Using Peptidomimetics and Constrained Peptides as Valuable Tools for Inhibiting Protein–Protein Interactions
Abstract
1. Introduction
2. General Structure of PPIs
3. Methods for Targeting PPIs
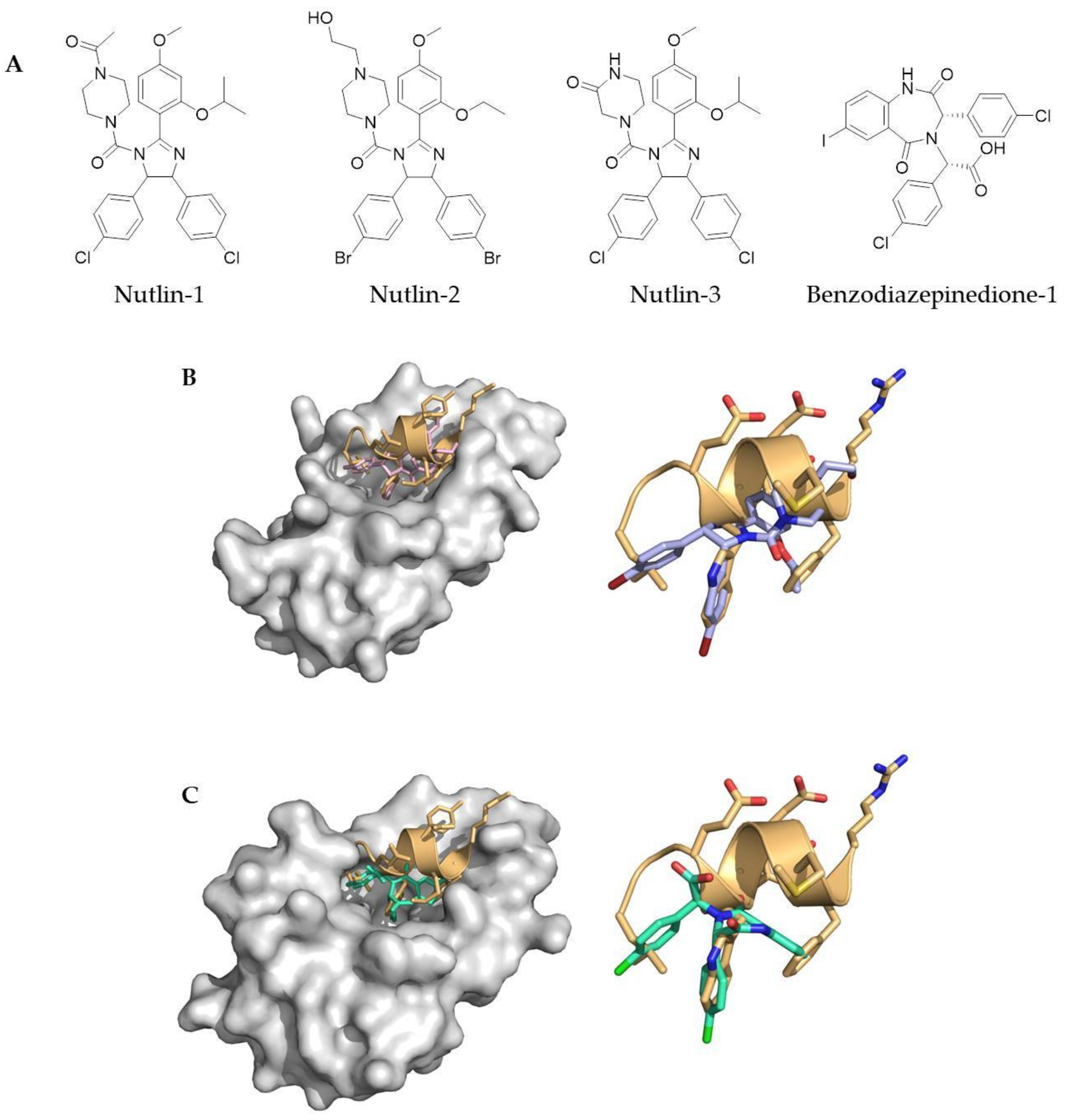
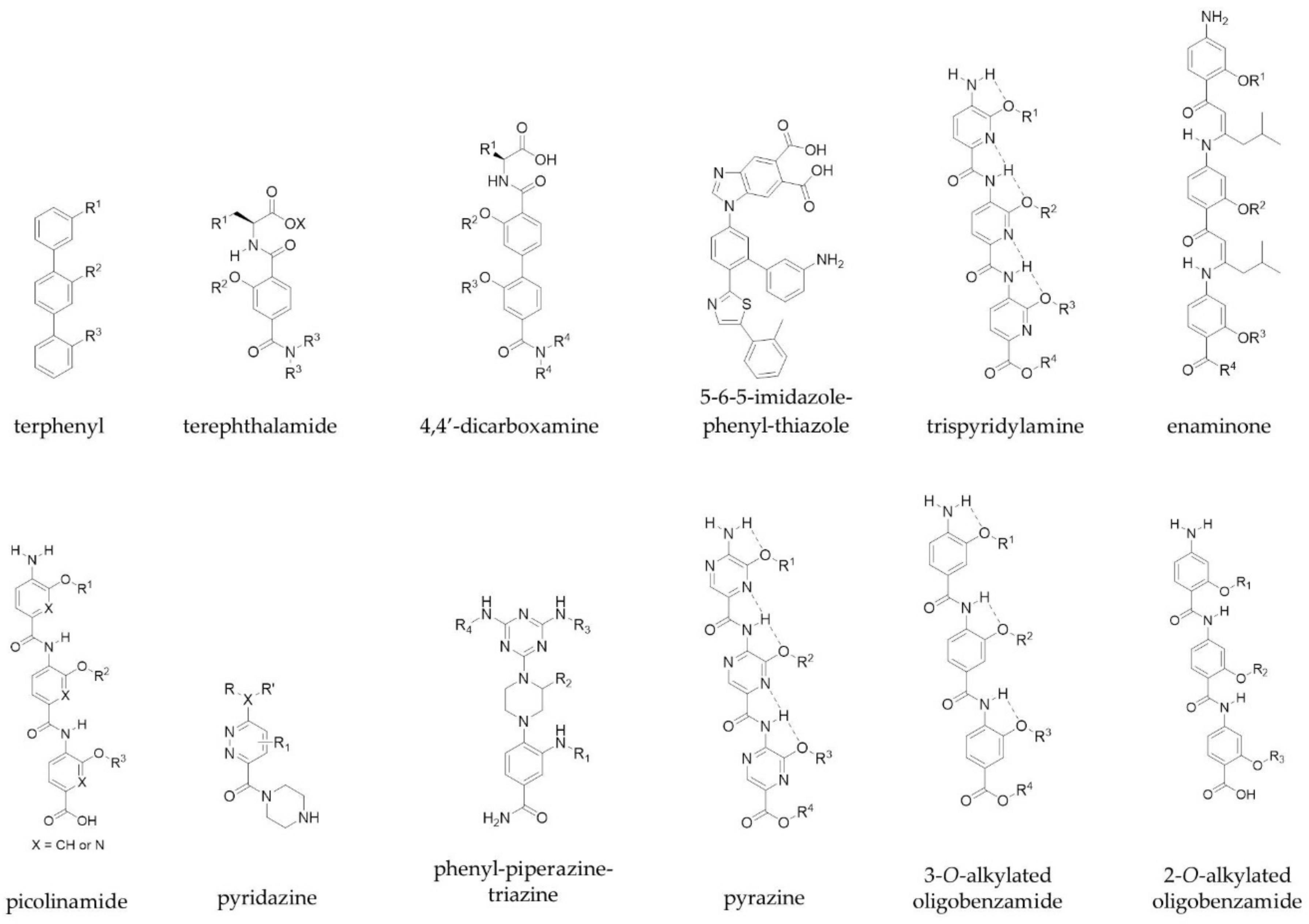
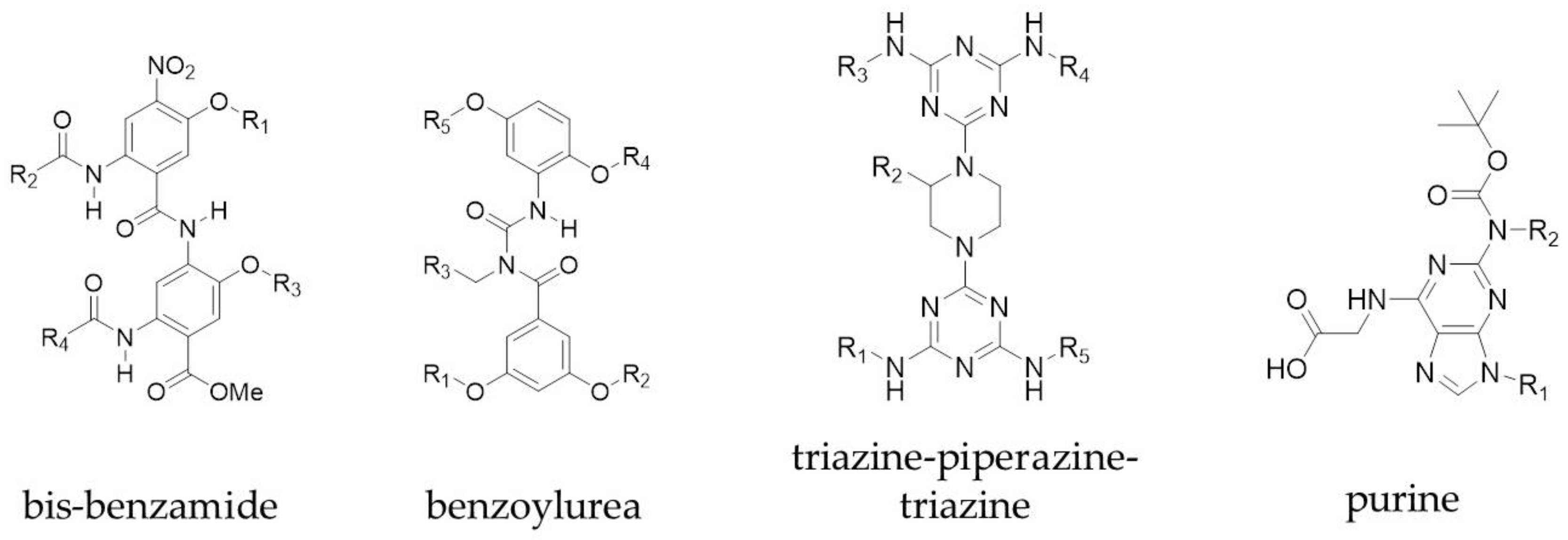
3.1. Small Molecules and Peptidomimetics
3.2. Peptides to Target PPIs
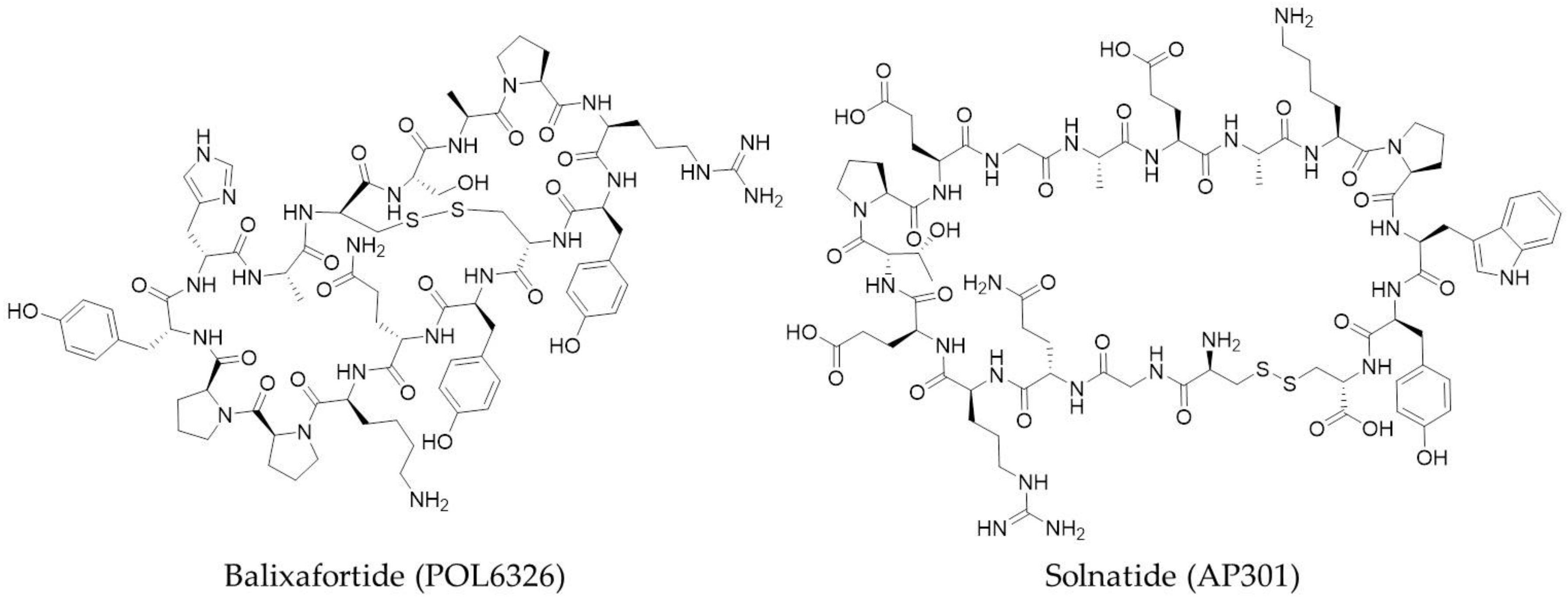
3.3. Macrocyclic Peptides
4. Stapled Peptides
4.1. One-Component Stapling
4.2. Two-Component Stapling
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Keskin, O.; Gursoy, A.; Ma, B.; Nussinov, R. Principles of protein-protein interactions: What are the preferred ways for proteins to interact? Chem. Rev. 2008, 108, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.P.; Matthews, J.M. Protein-protein interactions in human disease. Curr. Opin. Struct. Biol. 2005, 15, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.W.; Kann, M.G. Chapter 4: Protein Interactions and Disease. PLoS Comput. Biol. 2012, 8, e1002819. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, M.P.H.; Thorne, T.; de Silva, E.; Stewart, R.; An, H.J.; Lappe, M.; Wiuf, C. Estimating the size of the human interactome. Proc. Natl. Acad. Sci. USA 2008, 105, 6959–6964. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, K.; Rual, J.-F.; Vazquez, A.; Stelzl, U.; Lemmens, I.; Hirozane-Kishikawa, T.; Hao, T.; Zenkner, M.; Xin, X.; Goh, K.-I.; et al. An empirical framework for binary interactome mapping. Nat. Methods 2009, 6, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, L. Protein-protein interactions: Interactome under construction. Nature 2010, 468, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Hamilton, A.D. Strategies for targeting protein-protein interactions with synthetic agents. Angew. Chem. Int. Ed. 2005, 44, 4130–4163. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Thornton, J.M. Principles of protein-protein interactions. Proc. Natl. Acad. Sci. USA 1996, 93, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.L.; Chothia, C.; Janin, J. The atomic structure of protein-protein recognition sites. J. Mol. Biol. 1999, 285, 2177–2198. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.A.; McClendon, C.L. Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature 2007, 450, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Cukuroglu, E.; Engin, H.B.; Gursoy, A.; Keskin, O. Hot spots in protein-protein interfaces: Towards drug discovery. Prog. Biophys. Mol. Biol. 2014, 116, 165–173. [Google Scholar] [CrossRef] [PubMed]
- La, D.; Kong, M.; Hoffman, W.; Choi, Y.I.; Kihara, D. Predicting permanent and transient protein-protein interfaces. Proteins 2013, 81, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gullá, S.; Chen, T.S.; Fire, E.; Grant, R.A.; Keating, A.E. Determinants of BH3 Binding Specificity for Mcl-1 versus Bcl-xL. J. Mol. Biol. 2010, 398, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Clackson, T.; Wells, J.A. A hot spot of binding energy in a hormone-receptor interface. Science 1995, 267, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Bogan, A.A.; Thorn, K.S. Anatomy of hot spots in protein interfaces. J. Mol. Biol. 1998, 280, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Keskin, O.; Ma, B.; Nussinov, R. Hot regions in protein—Protein interactions: The organization and contribution of structurally conserved hot spot residues. J. Mol. Biol. 2005, 345, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Guharoy, M.; Chakrabarti, P. Secondary structure based analysis and classification of biological interfaces: Identification of binding motifs in protein-protein interactions. Bioinformatics 2007, 23, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Jochim, A.L.; Arora, P.S. Systematic analysis of helical protein interfaces reveals targets for synthetic inhibitors. ACS Chem. Biol. 2010, 5, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.; Bullock, B.N.; Arora, P.S. Plucking the high hanging fruit: A systematic approach for targeting protein–protein interactions. Bioorg. Med. Chem. 2013, 21, 4051–4057. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.R.; Dunyak, B.M.; Komiyama, T.; Shao, H.; Ran, X.; Assimon, V.A.; Kalyanaraman, C.; Rauch, J.N.; Jacobson, M.P.; Zuiderweg, E.R.P.; et al. High Throughput Screen for Inhibitors of Protein-Protein Interactions in a Reconstituted Heat Shock Protein 70 (Hsp70) Complex. J. Biol. Chem. 2018, 293, 4014–4025. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.P.; Tung, F.; Antonysamy, S.; Wasserman, S.; Jones, S.B.; Zhang, F.F.; Espada, A.; Broughton, H.; Chalmers, M.J.; Woodman, M.E.; et al. Utilization of peptide phage display to investigate hotspots on IL-17A and what it means for drug discovery. PLoS ONE 2018, 13, e0190850. [Google Scholar] [CrossRef] [PubMed]
- Suchanek, M.; Radzikowska, A.; Thiele, C. Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nat. Methods 2005, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Melagraki, G.; Ntougkos, E.; Rinotas, V.; Papaneophytou, C.; Leonis, G.; Mavromoustakos, T.; Kontopidis, G.; Douni, E.; Afantitis, A.; Kollias, G. Cheminformatics-aided discovery of small-molecule Protein-Protein Interaction (PPI) dual inhibitors of Tumor Necrosis Factor (TNF) and Receptor Activator of NF-κB Ligand (RANKL). PLoS Comput. Biol. 2017, 13, e1005372. [Google Scholar] [CrossRef] [PubMed]
- Ripka, A.S.; Rich, D.H. Peptidomimetic design. Curr. Opin. Chem. Biol. 1998, 2, 441–452. [Google Scholar] [CrossRef]
- Azzarito, V.; Long, K.; Murphy, N.S.; Wilson, A.J. Inhibition of α-helix-mediated protein-protein interactions using designed molecules. Nat. Chem. 2013, 5, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Pelay-Gimeno, M.; Glas, A.; Koch, O.; Grossmann, T.N. Structure-Based Design of Inhibitors of Protein-Protein Interactions: Mimicking Peptide Binding Epitopes. Angew. Chem. Int. Ed. 2015, 54, 8896–8927. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.F.; Czabotar, P.E.; Smith, B.J.; Deshayes, K.; Zobel, K.; Colman, P.M.; Fairlie, W.D. Crystal structure of ABT-737 complexed with Bcl-xL: Implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 2007, 14, 1711–1713. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Grasberger, B.L.; Lu, T.; Schubert, C.; Parks, D.J.; Carver, T.E.; Koblish, H.K.; Cummings, M.D.; LaFrance, L.V.; Milkiewicz, K.L.; Calvo, R.R.; et al. Discovery and cocrystal structure of benzodiazepinedione HDM2 antagonists that activate p53 in cells. J. Med. Chem. 2005, 48, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Orner, B.P.; Ernst, J.T.; Hamilton, A.D. Toward Proteomimetics: Terphenyl Derivatives as Structural and Functional Mimics of Extended Regions of an α-Helix. J. Am. Chem. Soc. 2001, 123, 5382–5383. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Lee, G.-I.; Sedey, K.A.; Rodriguez, J.M.; Wang, H.-G.; Sebti, S.M.; Hamilton, A.D. Terephthalamide derivatives as mimetics of helical peptides: Disruption of the Bcl-x(L)/Bak interaction. J. Am. Chem. Soc. 2005, 127, 5463–5468. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.; Nevola, L.; Ross, N.T.; Lee, G.; Hamilton, A.D. Synthetic inhibitors of extended helix-protein interactions based on a biphenyl 4,4′-dicarboxamide scaffold. Chembiochem 2009, 10, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.G.; Ross, N.T.; Katt, W.P.; Hamilton, A.D. Synthesis and biological evaluation of a 5-6-5 imidazole-phenyl-thiazole based alpha-helix mimetic. Org. Lett. 2009, 11, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.T.; Becerril, J.; Park, H.S.; Yin, H.; Hamilton, A.D. Design and application of an alpha-helix-mimetic scaffold based on an oligoamide-foldamer strategy: Antagonism of the Bak BH3/Bcl-xL complex. Angew. Chem. Int. Ed. 2003, 42, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Adler, M.J.; Scott, R.T.W.; Hamilton, A.D. Enaminone-based mimics of extended and hydrophilic α-helices. Chemistry 2012, 18, 12974–12977. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.L.; Cao, X.; Vanommeslaeghe, K.; Jung, K.-Y.; Peddaboina, C.; Wilder, P.T.; Nan, A.; MacKerell, A.D.; Smythe, W.R.; Fletcher, S. Relaxation of the rigid backbone of an oligoamide-foldamer-based α-helix mimetic: Identification of potent Bcl-xL inhibitors. Org. Biomol. Chem. 2012, 10, 2928–2933. [Google Scholar] [CrossRef] [PubMed]
- Biros, S.M.; Moisan, L.; Mann, E.; Carella, A.; Zhai, D.; Reed, J.C.; Rebek, J. Heterocyclic alpha-helix mimetics for targeting protein-protein interactions. Bioorg. Med. Chem. Lett. 2007, 17, 4641–4645. [Google Scholar] [CrossRef] [PubMed]
- Londregan, A.T.; Piotrowski, D.W.; Wei, L. Synthesis of Pyridazine-Based α-Helix Mimetics. ACS Comb. Sci. 2016, 18, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Lee, W.S.; Oh, M.; Lee, H.; Lee, J.H.; Im, W.; Lim, H.-S. Design, Solid-Phase Synthesis, and Evaluation of a Phenyl-Piperazine-Triazine Scaffold as α-Helix Mimetics. ACS Comb. Sci. 2014, 16, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Van Mileghem, S.; Egle, B.; Gilles, P.; Veryser, C.; Van Meervelt, L.; De Borggraeve, W.M. Carbonylation as a novel method for the assembly of pyrazine based oligoamide alpha-helix mimetics. Org. Biomol. Chem. 2017, 15, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Plante, J.P.; Burnley, T.; Malkova, B.; Webb, M.E.; Warriner, S.L.; Edwards, T.A.; Wilson, A.J. Oligobenzamide proteomimetic inhibitors of the p53-hDM2 protein-protein interaction. Chem. Commun. 2009, 5091–5093. [Google Scholar] [CrossRef] [PubMed]
- Azzarito, V.; Prabhakaran, P.; Bartlett, A.I.; Murphy, N.S.; Hardie, M.J.; Kilner, C.A.; Edwards, T.A.; Warriner, S.L.; Wilson, A.J. 2-O-alkylated para-benzamide α-helix mimetics: The role of scaffold curvature. Org. Biomol. Chem. 2012, 10, 6469–6472. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.; Plante, J.P.; Edwards, T.A.; Warriner, S.L.; Wilson, A.J. N-alkylated oligoamide alpha-helical proteomimetics. Org. Biomol. Chem. 2010, 8, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Long, K.; Edwards, T.A.; Wilson, A.J. Microwave assisted solid phase synthesis of highly functionalized N-alkylated oligobenzamide α-helix mimetics. Bioorg. Med. Chem. 2013, 21, 4034–4040. [Google Scholar] [CrossRef] [PubMed]
- Barnard, A.; Long, K.; Yeo, D.J.; Miles, J.A.; Azzarito, V.; Burslem, G.M.; Prabhakaran, P.; Edwards, A.T.; Wilson, A.J. Orthogonal functionalisation of α-helix mimetics. Org. Biomol. Chem. 2014, 12, 6794–6799. [Google Scholar] [CrossRef] [PubMed]
- Marimganti, S.; Cheemala, M.N.; Ahn, J.-M. Novel Amphiphilic α-Helix Mimetics Based on a Bis-benzamide Scaffold. Org. Lett. 2009, 11, 4418–4421. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Vallinayagam, R.; Adler, M.J.; Scott, R.T.W.; Hamilton, A.D. Double-sided α-helix mimetics. Tetrahedron 2012, 68, 4501–4505. [Google Scholar] [CrossRef]
- Thompson, S.; Hamilton, A.D. Amphiphilic α-helix mimetics based on a benzoylurea scaffold. Org. Biomol. Chem. 2012, 10, 5780–5782. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, M.; Kim, H.S.; Lee, H.; Im, W.; Lim, H.-S. Converting One-Face α-Helix Mimetics into Amphiphilic α-Helix Mimetics as Potent Inhibitors of Protein–Protein Interactions. ACS Comb. Sci. 2016, 18, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Lanning, M.E.; Wilder, P.T.; Bailey, H.; Drennen, B.; Cavalier, M.; Chen, L.; Yap, J.L.; Raje, M.; Fletcher, S. Towards more drug-like proteomimetics: Two-faced, synthetic α-helix mimetics based on a purine scaffold. Org. Biomol. Chem. 2015, 13, 8642–8646. [Google Scholar] [CrossRef] [PubMed]
- Lanning, M.; Fletcher, S. Multi-Facial, Non-Peptidic α-Helix Mimetics. Biology 2015, 4, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Geotti-Bianchini, P.; Moretto, A.; Peggion, C.; Beyrath, J.; Bianco, A.; Formaggio, F. Replacement of Ala by Aib improves structuration and biological stability in thymine-based α-nucleopeptides. Org. Biomol. Chem. 2010, 8, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Kanthala, S.; Gauthier, T.; Satyanarayanajois, S. Structure-activity relationships of peptidomimetics that inhibit PPI of HER2-HER3. Biopolymers 2014, 101, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, X.; Shih, T.-C.; Lee, J.S.; Lam, K.S. CHAPTER 10. Peptide Therapeutics: Oncology. In Peptide-Based Drug Discovery: Challenges and New Therapeutics; RSC Publishing: London, UK, 2017; pp. 278–325. [Google Scholar]
- Garton, M.; Nim, S.; Stone, T.A.; Wang, K.E.; Deber, C.M.; Kim, P.M. Method to generate highly stable d-amino acid analogs of bioactive helical peptides using a mirror image of the entire PDB. Proc. Natl. Acad. Sci. USA 2018, 115, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Luo, Z.; Luo, J.; Fan, X.; Cayabyab, M.; Hiraoka, M.; Liu, D.; Han, X.; Pesavento, J.; Dong, C.-Z.; et al. Exploring the stereochemistry of CXCR4-peptide recognition and inhibiting HIV-1 entry with D-peptides derived from chemokines. J. Biol. Chem. 2002, 277, 17476–17485. [Google Scholar] [CrossRef] [PubMed]
- Checco, J.W.; Lee, E.F.; Evangelista, M.; Sleebs, N.J.; Rogers, K.; Pettikiriarachchi, A.; Kershaw, N.J.; Eddinger, G.A.; Belair, D.G.; Wilson, J.L.; et al. α/β-Peptide Foldamers Targeting Intracellular Protein–Protein Interactions with Activity in Living Cells. J. Am. Chem. Soc. 2015, 137, 11365–11375. [Google Scholar] [CrossRef] [PubMed]
- Funke, S.A.; Willbold, D. Mirror image phage display—A method to generate d-peptide ligands for use in diagnostic or therapeutical applications. Mol. Biosyst. 2009, 5, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Tal-Gan, Y.; Freeman, N.S.; Klein, S.; Levitzki, A.; Gilon, C. Metabolic Stability of Peptidomimetics: N-Methyl and Aza Heptapeptide Analogs of a PKB/Akt Inhibitor. Chem. Biol. Drug Des. 2011, 78, 887–892. [Google Scholar] [CrossRef] [PubMed]
- McDougall, L.; Draper, E.R.; Beadle, J.D.; Shipman, M.; Raubo, P.; Jamieson, A.G.; Adams, D.J. Enzymatically-stable oxetane-based dipeptide hydrogels. Chem. Commun. 2018, 54, 1793–1796. [Google Scholar] [CrossRef] [PubMed]
- Walensky, L.D.; Bird, G.H. Hydrocarbon-stapled peptides: Principles, practice, and progress. J. Med. Chem. 2014, 57, 6275–6288. [Google Scholar] [CrossRef] [PubMed]
- Cardote, T.A.F.; Ciulli, A. Cyclic and Macrocyclic Peptides as Chemical Tools To Recognise Protein Surfaces and Probe Protein-Protein Interactions. ChemMedChem 2016, 11, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kunciw, D.L.; Xu, W.; Wiedmann, M.M.; Wu, Y.; Sore, H.F.; Galloway, W.R.J.D.; Lau, Y.H.; Itzhaki, L.S.; Spring, D.R. CHAPTER 8. Double-click Stapled Peptides for Inhibiting Protein–Protein Interactions. In Cyclic Peptides: From Bioorganic Synthesis to Applications; RSC Publishing: London, UK, 2017; pp. 164–187. [Google Scholar]
- Dougherty, P.G.; Qian, Z.; Pei, D. Macrocycles as protein–protein interaction inhibitors. Biochem. J. 2017, 474, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M. Interface Peptide Mimetics-Rationale and Application as Therapeutic Agents. Med. Chem. 2016, 6, 189–194. [Google Scholar] [CrossRef]
- Robertson, N.; Jamieson, A. Regulation of protein-protein interactions using stapled peptides. Rep. Org. Chem. 2015, 5, 65–74. [Google Scholar]
- Atzori, A.; Baker, A.E.; Chiu, M.; Bryce, R.A.; Bonnet, P. Effect of Sequence and Stereochemistry Reversal on p53 Peptide Mimicry. PLoS ONE 2013, 8, e68723. [Google Scholar] [CrossRef] [PubMed]
- Frackenpohl, J.; Arvidsson, P.I.; Schreiber, J.V.; Seebach, D. The Outstanding Biological Stability of β- and γ-Peptides toward Proteolytic Enzymes: An In Vitro Investigation with Fifteen Peptidases. ChemBioChem 2001, 2, 445–455. [Google Scholar] [CrossRef]
- Wiegand, H.; Wirz, B.; Schweitzer, A.; Camenisch, G.P.; Perez, M.I.R.; Gross, G.; Woessner, R.; Voges, R.; Arvidsson, P.I.; Frackenpohl, J.; et al. The outstanding metabolic stability of a 14C-labeled beta-nonapeptide in rats—In vitro and in vivo pharmacokinetic studies. Biopharm. Drug Dispos. 2002, 23, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Cheng, K.; Yin, H. Targeting protein-protein interfaces using macrocyclic peptides. Biopolymers 2015, 104, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Villar, E.A.; Beglov, D.; Chennamadhavuni, S.; Porco, J.A.; Kozakov, D.; Vajda, S.; Whitty, A. How proteins bind macrocycles. Nat. Chem. Biol. 2014, 10, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Krüger, D.M.; Glas, A.; Bier, D.; Pospiech, N.; Wallraven, K.; Dietrich, L.; Ottmann, C.; Koch, O.; Hennig, S.; Grossmann, T.N. Structure-Based Design of Non-natural Macrocyclic Peptides That Inhibit Protein–Protein Interactions. J. Med. Chem. 2017, 60, 8982–8988. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martin, M.; Pardo, P.G.; Lopez-Tarruella, S.; Manso, L.; Perez-Fidalgo, J.A.; Ademuyiwa, F.O.; Mayer, I.A.; Pluard, T.J.; Garcia, M.M.; Kaufman, P.A.; et al. Phase I study of the combination of balixafortide (CXCR4 inhibitor) and eribulin in HER2-negative metastatic breast cancer (MBC) patients (pts). J. Clin. Oncol. 2017, 35, 2555. [Google Scholar]
- Von Nussbaum, F.; Li, V.M.-J. Neutrophil elastase inhibitors for the treatment of (cardio)pulmonary diseases: Into clinical testing with pre-adaptive pharmacophores. Bioorg. Med. Chem. Lett. 2015, 25, 4370–4381. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.; Harrison, S.; Campbell, S.; Teufel, D.; Langforn, G.; Watt, A.; Bonny, C. Development of BT1718, a Bicycle Drug Conjugate® (BDC) targeting MT1-MMP for treatment of solid tumours. Eur. J. Cancer 2016, 69, S21. [Google Scholar] [CrossRef]
- Schwameis, R.; Eder, S.; Pietschmann, H.; Fischer, B.; Mascher, H.; Tzotzos, S.; Fischer, H.; Lucas, R.; Zeitlinger, M.; Hermann, R. A FIM Study to Assess Safety and Exposure of Inhaled Single Doses of AP301-A Specific ENaC Channel Activator for the Treatment of Acute Lung Injury. J. Clin. Pharmacol. 2014, 54, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, A.; Arata, M.; DeMarco, S.; Dhamnaskar, K.; Hammer, R.; Fridkis-Hareli, M.; Rajagopal, V.; Seyb, K.; Tang, G.-Q.; Tobe, S.; et al. Preclinical evaluation of RA101495, a potent cyclic peptide inhibitor of C5 for the treatment of paroxysmal nocturnal hemoglobinuria. Blood 2015, 126, 939. [Google Scholar]
- Rennie, Y.K.; McIntyre, P.J.; Akindele, T.; Bayliss, R.; Jamieson, A.G. A TPX2 Proteomimetic Has Enhanced Affinity for Aurora-A Due to Hydrocarbon Stapling of a Helix. ACS Chem. Biol. 2016, 11, 3383–3390. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Okamura, H. Conformational change of Sos-derived proline-rich peptide upon binding Grb2 N-terminal SH3 domain probed by NMR. Sci. Rep. 2013, 3, 2913. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Moellering, R.E.; Hilinski, G.J.; Kim, Y.-W.; Grossmann, T.N.; Yeh, J.T.-H.; Verdine, G.L. Towards understanding cell penetration by stapled peptides. Med. Chem. Commun. 2015, 6, 111–119. [Google Scholar] [CrossRef]
- Scholtz, J.M.; Qian, H.; Robbins, V.H.; Baldwin, R.L. The energetics of ion-pair and hydrogen-bonding interactions in a helical peptide. Biochemistry 1993, 32, 9668–9676. [Google Scholar] [CrossRef] [PubMed]
- Marqusee, S.; Baldwin, R.L. Helix stabilization by Glu−...Lys+ salt bridges in short peptides of de novo design. Proc. Natl. Acad. Sci. USA 1987, 84, 8898–8902. [Google Scholar] [CrossRef] [PubMed]
- Chorev, M.; Roubini, E.; McKee, R.L.; Gibbons, S.W.; Goldman, M.E.; Caulfield, M.P.; Rosenblatt, M. Cyclic parathyroid hormone-related protein antagonists: Lysine 13 to aspartic acid 17 [i to (i+4)] side chain to side chain lactamization. Biochemistry 1991, 30, 5968–5974. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.E.; Gannon, C.L.; Kay, C.M.; Hodges, R.S. Lactam bridge stabilization of alpha-helical peptides: Ring size, orientation and positional effects. J. Pept. Sci. 1995, 1, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.J.; Schmidt, S.; Wadhwani, P.; Bürck, J.; Reichert, J.; Afonin, S.; Berditsch, M.; Schober, T.; Brock, R.; Kansy, M.; et al. Lactam-Stapled Cell-Penetrating Peptides: Cell Uptake and Membrane Binding Properties. J. Med. Chem. 2017, 60, 8071–8082. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, S.A.; Coleska, A.; Ran, X.; Yang, C.-Y.; Wang, S. Design of Triazole-Stapled BCL9 α-Helical Peptides to Target the β-Catenin/B-Cell CLL/lymphoma 9 (BCL9) Protein–Protein Interaction. J. Med. Chem. 2012, 55, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Walensky, L.D.; Kung, A.L.; Escher, I.; Malia, T.J.; Barbuto, S.; Wright, R.D.; Wagner, G.; Verdine, G.L.; Korsmeyer, S.J. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 2004, 305, 1466–1470. [Google Scholar] [CrossRef] [PubMed]
- Bernal, F.; Tyler, A.F.; Korsmeyer, S.J.; Walensky, L.D.; Verdine, G.L. Reactivation of the p53 tumor suppressor pathway by a stapled p53 peptide. J. Am. Chem. Soc. 2007, 129, 2456–2457. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-W.; Kutchukian, P.S.; Verdine, G.L. Introduction of all-hydrocarbon i,i+3 staples into alpha-helices via ring-closing olefin metathesis. Org. Lett. 2010, 12, 3046–3049. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-W.; Grossmann, T.N.; Verdine, G.L. Synthesis of all-hydrocarbon stapled α-helical peptides by ring-closing olefin metathesis. Nat. Protoc. 2011, 6, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Bird, G.H.; Bernal, F.; Pitter, K.; Walensky, L.D. Synthesis and biophysical characterization of stabilized alpha-helices of BCL-2 domains. Methods Enzymol. 2008, 446, 369–386. [Google Scholar] [PubMed]
- LaBelle, J.L.; Katz, S.G.; Bird, G.H.; Gavathiotis, E.; Stewart, M.L.; Lawrence, C.; Fisher, J.K.; Godes, M.; Pitter, K.; Kung, A.L.; et al. A stapled BIM peptide overcomes apoptotic resistance in hematologic cancers. J. Clin. Investig. 2012, 122, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, N.E.; Hoang, H.N.; Abbenante, G.; Fairlie, D.P. Single turn peptide alpha helices with exceptional stability in water. J. Am. Chem. Soc. 2005, 127, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, N.E.; Abbenante, G.; Fairlie, D.P. Consecutive Cyclic Pentapeptide Modules Form Shortα-Helices that are Very Stable to Water and Denaturants. Angew. Chem. Int. Ed. 2004, 43, 2687–2690. [Google Scholar] [CrossRef]
- Harrison, R.S.; Ruiz-Gómez, G.; Hill, T.A.; Chow, S.Y.; Shepherd, N.E.; Lohman, R.-J.; Abbenante, G.; Hoang, H.N.; Fairlie, D.P. Novel Helix-Constrained Nociceptin Derivatives Are Potent Agonists and Antagonists of ERK Phosphorylation and Thermal Analgesia in Mice. J. Med. Chem. 2010, 53, 8400–8408. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, Y.; Ashigai, H.; Goto, Y.; Murakami, H.; Suga, H. Ribosomal Synthesis of Cyclic Peptides with a Fluorogenic Oxidative Coupling Reaction. ChemBioChem 2009, 10, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.J.; Grubbs, R.H. Synthesis of Conformationally Restricted Amino Acids and Peptides Employing Olefin Metathesis. J. Am. Chem. Soc. 1995, 117, 5855–5856. [Google Scholar] [CrossRef]
- Miller, S.J.; Blackwell, H.E.; Grubbs, R.H. Application of Ring-Closing Metathesis to the Synthesis of Rigidified Amino Acids and Peptides. J. Am. Chem. Soc. 1996, 118, 9606–9614. [Google Scholar] [CrossRef]
- Blackwell, H.E.; Grubbs, R.H. Highly Efficient Synthesis of Covalently Cross-Linked Peptide Helices by Ring-Closing Metathesis. Angew. Chem. Int. Ed. 1998, 37, 3281–3284. [Google Scholar] [CrossRef]
- Schafmeister, C.E.; Po, J.; Verdine, G.L. An All-Hydrocarbon Cross-Linking System for Enhancing the Helicity and Metabolic Stability of Peptides. J. Am. Chem. Soc. 2000, 122, 5891–5892. [Google Scholar] [CrossRef]
- Walensky, L.D.; Pitter, K.; Morash, J.; Oh, K.J.; Barbuto, S.; Fisher, J.; Smith, E.; Verdine, G.L.; Korsmeyer, S.J. A stapled BID BH3 helix directly binds and activates BAX. Mol. Cell 2006, 24, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Verdine, G.L.; Hilinski, G.J. Stapled peptides for intracellular drug targets. Methods Enzymol. 2012, 503, 3–33. [Google Scholar] [PubMed]
- Aillard, B.; Robertson, N.S.; Baldwin, A.R.; Robins, S.; Jamieson, A.G. Robust asymmetric synthesis of unnatural alkenyl amino acids for conformationally constrained α-helix peptides. Org. Biomol. Chem. 2014, 12, 8775–8782. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.J.; Warriner, S.L.; Wilson, A.J. Monosubstituted alkenyl amino acids for peptide “stapling”. Chem. Commun. 2013, 49, 9131–9133. [Google Scholar] [CrossRef] [PubMed]
- McWhinnie, F.S.; Sepp, K.; Wilson, C.; Kunath, T.; Hupp, T.R.; Baker, T.S.; Houston, D.R.; Hulme, A.N. Mono-Substituted Hydrocarbon Diastereomer Combinations Reveal Stapled Peptides with High Structural Fidelity. Chem. A Eur. J. 2018, 24, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.Y.; Kim, Y.-W.; Verdine, G.L. A new i, i + 3 peptide stapling system for α-helix stabilization. Chem. Biol. Drug Des. 2013, 82, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Bird, G.H.; Madani, N.; Perry, A.F.; Princiotto, A.M.; Supko, J.G.; He, X.; Gavathiotis, E.; Sodroski, J.G.; Walensky, L.D. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proc. Natl. Acad. Sci. USA 2010, 107, 14093–14098. [Google Scholar] [CrossRef] [PubMed]
- Hilinski, G.J.; Kim, Y.-W.; Hong, J.; Kutchukian, P.S.; Crenshaw, C.M.; Berkovitch, S.S.; Chang, A.; Ham, S.; Verdine, G.L. Stitched α-helical peptides via bis ring-closing metathesis. J. Am. Chem. Soc. 2014, 136, 12314–12322. [Google Scholar] [CrossRef] [PubMed]
- Grigoryev, Y. Stapled peptide to enter human testing, but affinity questions remain. Nat. Med. 2013, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Payton, M.; Pinchasik, D.; Mehta, A.; Goel, S.; Zain, J.M.; Sokol, L.; Jacobsen, E.; Patel, M.R.; Horwitz, S.M.; Meric-Bernstam, F.; et al. Phase 2a study of a novel stapled peptide ALRN-6924 disrupting MDMX- and MDM2-mediated inhibition of wild-type TP53 in patients with peripheral t-cell lymphoma. Ann. Oncol. 2017, 28, v355–v371. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Saleh, M.N.; Infante, J.R.; Goel, S.; Falchook, G.S.; Shapiro, G. Phase I trial of a novel stapled peptide ALRN-6924 disrupting MDMX- and MDM2-mediated inhibition of WT p53 in patients with solid tumors and lymphomas. J. Clin. Oncol. 2017, 26, 153–160. [Google Scholar]
- Kumita, J.R.; Smart, O.S.; Woolley, G.A. Photo-control of helix content in a short peptide. Proc. Natl. Acad. Sci. USA 2000, 97, 3803–3808. [Google Scholar] [CrossRef] [PubMed]
- Flint, D.G.; Kumita, J.R.; Smart, O.S.; Woolley, G.A. Using an Azobenzene Cross-Linker to Either Increase or Decrease Peptide Helix Content upon Trans-to-Cis Photoisomerization. Chem. Biol. 2002, 9, 391–397. [Google Scholar] [CrossRef]
- Woolley, G.A. Photocontrolling peptide α helices. Acc. Chem. Res. 2005, 38, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Kneissl, S.; Loveridge, E.J.; Williams, C.; Crump, M.P.; Allemann, R.K. Photocontrollable peptide-based switches target the anti-apoptotic protein Bcl-xL. Chembiochem 2008, 9, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chou, D.H.-C. A Thiol-Ene Coupling Approach to Native Peptide Stapling and Macrocyclization. Angew. Chem. Int. Ed. 2015, 54, 10931–10934. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Meinhardt, N.; Wu, Y.; Kulkarni, S.; Hu, X.; Low, K.E.; Davies, P.L.; DeGrado, W.F.; Greenbaum, D.C. Development of α-helical calpain probes by mimicking a natural protein-protein interaction. J. Am. Chem. Soc. 2012, 134, 17704–17713. [Google Scholar] [CrossRef] [PubMed]
- Peraro, L.; Zou, Z.; Makwana, K.M.; Cummings, A.E.; Ball, H.L.; Yu, H.; Lin, Y.-S.; Levine, B.; Kritzer, J.A. Diversity-Oriented Stapling Yields Intrinsically Cell-Penetrant Inducers of Autophagy. J. Am. Chem. Soc. 2017, 139, 7792–7802. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.J.; Zhang, C.; Vinogradova, E.V.; Buchwald, N.H.; Reilly, J.; Pentelute, B.L.; Buchwald, S.L. Divergent unprotected peptide macrocyclisation by palladium-mediated cysteine arylation. Chem. Sci. 2017, 8, 4257–4263. [Google Scholar] [CrossRef] [PubMed]
- St. Louis, L.E.; Rodriguez, T.M.; Waters, M.L. A study of 2-component i, i + 3 peptide stapling using thioethers. Bioorg. Med. Chem. 2018, 26, 1203–1205. [Google Scholar]
- Micewicz, E.D.; Sharma, S.; Waring, A.J.; Luong, H.T.; McBride, W.H.; Ruchala, P. Bridged Analogues for p53-Dependent Cancer Therapy Obtained by S-Alkylation. Int. J. Pept. Res. Ther. 2016, 22, 67–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spokoyny, A.M.; Zou, Y.; Ling, J.J.; Yu, H.; Lin, Y.-S.; Pentelute, B.L. A Perfluoroaryl-Cysteine SNAr Chemistry Approach to Unprotected Peptide Stapling. J. Am. Chem. Soc. 2013, 135, 5946–5949. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.P.; Smith, A.B. Peptide/Protein Stapling and Unstapling: Introduction of s-Tetrazine, Photochemical Release, and Regeneration of the Peptide/Protein. J. Am. Chem. Soc. 2015, 137, 4034–4037. [Google Scholar] [CrossRef] [PubMed]
- Lautrette, G.; Touti, F.; Lee, H.G.; Dai, P.; Pentelute, B.L. Nitrogen Arylation for Macrocyclization of Unprotected Peptides. J. Am. Chem. Soc. 2016, 138, 8340–8343. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.Y.-L.; Rout, B.; Tan, Y.S.; Verma, C.S.; Chan, K.-P.; Johannes, C.W. An intramolecular tryptophan-condensation approach for peptide stapling. Org. Biomol. Chem. 2018, 16, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.H.; de Andrade, P.; Quah, S.-T.; Rossmann, M.; Laraia, L.; Sköld, N.; Sum, T.J.; Rowling, P.J.E.; Joseph, T.L.; Verma, C.; et al. Functionalised staple linkages for modulating the cellular activity of stapled peptides. Chem. Sci. 2014, 5, 1804–1809. [Google Scholar] [CrossRef]
- Wu, Y.; Olsen, L.B.; Lau, Y.H.; Jensen, C.H.; Rossmann, M.; Baker, Y.R.; Sore, H.F.; Collins, S.; Spring, D.R. Development of a Multifunctional Benzophenone Linker for Peptide Stapling and Photoaffinity Labelling. ChemBioChem 2016, 17, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Villa, F.; Maman, J.; Lau, Y.H.; Dobnikar, L.; Simon, A.C.; Labib, K.; Spring, D.R.; Pellegrini, L. Targeting the Genome-Stability Hub Ctf4 by Stapled-Peptide Design. Angew. Chem. Int. Ed. 2017, 56, 12866–12872. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.H.; Wu, Y.; Rossmann, M.; Tan, B.X.; de Andrade, P.; Tan, Y.S.; Verma, C.; McKenzie, G.J.; Venkitaraman, A.R.; Hyvönen, M.; et al. Double Strain-Promoted Macrocyclization for the Rapid Selection of Cell-Active Stapled Peptides. Angew. Chem. Int. Ed. 2015, 54, 15410–15413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Navaratna, T.; Liao, J.; Thurber, G.M. Dual-Purpose Linker for Alpha Helix Stabilization and Imaging Agent Conjugation to Glucagon-Like Peptide-1 Receptor Ligands. Bioconjug. Chem. 2015, 26, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Boutureira, O.; Bernardes, G.J.L. Advances in Chemical Protein Modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef] [PubMed]
- Fairlie, D.P.; De Araujo, A.D. Review Stapling Peptides Using Cysteine Crosslinking. Pept. Sci. 2016, 106, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sadovski, O.; Xin, S.J.; Woolley, G.A. Stabilization of folded peptide and protein structures via distance matching with a long, rigid cross-linker. J. Am. Chem. Soc. 2007, 129, 14154–14155. [Google Scholar] [CrossRef] [PubMed]
- Mart, R.J.; Allemann, R.K. Azobenzene photocontrol of peptides and proteins. Chem. Commun. 2016, 52, 12262–12277. [Google Scholar] [CrossRef] [PubMed]
- Heinis, C.; Rutherford, T.; Freund, S.; Winter, G. Phage-encoded combinatorial chemical libraries based on bicyclic peptides. Nat. Chem. Biol. 2009, 5, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Cendron, L.; Chen, S.; Touati, J.; Winter, G.; Zanotti, G.; Heinis, C. Bicyclic Peptide Inhibitor Reveals Large Contact Interface with a Protease Target. ACS Chem. Biol. 2012, 7, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bertoldo, D.; Angelini, A.; Pojer, F.; Heinis, C. Peptide Ligands Stabilized by Small Molecules. Angew. Chem. Int. Ed. 2014, 53, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Assem, N.; Ferreira, D.J.; Wolan, D.W.; Dawson, P.E. Acetone-Linked Peptides: A Convergent Approach for Peptide Macrocyclization and Labeling. Angew. Chem. Int. Ed. 2015, 54, 8665–8668. [Google Scholar] [CrossRef] [PubMed]
- Grison, C.M.; Burslem, G.M.; Miles, J.A.; Pilsl, L.K.A.; Yeo, D.J.; Imani, Z.; Warriner, S.L.; Webb, M.E.; Wilson, A.J. Double quick, double click reversible peptide “stapling”. Chem. Sci. 2017, 8, 5166–5171. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, R.; Xu, H.; Chen, J.; Zhan, Y.; Zhou, X.; Chen, H.; Jiang, B. Divinylsulfonamides as Specific Linkers for Stapling Disulfide Bonds in Peptides. Org. Lett. 2017, 19, 4972–4975. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.H.; Wu, Y.; de Andrade, P.; Galloway, W.R.J.D.; Spring, D.R. A two-component “double-click” approach to peptide stapling. Nat. Protoc. 2015, 10, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.H.; de Andrade, P.; McKenzie, G.J.; Venkitaraman, A.R.; Spring, D.R. Linear Aliphatic Dialkynes as Alternative Linkers for Double-Click Stapling of p53-Derived Peptides. ChemBioChem 2014, 15, 2680–2683. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.H.; de Andrade, P.; Sköld, N.; McKenzie, G.J.; Venkitaraman, A.R.; Verma, C.; Lane, D.P.; Spring, D.R.; Lukacs, C.; Klein, C.; et al. Investigating peptide sequence variations for “double-click” stapled p53 peptides. Org. Biomol. Chem. 2014, 12, 4074–4077. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, M.M.; Tan, Y.S.; Wu, Y.; Aibara, S.; Xu, W.; Sore, H.F.; Verma, C.S.; Itzhaki, L.; Stewart, M.; Brenton, J.D.; et al. Development of Cell-Permeable, Non-Helical Constrained Peptides to Target a Key Protein-Protein Interaction in Ovarian Cancer. Angew. Chem. Int. Ed. 2017, 56, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lau, Y.H.; Fischer, G.; Tan, Y.S.; Chattopadhyay, A.; de la Roche, M.; Hyvönen, M.; Verma, C.; Spring, D.R.; Itzhaki, L.S. Macrocyclized Extended Peptides: Inhibiting the Substrate-Recognition Domain of Tankyrase. J. Am. Chem. Soc. 2017, 139, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Navaratna, T.; Thurber, G.M. A Helix-Stabilizing Linker Improves Subcutaneous Bioavailability of a Helical Peptide Independent of Linker Lipophilicity. Bioconjug. Chem. 2016, 27, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.T.; Larsen, C.Ø.; Røndbjerg, T.; De Foresta, M.; Kunze, M.B.A.; Marek, A.; Løper, J.H.; Boyhus, L.-E.; Knuhtsen, A.; Lindorff-Larsen, K.; et al. Diversity-Oriented Peptide Stapling: A Third Generation Copper-Catalysed Azide-Alkyne Cycloaddition Stapling and Functionalisation Strategy. Chem. A Eur. J. 2017, 23, 3490–3495. [Google Scholar] [CrossRef] [PubMed]
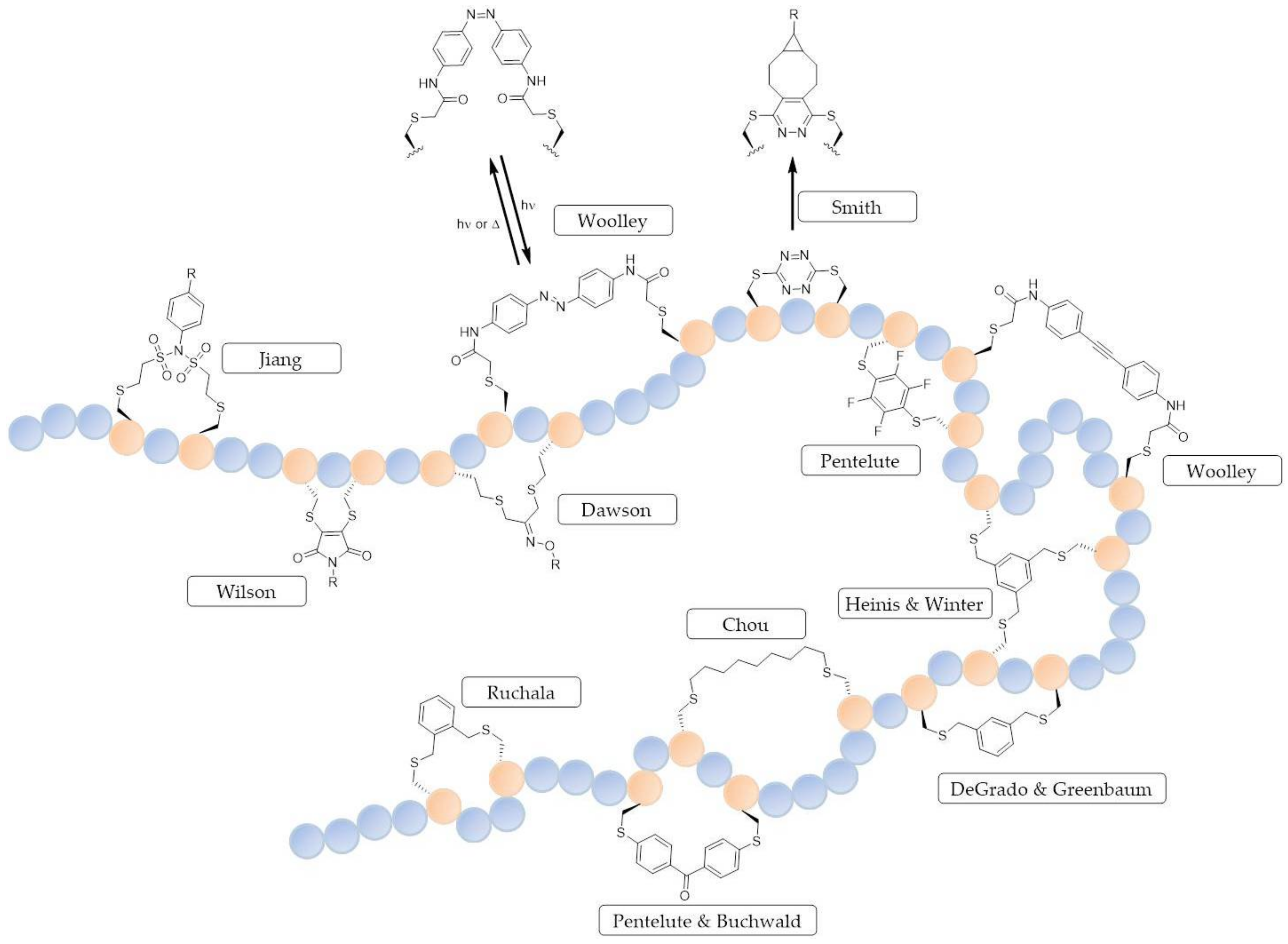
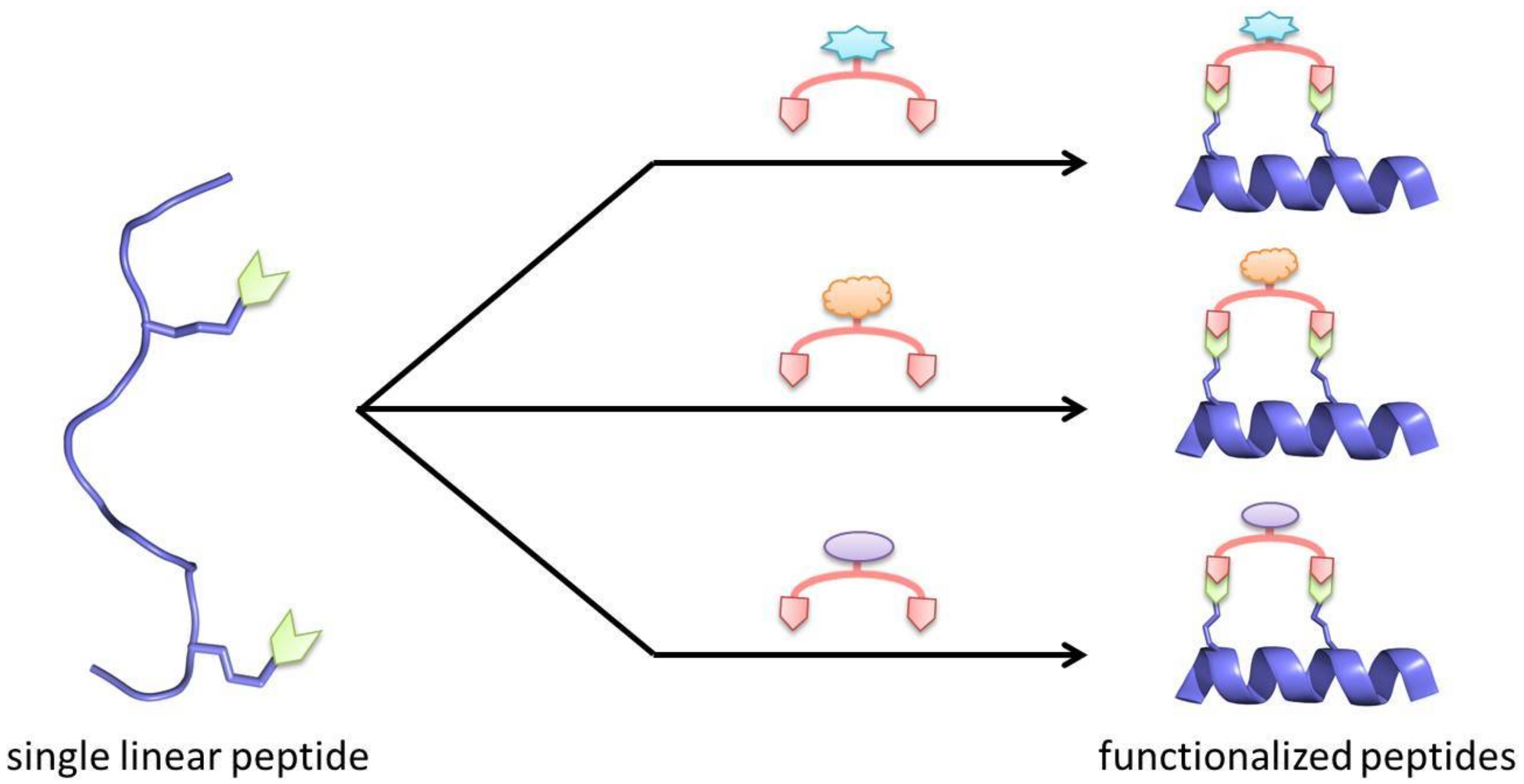
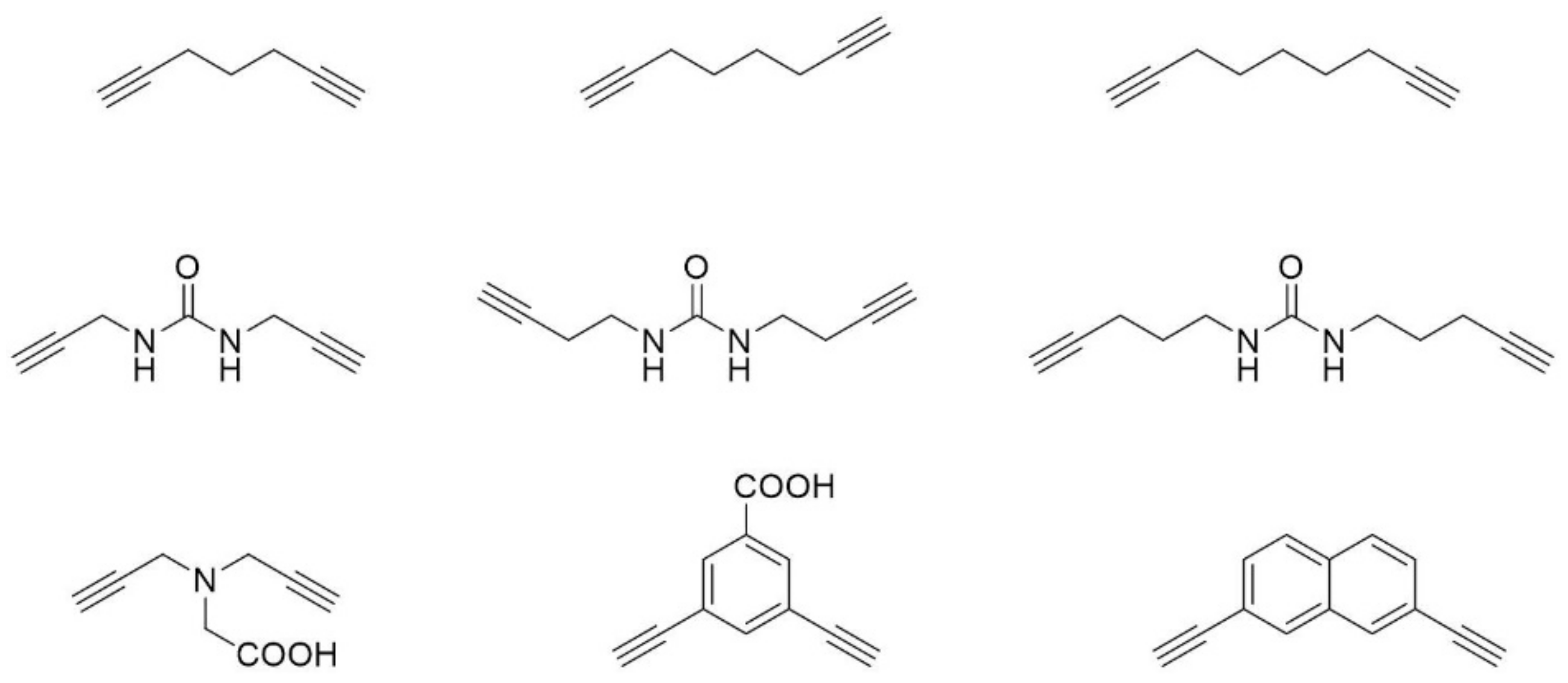
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robertson, N.S.; Spring, D.R. Using Peptidomimetics and Constrained Peptides as Valuable Tools for Inhibiting Protein–Protein Interactions. Molecules 2018, 23, 959. https://doi.org/10.3390/molecules23040959
Robertson NS, Spring DR. Using Peptidomimetics and Constrained Peptides as Valuable Tools for Inhibiting Protein–Protein Interactions. Molecules. 2018; 23(4):959. https://doi.org/10.3390/molecules23040959
Chicago/Turabian StyleRobertson, Naomi S., and David R. Spring. 2018. "Using Peptidomimetics and Constrained Peptides as Valuable Tools for Inhibiting Protein–Protein Interactions" Molecules 23, no. 4: 959. https://doi.org/10.3390/molecules23040959
APA StyleRobertson, N. S., & Spring, D. R. (2018). Using Peptidomimetics and Constrained Peptides as Valuable Tools for Inhibiting Protein–Protein Interactions. Molecules, 23(4), 959. https://doi.org/10.3390/molecules23040959




