=distribution (accessed on 12 February 2017).
- Moraes, M.R.; Borchsenius, F.; Blicher-Mathiesen, U. Notes on the Biology and Uses of the Motacú Palm (Attalea phalerata, Arecaceae) from Bolivia. Econ. Bot. 1996, 50, 423–428. [Google Scholar] [CrossRef]
- Li, S.C.; Liu, Y.H.; Liu, J.F.; Chang, W.H.; Chen, C.M.; Chen, C.Y.O. Nut consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 60, 474–479. [CrossRef] [PubMed]
- Bento, A.P.; Cominetti, C.; Simões Filho, A.; Naves, M.M.V. Baru nut improves lipid profile in mildly hypercholesterolemic subjects: A randomized, controlled, crossover study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.Y.; Zhang, Q.A.; Zhang, Z.Q.; Wang, Y.; Yuan, J.F.; Wang, H.Y.; Zhao, D. Hepatoprotective effects of nut oil against carbon tetrachloride induced liver injury in rats. Food Chem. 2011, 125, 673–678. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668, S50–S58. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, P.; Lokesh, B.R.; Krishna, A.G.G. Hypolipidemic effect of blends of coconut oil with soybean oil or sunflower oil in experimental rats. Food Chem. 2010, 123, 728–733. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Jones, P.J. Physiological effects of medium-chain triglycerides: Potential agents in the prevention of obesity. J. Nutr. 2002, 132, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Tvrzicka, E.; Kremmyda, L.S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease–A review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. 2011, 155, 117–130. [Google Scholar] [CrossRef]
- Thies, F.; Garry, J.M.C.; Yaqoob, P.; Rerkasem, K.; Williams, J.; Shearman, C.P.; Gallagher, P.J.; Calder, P.C.; Grimble, R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 2003, 361, 477–485. [Google Scholar] [CrossRef]
- Codex Alimentarius (FAO/WHO). Codex Standard for Named Vegetable Oils. Codex Stan 210. Available online: http://www.codexalimentarius.org/ (accessed on 21 January 2017).
- Vögler, O.; López-Bellan, A.; Alemany, R.; Tofé, S.; González, M.; Quevedo, J.; Pereq, V.; Barceló, F.; Escriba, P.V. Structure-Effect relation of C18 long-chain fatty acids in the reduction of body weight in rats. Int. J. Obes. 2008, 32, 464–473. [Google Scholar] [CrossRef] [PubMed]
- DeLany, J.P.; Windhauser, M.M.; Champagne, C.M.; Bray, G.A. Differential oxidation of individual dietary fatty acids in humans. Am. J. Clin. Nutr. 2000, 72, 905–911. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Jones, P.J. Greater rise in fat oxidation with medium-chain triglyceride consumption relative to long-chain triglyceride is associated with lower initial body weight and greater loss of subcutaneous adipose tissue. Int. J. Obes. 2003, 27, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Voon, P.T.; Ng, T.K.; Lee, V.K.; Nesaretnam, K. Diets high in palmitic acid (16:0), lauric and myristic acids (12:0 + 14:0), or oleic acid (18:1) do not alter postprandial or fasting plasma homocysteine and inflammatory markers in healthy Malaysian adults. Am. J. Clin. Nutr. 2011, 94, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Czernichow, S.; Thomas, D.; Bruckert, E. n-6 Fatty acids and cardiovascular health: A review of the evidence for dietary intake recommendations. Br. J. Nutr. 2010, 104, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Mozaffarian, D.; Rimm, E.; Kris-Etherton, P.; Rudel, L.L.; Appel, L.J.; Engler, M.M.; Engler, M.B.; Sacks, F. Omega-6 fatty acids and risk for cardiovascular disease. Circulation 2009, 119, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations: Fats and Fatty Acids in Human Nutrition Report of an Expert Consultation, 10–14 November, 2008. Geneva, FAO Food and Nutrition Paper 91. Rome, 2010. Available online: http://www.fao.org/docrep/013/i1953e/i1953e0.pdf (accessed on 14 January 2017).
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172. [Google Scholar] [PubMed]
- Grimm, H.; Mayer, K.; Mayser, P.; Eigenbrodt, E. Regulatory potential of n-3 fatty acids in immunological and inflammatory processes. Br. J. Nutr. 2002, 87, S59–S67. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Bosaeus, I. Fibre effects on intestinal functions (diarrhoea, constipation and irritable bowel syndrome). Clin. Nutr. 2004, 1, 33–38. [Google Scholar] [CrossRef]
- Jena, P.K.; Singh, S.; Prajapati, B.; Nareshkumar, G.; Mehta, T.; Seshadri, S. Impact of targeted specific antibiotic delivery for gut microbiota modulation on high-fructose-fed rats. Appl. Biochem. Biotechnol. 2014, 172, 3810–3826. [Google Scholar] [CrossRef] [PubMed]
- Putakala, M.; Gujjala, S.; Nukala, S.; Desireddy, S. Beneficial effects of Phyllanthus amarus against high fructose diet induced insulin resistance and hepatic oxidative stress in male wistar rats. Appl. Biochem. Biotechnol. 2017, 183, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Lamba, D.; Kumar, R.; Nath, P.; Gauttam, S. Antihyperlipidemic activity of Aloe succotrina in rats: Possibly mediated by inhibition of HMG-COA reductase. ISRN Pharmacol. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Manabe, I.; Nagai, R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov. Med. 2009, 8, 55–60. [Google Scholar] [PubMed]
- Poudyal, H.; Kumar, S.A.; Iyer, A.; Waanders, J.; Ward, L.C.; Brown, L. Responses to oleic, linoleic and α-linolenic acids in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J. Nutr. Biochem. 2013, 24, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Assunção, M.L.; Ferreira, H.S.; dos Santos, A.F.; Cabral Jr, C.R.; Florêncio, T.M.M.T. Effects of dietary coconut oil on the biochemical and anthropometric profiles of women presenting abdominal obesity. Lipids 2009, 44, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, O.; Takeuchi, H.; Kubota, F.; Tsuji, H.; Aoyama, T. Larger diet-induced thermogenesis and less body fat accumulation in rats fed medium-chain triacylglycerols than in those fed long-chain triacylglycerols. J. Nutr. Sci. Vitaminol. 2002, 48, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Edem, D.O. Palm oil: Biochemical, physiological, nutritional, hematological, and toxicological aspects: A review. Plant Foods Hum. Nutr. 2002, 57, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Freitas de Lima, F.; Menegati, S.E.L.T.; Traesel, G.K.; Souza de Araújo, F.H.; Lescano, C.H.; Peixoto, S.M.; Silva, F.A.M.; Vieira, S.C.H.; Vieira, M.C.; Oesterreich, S.A. Study on the cytotoxic, genotoxic and clastogenic potential of Attalea phalerata Mart. ex Spreng. Oil pulp in vitro and in vivo experimental models. PLoS ONE 2016, 11, e0165258. [Google Scholar] [CrossRef] [PubMed]
- Freitas de Lima, F.; Traesel, G.K.; Menegati, S.E.; Santos, A.C.; Souza, R.I.; de Oliveira, V.S.; Sanjinez-Argandoña, E.J.; Cardoso, C.A.; Oesterreich, S.A.; Vieira, M.D. Acute and subacute oral toxicity assessment of the oil extracted from Attalea phalerata Mart ex Spreng. Pulp fruit in rats. Food Res. Int. 2017, 91, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Varela-Lopez, A.; Pérez-López, M.P.; Ramirez-Tortosa, C.L.; Battino, M.; Granados-Principal, S.; Ramirez-Tortosa, M.D.C.; Ochoa, J.J.; Vera-Ramirez, L.; Giampieri, F.; Quiles, J.L. Gene pathways associated with mitochondrial function, oxidative stress and telomere length are differentially expressed in the liver of rats fed lifelong on virgin olive, sunflower or fish oils. J. Nutr. Biochem. 2018, 52, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Martínez-Lara, E.; Cañuelo, A.; del Moral, M.L.; Blanco, S.; Siles, E.; Jiménez, A.; Pedrosa, J.A.; Peinado, M.A. Steatosis recovery after treatment with a balanced sunflower or olive oil-based diet, involvement of perisinusoidal stellate cells. World J. Gastroenterol. 2005, 11, 7480–7485. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Pearson, T.A.; Wan, Y.; Hargrove, R.L.; Moriarty, K.; Fishell, V.; Etherton, T.D. High-Monounsaturated fatty acid diets lower both plasma cholesterol and triacylglycerol concentrations. Am. J. Clin. Nutr. 1999, 70, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- O'Driscoll, G.; Green, D.; Taylor, R.R. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation 1997, 95, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Strauss, V.; Mellert, W.; Wiemer, J.; Leibold, E.; Kamp, H.; Walk, T.; Looser, R.; Prokoudine, A.; Fabian, E.; Krennrich, G.; et al. Increased toxicity when fibrates and statins are administered in combination–A metabolomics approach with rats. Toxicol. Lett. 2012, 211, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Berbée, J.F.; Boon, M.R.; Khedoe, P.P.; Bartelt, A.; Schlein, C.; Worthmann, A.; Kooijman, S.; Hoeke, G.; Mol, I.M.; John, C.; et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 2015, 6, 6356. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; John, C.; Schaltenberg, N.; Berbée, J.F.P.; Worthmann, A.; Cherradi, M.L.; Schlein, C.; Piepenburg, J.; Boon, M.R.; Rinninger, F.; et al. Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat. Commun. 2017, 19, 15010. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, P. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 1996. [Google Scholar]
- Hartman, L. Rapid preparation of fatty acid methyl esters from lipids. Lab. Pract. 1973, 22, 475–476. [Google Scholar] [PubMed]
- Barminas, J.T.; James, M.K.; Abubakar, U.M. Chemical composition of seeds and oil of Xylopia aethiopica grown in Nigeria. Plant Foods Hum. Nutr. 1999, 53, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
Sample Availability: Samples of the fatty acid compounds are available from the authors. |
| Fatty Acid | APNO (g·100 g−1 of Total Fatty Acid) |
|---|---|
| SAFA | |
| Caproic acid (C 6:0) | 0.27 |
| Caprylic acid (C 8:0) | 3.84 |
| Capric acid (C 10:0) | 4.13 |
| Lauric acid (C 12:0) | 28.87 |
| Myristic acid (C 14:0) | 12.00 |
| Pentadecanoic acid (C 15:0) | 0.03 |
| Palmitic acid (C 16:0) | 10. 70 |
| Margaric acid (C 17:0) | 0.04 |
| Stearic acid (C18:0) | 4.24 |
| Eicosanoic acid (C 20:0) | 0.10 |
| Docosanoic acid (C 22:0) | 0.02 |
| Lignoceric acid (C 24:0) | 0.07 |
| Total SAFA | 64.31 |
| MUFA | |
| Palmitoleic acid (C16:1 n-7) | 0.06 |
| cis-10-heptadecenoic acid (C 17:1) | 0.03 |
| Oleic acid (C18:1 n-9) | 30.70 |
| Eicosenoic acid (C 20:1 n-9) | 0.11 |
| Total MUFA | 30.90 |
| PUFA | |
| Linoleic acid (C 18:2 n-6) | 4.75 |
| α-Linolenic (C 18:3 n-3) | 0.04 |
| Total PUFA | 4.79 |
| Total fatty acid | 100 |
| Parameters | Groups | ||
|---|---|---|---|
| Control (n = 5) | HFD (n = 8) | HFD + APNO (n = 8) | |
| Daily mean by animal | |||
| Food intake (g/day) | 23.40 ± 0.70 a | 26.74 ± 1.07 b | 25.30 ± 1.03 ab |
| Water intake (mL/day) | 47.97 ± 1.32 a | 36.71 ± 0.82 b | 31.06 ± 0.61 c |
| White adipose tissue (g·100 g–1 BW) | |||
| Mesenteric | 0.80 ± 0.05 a | 0.96 ± 0.09 ab | 0.72 ± 0.04 ac |
| Retroperitoneal | 2.27 ± 0.22 a | 1.92 ± 0.10 a | 1.97 ± 0.19 a |
| Epididymal | 1.77 ± 0.14 a | 1.17 ± 0.15 b | 1.32 ± 0.10 b |
| Inguinal subcutaneous | 0.14 ± 0.03 a | 0.28 ± 0.01 b | 0.28 ± 0.03 b |
| Skeletal muscle (mg·g–1 BW) | |||
| EDL | 0.014 ± 0.001 a | 0.014 ± 0.001 a | 0.016 ± 0.002 a |
| Soleus | 0.033 ± 0.002 a | 0.028 ± 0.002 a | 0.029 ± 0.001 a |
| Organs (g·100 g–1 BW) | |||
| Spleen | 0.117 ± 0.040 a | 0.115 ± 0.002 a | 0.105 ± 0.003 a |
| Heart | 0.321 ± 0.013 a | 0.347 ± 0.010 ab | 0.320 ± 0.006 ac |
| Liver | 2.860 ± 0.045 a | 2.720 ± 0.054 a | 3.230 ± 0.135 b |
| Lung | 0.522 ± 0.068 a | 0.486 ± 0.027 a | 0.436 ± 0.023 a |
| Kidney | 0.601 ± 0.026 a | 0.545 ± 0.018 ab | 0.602 ± 0.014 ac |
| Lipids (%) | |||
| Liver | 13.92 ± 0.76 a | 8.83 ± 0.79 b | 17.04 ± 1.70 a |
| Parameters | Experimental Groups | |
|---|---|---|
| HFD (n = 8) | HFD + APNO (n = 8) | |
| Food intake (g) | 17.90 ± 1.38 | 19.86 ± 1.55 |
| Water intake (mL) | 17.93 ± 1.91 | 14.88 ± 0.85 |
| Urinary excretion (mL) | 5.00 ± 1.13 | 1.64 ± 0.34 ** |
| Feces (g) | 4.72 ± 0.50 | 4.69 ± 0.51 |
| Humidity of feces (%) | 24.24 ± 0.13 | 48.93 ± 0.23 *** |
| Dry feces (%) | 75.75 ± 0.12 | 51.06 ± 0.23 *** |
| Lipid of feces (%) | 2.26 ± 0.07 | 2.99 ± 0.11 * |
| Anal temperature (° C) | 35.67 ± 0.55 | 36.95 ± 0.24 * |
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Control (n = 5) | HFD (n = 8) | HFD + C (n = 7) | HFD + S (n = 6) | HFD + APNO (n = 8) | |
| TG (mg·dL−1) | 113.0 ± 5.2 a | 241.2 ± 28.1 b | 155.1 ± 14.2 a | 118.9 ± 7.0 a | 243.0 ± 20.0 b |
| TC (mg·dL−1) | 79.0 ± 2.01 a | 117.2 ± 12.9 b | 84.4 ± 4.3 a | 90.3 ± 5.2 a | 93.3 ± 2.5 a |
| HDL (mg·dL−1) | 45.08 ± 2.44 a | 54.59 ± 3.79 a | 47.54 ± 2.35 a | 54.11 ± 3.11 a | 49.07 ± 1.73 a |
| LDL (mg·dL−1) | 11.32 ± 2.40 a | 19.73 ± 3.19a | 8.74 ± 2.83 a | 17.25 ± 5.18 a | 13.94 ± 7.07 a |
| ALT (U·L−1) | 53.2 ± 3.3 a | 65.4 ± 7.0 a | 65.0 ± 4.1 a | 64.8 ± 5.7 a | 66.1 ± 7.5 a |
| AST (U·L−1) | 174.0 ± 6.5 a | 178.0 ± 14.5 a | 195.0 ± 16.0 a | 219.5 ± 19.7 a | 170.8 ± 11.9 a |
| CR (U·L−1) | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.3 ± 0.0 a |
| UREA | 22.9 ± 1.9 a | 30.0 ± 3.0 a | 25.9 ± 3.7 a | 20.9 ± 1.4 a | 19.8 ± 1.9 a |
| Diet | Humidity (%) | Lipids (%) | Ashes (%) | Proteins (%) | Carbohydrate (%) | Energy (Kcal∙100g−1) |
|---|---|---|---|---|---|---|
| CD | 10.70 ± 0.04 | 1.50 ± 0.12 | 8.21 ± 0.14 | 26.14 ± 0.95 | 59.40 ± 0.64 | 332.51 ± 0.46 |
| HFD | 3.98 ± 0.12 | 0.35 ± 0.00 | 3.04 ± 0.08 | 14.28 ± 1.80 | 84.39 ± 0.43 | 374.26 ± 0.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldivia, D.D.S.; Sanjinez-Argandonã, E.J.; Antunes, K.Á.; Moraes, I.C.F.; Dos Santos, E.L.; De Picoli Souza, K. The Chemical Composition and Metabolic Effects of Attalea phalerata Nut Oil in Hyperlipidemic Rats Induced by a High-Fructose Diet. Molecules 2018, 23, 960. https://doi.org/10.3390/molecules23040960
Baldivia DDS, Sanjinez-Argandonã EJ, Antunes KÁ, Moraes ICF, Dos Santos EL, De Picoli Souza K. The Chemical Composition and Metabolic Effects of Attalea phalerata Nut Oil in Hyperlipidemic Rats Induced by a High-Fructose Diet. Molecules. 2018; 23(4):960. https://doi.org/10.3390/molecules23040960
Chicago/Turabian StyleBaldivia, Débora Da Silva, Eliana Janet Sanjinez-Argandonã, Kátia Ávila Antunes, Izabel Cristina Freitas Moraes, Edson Lucas Dos Santos, and Kely De Picoli Souza. 2018. "The Chemical Composition and Metabolic Effects of Attalea phalerata Nut Oil in Hyperlipidemic Rats Induced by a High-Fructose Diet" Molecules 23, no. 4: 960. https://doi.org/10.3390/molecules23040960
APA StyleBaldivia, D. D. S., Sanjinez-Argandonã, E. J., Antunes, K. Á., Moraes, I. C. F., Dos Santos, E. L., & De Picoli Souza, K. (2018). The Chemical Composition and Metabolic Effects of Attalea phalerata Nut Oil in Hyperlipidemic Rats Induced by a High-Fructose Diet. Molecules, 23(4), 960. https://doi.org/10.3390/molecules23040960







