Stryphnodendron Species Known as “Barbatimão”: A Comprehensive Report
Abstract
:1. Introduction
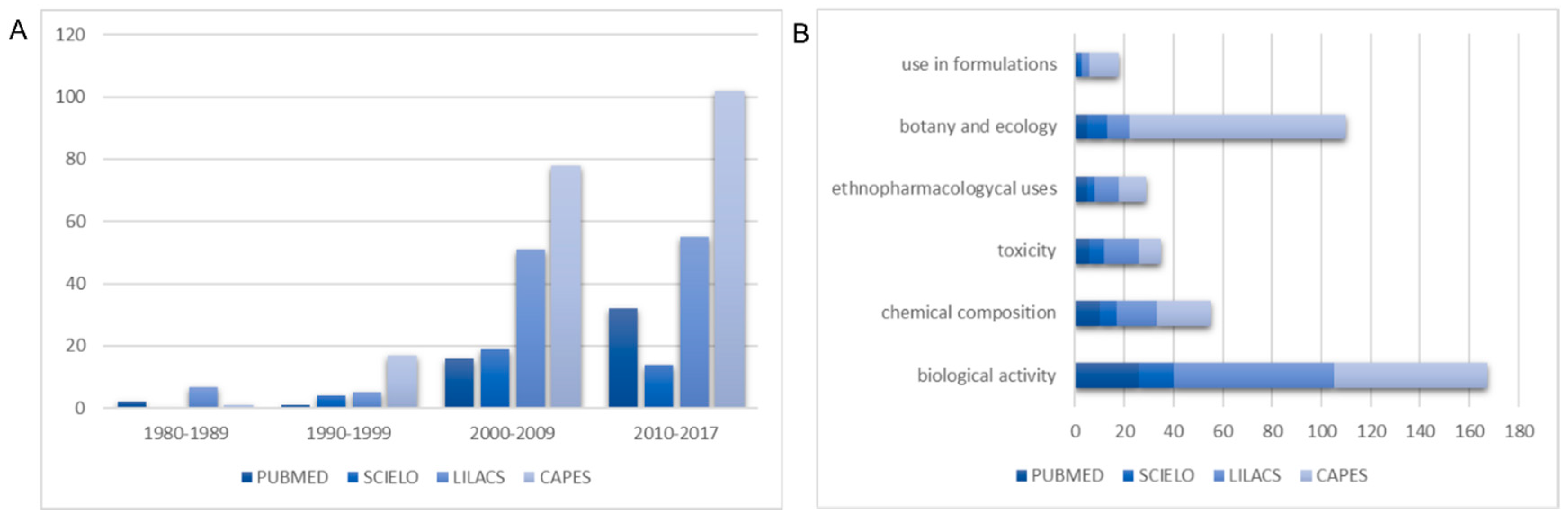
2. Data Collection Methodology
3. General Aspects on Literature about Stryphnodendron
4. Botanical Features and Sustainable Management Aspects
5. Ethnopharmacological Uses
6. Chemical Composition
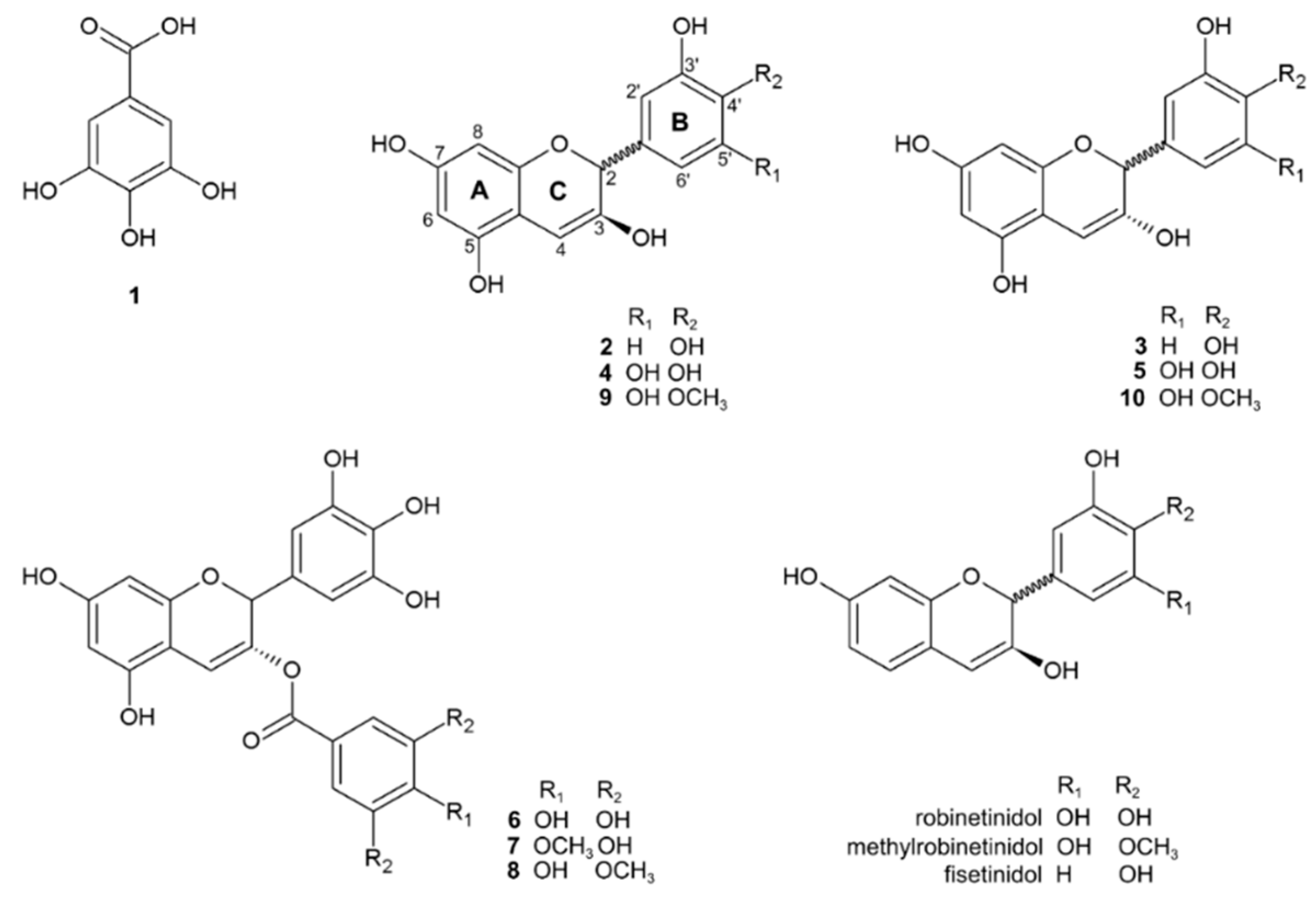
6.1. Metabolites Identified in Stryphnodendron Species
6.2. Extraction and Analysis of Tannin Metabolites of Stryphnodendron Species
7. Correlated Biological Activities
7.1. “Barbatimão” Bark Promotes Wound Healing
7.2. Anti-inflammatory Activity of “barbatimão” and Correlation to Antinociception
7.3. Antioxidant Property Might Mediate Claimed Anticancer Activity
7.4. Antimicrobial Activity Corroborates Oral and Genitourinary Use
7.5. Effect on Diabetes, Blood Pressure, and Diuresis
7.6. Anthelmintic Activity and Other Promising Activities
8. Cytotoxicity and Toxicology
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heinrich, M.; Heinrich, M. Ethnopharmacology: Quo vadis? Challenges for the future. Rev. Bras. Farmacogn. 2014, 24, 99–102. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, P.M.; Ferreira Júnior, W.S.; Ramos, M.A.; Silva, T.C.; Ladio, A.H.; Albuquerque, U.P. Why do people use exotic plants in their local medical systems? A systematic review based on Brazilian local communities. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Tomazzoni, M.I.; Negrelle, R.R.B.; Centa, M.L. Popular phytotherapy: The instrumental search as therapy. Texto Contexto Enferm. 2006, 15, 115–121. [Google Scholar] [CrossRef]
- Santos, R.L.; Guimaraes, G.P.; Nobre, M.S.C.; Portela, A.S. Analysis about phytotherapy as an integrating practice in the Brazilian Unified Health System (UHS). Rev. Bras. Plantas Med. 2011, 13, 486–491. [Google Scholar] [CrossRef]
- Nogueira, R.C.; Cerqueira, H.F.; Soares, M.B. Patenting bioactive molecules from biodiversity: The Brazilian experience. Expert Opin. Ther. Pat. 2010, 20, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Da Saúde, M.; De Ciência, S.; Estratégicos, T.I.; Estratégicos, F.I. Programa Nacional de Plantas Medicinais e Fitoterápicos; Ministério da Saúde: Brasília, Brazil, 2009; p. 136. ISBN 978-85-334-1597-3. [Google Scholar]
- Nascimento, M.W.A.; Veríssimo, R.C.S.S.; Bastos, M.L.A.; Bernardo, T.H.L. Medicinal plants indications from herbal healers for wound treatment. Rev. Eletrôn. Enferm. 2016, 18, 1–10. [Google Scholar]
- Passaretti, T.; Guarnieri, A.P.; Filipini, R.; Alves, B.C.A.; Fonseca, F.L.A. Effective use of barbatiman (Stryphnodendron barbatiman) in the healing process of lesions: A literature review. ABCS Health Sci. 2016, 41, 51–54. [Google Scholar]
- Riet-Correa, F.; Medeiros, R.M.T.; Schild, A.L. A review of poisonous plants that cause reproductive failure and malformations in the ruminants of Brazil. J. Appl. Toxicol. 2011, 32, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Bürger, M.E.; Ahlert, N.; Baldisserotto, B.; Langeloh, A.; Schirmer, B.; Foletto, R. Analysis of the abortive and/or infertilizing activity of Stryphnodendron adstringens (Mart. Coville). Braz. J. Vet. Res. Anim. Sci. 1999, 36. [Google Scholar] [CrossRef]
- Souza, V.C.; Lima, A.G. Flora Do Brasil—Stryphnodendron adstringens (Mart.) Coville. Available online: http://reflora.jbrj.gov.br/reflora/listaBrasil/FichaPublicaTaxonUC/FichaPublicaTaxonUC.do?id=FB19133 (accessed on 5 November 2017).
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas Do Brasil, 2nd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2000; p. 384. ISBN 978-85-8671-450-4. [Google Scholar]
- Occhioni, E.M.L. Considerações taxonômicas no gênero Stryphnodendron Mart. (Leguminosae-Mimosoideae) e distribuição geográfica das espécies. Acta Bot. Bras. 1990, 4, 153–158. [Google Scholar]
- Simon, M.F.; Pastore, J.F.B.; Souza, A.F.; Borges, L.M.; Scalon, V.R.; Ribeiro, P.G.; Santos-Silva, J.; Souza, V.C.; Queiroz, L.P. Molecular Phylogeny of Stryphnodendron (Mimosoideae, Leguminosae) and Generic Delimitations in the Piptadenia Group. Int. J. Plant Sci. 2015, 177, 44–59. [Google Scholar] [CrossRef]
- Gertsch, J. How scientific is the science in ethnopharmacology? Historical perspectives and epistemological problems. J. Ethnopharmacol. 2009, 122, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Agência Nacional de Vigilancia Sanitária. Farmacopeia Brasileira, 5th ed.; Agência Nacional de Vigilancia Sanitária: Brasília, Brazil, 2010. Available online: http://www.anvisa.gov.br/hotsite/cd_farmacopeia/index.htm (accessed on 5 November 2017).
- Agência Nacional de Vigilância Sanitária. Formulário de Fitoterápicos da Farmacopeia Brasileira; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2011; p. 126. Available online: http://www.anvisa.gov.br/hotsite/farmacopeiabrasileira/conteudo/Formulario_de_Fitoterapicos_da_Farmacopeia_Brasileira.pdf (accessed on 3 January 2018).
- Agência Nacional de Vigilância Sanitária. Memento Fitoterápico da Farmacopeia Brasileira; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2016; p. 115. Available online: http://portal.anvisa.gov.br/documents/33832/2909630/Memento+Fitoterapico/a80ec477-bb36-4ae0-b1d2-e2461217e06b (accessed on 3 January 2018).
- Scalon, V.R. Revisão Taxonômica de Stryphnodendron Mart. (Leguminosae-Mimosoideae). Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2007. [Google Scholar]
- Sanches, A.C.C.; Lopes, G.C.; Toledo, C.E.M.; Sacramento, L.V.S.; Sakuragui, C.M.; Mello, J.C.P. Estudo morfológico comparativo das cascas e folhas de Stryphnodendron adstringens, S. polyphyllum e S. obovatum—Leguminosae. Lat. Am. J. Pharm. 2007, 26, 362–368. [Google Scholar]
- Lima, L.C.P.; Garcia, F.C.P.; Sartori, Â.L.B. As Leguminosae arbóreas das florestas estacionais do Parque Estadual do Itacolomi, Minas Gerais, Brasil. Rodriguésia 2010, 61, 441–466. [Google Scholar] [CrossRef]
- Ortiz, P.L.; Arista, M.; Oliveira, P.E.; Talavera, S. Pattern of Flower and Fruit Production in Stryphnodendron adstringens, an Andromonoecious Legume Tree of Central Brazil. Plant Biol. 2003, 5, 592–599. [Google Scholar] [CrossRef]
- Bitu, V.C.N.; Matias, E.F.; Lima, W.P.; Costa Portelo, A.; Coutinho, H.D.; Menezes, I.R. Ethnopharmacological study of plants sold for therapeutic purposes in public markets in Northeast Brazil. J. Ethnopharmacol. 2015, 172, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Meira, M.R.; Nobre, D.A.C. Avaliação da qualidade de sementes de barbatimão oriundas de três locais no Norte de Minas Gerais. Rev. Ciênc. Agrár. 2014, 37, 50–58. [Google Scholar]
- Martins, C.C.; Camara, A.T.R.; Machado, C.G.; Nakagawa, J. Methods of breaking dormancy for seeds of Stryphnodendron. Acta Sci. Agron. 2008, 30, 381–385. [Google Scholar]
- Martins, C.C.; Martins, C.C.; Machado, C.G.; Nakagawa, J. Temperature and substrate for germination test of Stryphnodendron adstringens (Mart) Coville (Leguminosae). Rev. Árvore 2008, 32, 633–639. [Google Scholar] [CrossRef]
- Nicioli, P.M.; Paiva, R.; Nogueira, R.C.; Santana, J.R.F.; Silva, L.C.; Silva, D.P.C.; Porto, J.M.P. Adjustment of the process of micropropagation of Stryphnodendron adstringens (Mart.) Coville. Cienc. Rural 2008, 38, 685–689. [Google Scholar] [CrossRef]
- Castro, A.H.F.; Paiva, R.; Alvarenga, A.A.; Castro, E.M.; Vitor, S.M.M.; Fernandes, A.M. Cultivo in vitro e aspectos da anatomia foliar de barbatimão [Stryphnodendronadstringens (Mart.) Coville Fabaceae Papilionoideae]. Plant Cell Cult. Micropropag. 2007, 3, 61–68. [Google Scholar]
- Castro, A.H.F.; Paiva, R.; Alvarenga, A.A.; Vitor, S.M.M. Callogenesis and contents of total phenols and tannins in barbatimão [Stryphnodendron adsrtingens (Mart.) Coville]. Ciênc. Agrotec. 2009, 33, 385–390. [Google Scholar] [CrossRef]
- Castro, A.H.F.; Lima, M.M.; Paiva, R.; Alvarenga, A.A.; Sóter, M.O. Curva de crescimento, atividade da fenilalanina amônialiase e teores de fenóis e taninos totais em calos de Stryphnodendron adstringens (Mart.) Coville (Fabaceae-Mimosoideae). Plant Cell Cult. Micropropag. 2008, 4, 99–104. [Google Scholar]
- Glasenapp, J.S.; Martins, E.R.; Casali, V.W.D.; Cruz, C.D.; Barbosa, P.B. Characterization of diversity and genetic structure in natural populations of Stryphnodendron adstringens (Mart.) Coville by means of allozyme markers. Rev. Bras. Plantas Med. 2014, 16, 216–224. [Google Scholar] [CrossRef]
- Mendonça, P.; Bertoni, B.W.; Amui, S.F.; Giuliatti, S.; Corrêa, V.S.C.; França, S.C.; Pereira, A.N.S. Genetic diversity of Stryphnodendron adstringens (Mart.) Coville determined by AFLP molecular markers. Biochem. Syst. Ecol. 2017, 41, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Meira, M.R.; Cabacinha, C.D. Sustainable Management of Barbatimão in the Northern Minas Gerais State. Floresta Ambient 2016, 23, 61–69. [Google Scholar] [CrossRef]
- Borges Filho, H.C.; Felfili, J.M. Evaluation of exploitation levels of barbatimão bark [Stryphnodendron adstringens (Mart.) Coville] in Distrito Federal, Brazil. Rev. Árvore 2003, 27, 735–745. [Google Scholar]
- Feitosa, I.S.; Albuquerque, U.P.; Monteiro, J.M. Knowledge and extractivism of Stryphnodendron rotundifolium Mart. in a local community of the Brazilian Savanna, Northeastern Brazil. J. Ethnobiol. Ethnomed. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, I.S.; Sobral, A.; Monteiro, J.M.; Araujo, E.L.; Albuquerque, U.P. Impact of collection on bark regeneration from Stryphnodendron rotundifolium Mart. in northeastern Brazil. Environ. Monit. Assess. 2017, 189. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.V.; Sebastiani, R. Medicinal plants used for university students in São Paulo City, São Paulo State. J. Health Sci. Inst. 2011, 29, 11–15. [Google Scholar]
- Santana, B.F.; Voeks, R.A.; Funch, L.S. Ethnomedicinal survey of a maroon community in Brazil’s Atlantic tropical forest. J. Ethnopharmacol. 2016, 181, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.A.; Oliveira, L.G.; Macedo, D.G.; Menezes, I.R.; Costa, J.G.; Silva, M.A.; Lacerda, S.R.; Souza, M.M. Promising medicinal plants for bioprospection in a Cerrado area of Chapada do Araripe, Northeastern Brazil. J. Ethnopharmacol. 2014, 155, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.M.; Moreira, R.R.D.; Pietro, R.C.L.R.; Isaac, V.L.B. Avaliação da atividade anti-séptica de extrato seco de Stryphnodendron adstringens (Mart.) Coville e de preparação cosmética contendo este extrato. Rev. Bras. Farmacogn. 2007, 17, 71–75. [Google Scholar] [CrossRef]
- Ishida, K.; Mello, J.C.; Cortez, D.A.; Dias Filho, B.P.; Ueda-Nakamura, T.; Nakamura, C.V. Influence of tannins from Stryphnodendron adstringens on growth and virulence factors of Candida albicans. J. Antimicrob. Chemother. 2006, 58, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Nunes, G.P.; Silva, M.F.; Resende, U.M.; Siqueira, J.M. Medicinal plants from herb sellers operating in downtown Campo Grande, Mato Grosso do Sul, Brazil. Rev. Bras. Farmacogn. 2003, 13, 83–92. [Google Scholar] [CrossRef]
- Macedo, M.; Ferreira, A.R. Plantas medicinais usadas para tratamentos dermatológicos, em comunidades da Bacia do Alto Paraguai, Mato Grosso. Rev. Bras. Farmacogn. 2004, 14, 40–44. [Google Scholar] [CrossRef]
- Botelho, N.M.; Brito, N.B.; Silva, N.M. The use of medicinal plants by canal da Visconde community. Rev. Para. Med. 2014, 28, 63–69. [Google Scholar]
- Fenner, R.; Betti, A.H.; Mentz, L.A.; Rates, S.M.K. Plants with potencial antifungal activity employed in Brazilian folk medicine. Rev. Bras. Cienc. Farm. 2006, 42, 369–394. [Google Scholar] [CrossRef]
- Souza, C.M.P.; Brandão, D.O.; Silva, M.S.P.; Palmeira, A.C.; Simões, M.O.S.; Medeiros, A.C.D. Use of medicinal plants with antimicrobial activity by users of the Public Health System in Campina Grande—Paraíba, Brazil. Rev. Bras. Plantas Med. 2013, 15, 188–193. [Google Scholar] [CrossRef]
- Souza, D.R.; Rodrigues, E.C.A.M.S. Medicinal plants: Traditional healers’ indications for the treatment of wounds. Rev. Bras. Promoç. Saúde 2016, 29, 197–203. [Google Scholar]
- Ustulin, M.; Figueiredo, B.B.; Tremea, C.; Pott, A.; Pott, V.J.; Bueno, N.R.; Castilho, R.O. Commercialized medicinal plants in the Mercado Municipal of Campo Grande-MS. Rev. Bras. Farmacogn. 2009, 19, 805–813. [Google Scholar] [CrossRef]
- Oliveira, D.R.; Ferreira Júnior, W.S.; Bitu, V.C.N.; Pinheiro, P.G.; Menezes, C.D.; Almino Brito Junior, F.E.; Albuquerque, U.P.; Kerntopf, M.R.; Coutinho, H.D.M.; Fachinetto, R.; et al. Ethnopharmacological study of Stryphnodendron rotundifolium in two communities in the semi-arid region of northeastern Brazil. Rev. Bras. Farmacogn. 2014, 24, 124–132. [Google Scholar] [CrossRef]
- Oliveira, D.R.; Brito Júnior, F.E.; Sampaio, L.A.; Torres, J.C.; Ramos, A.G.B.; Nunes, A.A. Ethnopharmacological usage of medicinal plants in genitourinary infections by residents of Chapada do Araripe, Crato, Ceará—Brazil. Rev. Bras. Promoç. Saúde 2012, 25, 278–286. [Google Scholar]
- Melo, N.D.P.; Ribeiro, S.C.; Barros, A.B. Etnoconhecimento de pequenos agricultores tradicionais sobre plantas medicinais no tratamento de dores provocadas pelo trabalho. Braz. J. Occup. Ther. 2016, 24, 563–574. [Google Scholar]
- Figueredo, C.A.; Gurgel, I.G.D.; Gurgel Junior, G.D. The National Policy on Medicinal Plants and Phytotherapy: Building, perspectives and challenges. Physis 2014, 24, 381–400. [Google Scholar] [CrossRef]
- Lopes, G.C.; Machado, F.A.V.; Toledo, C.E.M.; Sakuragui, C.M.; Mello, J.C.P. Chemotaxonomic significance of 5-deoxyproanthocyanidins in Stryphnodendron species. Biochem. Syst. Ecol. 2009, 36, 925–931. [Google Scholar] [CrossRef]
- Lopes, G.C.; Sanches, A.C.C.; Toledo, C.E.M.; Isler, A.C.; Mello, J.C.P. Determinação quantitativa de taninos em três espéciesde Stryphnodendron por cromatografia líquida de alta eficiência. Braz. J. Pharm. Sci. 2009, 45, 135–143. [Google Scholar] [CrossRef]
- Nascimento, A.M.; Guedes, P.T.; Castilho, R.O.; Vianna-Soares, C.D. Stryphnodendron adstringens (Mart.) Coville (Fabaceae) proanthocyanidins quantitation by RP-HPLC. Braz. J. Pharm. Sci. 2013, 49, 549–558. [Google Scholar] [CrossRef]
- Santos, S.C.; Costa, W.F.; Batista, F.; Santos, L.R.; Ferri, P.H.; Ferreira, H.D.; Seraphin, J.C. Seasonal variation in the content of tannins in barks of barbatimão species. Rev. Bras. Farmacogn. 2006, 16, 552–556. [Google Scholar] [CrossRef]
- Jacobson, T.K.B.; Garcia, J.; Santos, S.C.; Duarte, J.B.; Farias, J.G.; Kliemann, H.J. Influência de fatores edáficos na produçãode fenóis totais e taninos de duas espécies debarbatimão (Stryphnodendron sp.). Pesqui. Agropecu. Trop. 2005, 35, 163–169. [Google Scholar]
- Sabino, A.P.L.; Eustáquio, L.M.S.; Miranda, A.C.F.; Biojone, C.; Mariosa, T.N.; Gouvêa, C.M.C.P. Stryphnodendron adstringens (“Barbatimão”) Leaf Fraction: Chemical Characterization, Antioxidant Activity, and Cytotoxicity Towards Human Breast Cancer Cell Lines. Appl. Biochem. Biotechnol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.G.M.; Leite, G.O.; Dubois, A.F.; Seeger, R.L.; Boligon, A.A.; Athayde, M.L.; Campos, A.R.; Rocha, J.B.T. Antioxidant Effect of Stryphnodendron rotundifolium Martius Extracts from Cariri-Ceará State (Brazil): Potential Involvement in Its Therapeutic Use. Molecules 2012, 17, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.N.; Pedroso, N.B.; Borges, L.L.; Oliveira, G.A.; Paula, J.R.; Conceicao, E.C. Optimization of Ultrasound-assisted extraction of polyphenols, tannins and epigallocatechin gallate from barks of Stryphnodendron adstringens (Mart.) Coville bark extracts. Pharmacogn. Mag. 2014, 10, S318–S323. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.C.; Costa, W.F.; Ribeiro, J.P.; Guimaraes, D.O.; Ferri, P.H.; Ferreira, H.D.; Seraphin, J.C. Tannin composition of barbatimao species. Fitoterapia 2002, 73, 292–299. [Google Scholar] [CrossRef]
- Ardisson, L.; Godoy, J.S.; Ferreira, L.A.M.; Stehmann, J.R.; Brandão, M.G.L. Preparation and characterization of propylene glicol extracts obtained from dried stem barks of Stryphnodendron adstringens (Mart.) Coville (barbatimão). Rev. Bras. Farmacogn. 2002, 12, 27–34. [Google Scholar]
- Henriques, B.O.; Corrêa, O.; Azevedo, E.P.C.; Pádua, R.M.; Oliveira, V.L.S.; Oliveira, T.H.C.; Boff, D.; Dias, A.C.F.; Souza, D.G.; Amaral, F.A.; et al. In Vitro TNF-α Inhibitory Activity of Brazilian Plants and Anti-Inflammatory Effect of Stryphnodendron adstringens in an Acute Arthritis Model. Evid. Based Complement. Altern. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.G.; Nascimento, A.M.; Henriques, B.O.; Chavez-Fumagalli, M.A.; Franca, J.R.; Duarte, M.C.; Lage, P.S.; Andrade, P.H.; Lage, D.P.; Rodrigues, L.B.; et al. Antileishmanial activity of standardized fractions of Stryphnodendron obovatum (Barbatimao) extract and constituent compounds. J. Ethnopharmacol. 2015, 165, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.C.; Bueno, F.G.; Panizzon, G.P.; Morais, G.; Santos, P.V.; Baesso, M.L.; Leite-Mello, E.V.; Mello, J.C. Stryphnodendron adstringens: Clarifying Wound Healing in Streptozotocin-Induced Diabetic Rats. Planta Med. 2015, 81, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Felipe, A.M.; Rincao, V.P.; Benati, F.J.; Linhares, R.E.; Galina, K.J.; Toledo, C.E.; Lopes, G.C.; Mello, J.C.; Nozawa, C. Antiviral effect of Guazuma ulmifolia and Stryphnodendron adstringens on poliovirus and bovine herpesvirus. Biol. Pharm. Bull. 2006, 29, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Mello, J.C.P.; Petereit, F.; Nahrstedt, A. Flavan-3-ols and prodelphinidins from Stryphnodendron adstringens. Phytochemistry 1996, 41, 807–813. [Google Scholar] [CrossRef]
- Mello, J.C.P.; Petereit, F.; Nahrstedt, A. Prorobinetinidins from Stryphnodendron adstringens. Phytochemistry 1996, 42, 857–862. [Google Scholar] [CrossRef]
- Mello, J.C.P.; Petereit, F.; Nahrstedt, A. A dimeric proanthocyanidin from Stryphnodendron adstringens. Phytochemistry 1999, 51, 1105–1107. [Google Scholar]
- Oliveira, A.L.S.; Figueiredo, A.D.L. Prospecção Fitoquímica das Folhas de Stryphnodendron adstringens (Mart.) Coville (Leguminosae-Mimosoidae). Rev. Bras. Biocienc. 2007, 5, 384–386. [Google Scholar]
- Salvalaggio Mde, O.; Freitas, R.A.; Franquetto, E.M.; Koop, H.S.; Silveira, J.L. Influence of the extraction time on macromolecular parameters of galactomannans. Carbohydr. Polym. 2015, 116, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Simon, J.; Kitunen, V.; Adamczyk, S.; Smolander, A. Tannins and Their Complex Interaction with Different Organic Nitrogen Compounds and Enzymes: Old Paradigms versus Recent Advances. Chem. Open 2017, 6, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Panizza, S.; Rocha, A.B.; Gecchi, R.; Souza e Silva, R.A.P. Stryphnodendron barbadetiman (Vellozo) Martius: Teor em Tannino na casca e sua propriedade cicatrizante. Rev. Ciênc. Farm. 1988, 10, 101–106. [Google Scholar]
- Silva, C.; Vilella, H. Ação do Stryphnodendron barbadetiman sobre a cicatrização: Estudo experimental em ratos. HB Cient 1996, 3, 77–79. [Google Scholar]
- Jorge Neto, J.; Fracasso, J.F.; Camargo Neves, C.L.; Santos, L.E.; Banuth, V.L. Tratamento de ulcera varicosa e lesoes de pele com Calendula officinalis L. e/ou com Stryphnodendron barbadetiman (Vellozo) Martius. Rev. Cienc. Farm. 1996, 17, 181–186. [Google Scholar]
- Eurides, D.; Mazzanti, A.; Belleti, M.E.; Silva, L.A.F.; Fioravante, M.C.S.; Troncoso Neto, N.S.; Campos, V.A.; Lemos, R.C.; Silvestrini Junior, P.L. Morfologia e Morfometria da Reparação Tecidual de Feridas Cutâneas de Camundongos Tratadas com Solução Aquosa de Barbatimão (Stryphynodendron barbatiman Martius). Revista da Faculdade de Zootecnia Veterinária e Agronomia 1995/1996, 2/3, 30–40. [Google Scholar]
- Coelho, J.M.; Antoniolli, A.B.; Nunes e Silva, D.; Carvalho, T.M.; Pontes, E.R.; Odashiro, A.N. Effects of silver sulfadiazine, ipe roxo (Tabebuia avellanedae) extract and barbatimao (Stryphnodendron adstringens) extract on cutaneous wound healing in rats. Rev. Col. Bras. Cir. 2010, 37, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chaves, D.A.; Lemes, S.R.; Araujo, L.A.; Sousa, M.A.M.; Freitas, G.B.; Lino-Junior, R.S.; Mrue, F.; Melo-Reis, P.R. Angiogenic activity of the aqueous solution of Barbatimão (Stryphnodendron adstringens). Rev. Bras. Plantas Med. 2016, 18, 524–530. [Google Scholar] [CrossRef]
- Bauer, S.M.; Bauer, R.J.; Velazquez, O.C. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc. Endovasc. Surg. 2005, 39, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Minatel, D.G.; Pereira, A.M.S.; Chiaratti, T.M.; Pasqualin, L.; Oliveira, J.C.N.; Couto, L.B.; Lia, R.C.C.; Cintra, J.M.; Bezzon, M.F.A.; Franca, S.C. Estudo clínico para validação da eficácia de pomada contendo barbatimão (Stryphnodendron adstringens (Mart.) Coville) na cicatrização de ulceras de decúbito. Rev. Bras. Med. 2010, 67, 250–256. [Google Scholar]
- Franca, S.D.C.; Oliveira, J.C.N.; Pasqualin, L.; Couto, L.B.; Lia, R.C.C. Composition for Topic Use Containing an Extract of Stryphnodendron, Its Preparation as Well as Its Application. Eur. Pat. CN1878560 B, 29 December 2010. [Google Scholar]
- Hernandes, L.; Pereira, L.M.S.; Palazzo, F.; Mello, J.C.P. Wound-healing evaluation of ointment from Stryphnodendron adstringens (barbatimão) in rat skin. Braz. J. Pharm. Sci. 2010, 46, 431–436. [Google Scholar] [CrossRef]
- Rodrigues, D.F.; Mendes, F.F.; Menezes, L.B.; Carvalho, W.L.; Sá, S.; Silva, J.A.; Souza, L.A.; Silva, L.A.F. Treatment of excisional wound in rabbits with barbatiman extracts associated with autologous bone marrow mononuclear cells. Arq. Bras. Med. Vet. Zootec. 2017, 69, 1243–1250. [Google Scholar] [CrossRef]
- Kim, H.L.; Lee, J.H.; Kwon, B.J.; Lee, M.H.; Han, D.W.; Hyon, S.H.; Park, J.C. Promotion of full-thickness wound healing using epigallocatechin-3-O-gallate/poly (lactic-co-glycolic acid) membrane as temporary wound dressing. Artif. Organs 2014, 38, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kawazoe, T.; Han, D.W.; Matsumara, K.; Suzuki, S.; Tsutsumi, S.; Hyon, S.H. Enhanced wound healing by an epigallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Repair Regen. 2008, 16, 714–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, G.C.; Sanches, A.C.C.; Nakamura, C.V.; Dias Filho, B.P.; Hernandes, L.; Mello, J.C.P. Influence of extracts of Stryphnodendron polyphyllum Mart. and Stryphnodendron obovatum Benth. on the cicatrisation of cutaneous wounds in rats. J. Ethnopharmacol. 2005, 99, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Audi, E.A.; Toledo, D.P.; Peres, P.G.; Kimura, E.; Pereira, W.K.; Mello, J.C.P.; Nakamura, C.; Alves-do-Prado, W.; Cuman, R.K.; Bersani-Amado, C.A. Gastric antiulcerogenic effects of Stryphnodendron adstringens in rats. Phytother. Res. 1999, 13, 264–266. [Google Scholar] [CrossRef]
- Martins, D.T.; Lima, J.C.; Rao, V.S. The acetone soluble fraction from bark extract of Stryphnodendron adstringens (Mart.) Coville inhibits gastric acid secretion andexperimental gastric ulceration in rats. Phytother. Res. 2002, 16, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Muramatsu, M.; Otomo, S. Inhibitory effect of tannic acid on gastric H+, K(+)-ATPase. J. Nat. Prod. 1992, 55, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.C.; Kushima, H.; Andreo, M.; Hiruma-Lima, C.A.; Vilegas, W.; Takahira, R.K.; Pellizzon, C.H. Studies of gastric mucosa regeneration and safety promoted by Mouriri pusa treatment in acetic acid ulcer model. J. Ethnopharmacol. 2008, 115, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Jesus, N.Z.; Souza Falcao, H.; Gomes, I.F.; Almeida Leite, T.J.; Morais Lima, G.R.; Barbosa-Filho, J.M.; Tavares, J.F.; da Silva, M.S.; Athayde-Filho, P.F.; Batista, L.M. Tannins, peptic ulcers and related mechanisms. Int. J. Mol. Sci. 2012, 13, 3203–3228. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.C.S.; Martins, D.T.O.; Souza, P.T. Experimental evaluation of stem bark of Stryphnodendron adstringens (Mart.) Coville for antiinflammatory activity. Phytother Res. 1998, 12, 218–220. [Google Scholar] [CrossRef]
- Coutinho, H.; Pinto, D.S.; Ribeiro, J.E.G.; Friedman, H. Anti-edema action of the Stryphnodendron barbadetiman (Barbatimão) 1 per cent in comparison with clorhexidin 0, 12 per cent. Rev. Odonto Ciênc. 2004, 19, 201–206. [Google Scholar]
- Wang, T.; Zhou, H.; Xie, H.; Mu, Y.; Xu, Y.; Liu, J.; Zhang, X. Epigallocatechin-3-gallate inhibits TF and TNF-α expression induced by the anti-β2GPI/β2GPI complex in human THP-1 cells. Int. J. Mol. Med. 2014, 33, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Crouvezier, S.; Powell, B.; Keir, D.; Yaqoob, P. The effects of phenolic components of tea on the production of pro- and anti-inflammatory cytokines by human leukocytes in vitro. Cytokine 2001, 13, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, Y.; Suzuki, T.; Mochizuki, K.; Goda, T. Dietary Supplementation with a Low Dose of (−)-Epigallocatechin-3-Gallate Reduces Pro-Inflammatory Responses in Peripheral Leukocytes of Non-Obese Type 2 Diabetic GK Rats. J. Nutr. Sci. Vitaminol. 2013, 59, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Melo, J.O.; Endo, T.H.; Bersani-Amado, L.E.; Svidzinski, A.E.; Baroni, S.; Mello, J.C.P.; Bersani-Amado, C.A. Effect of Stryphnodendron adstringens (barbatimão) bark on animal models of nociception. Rev. Bras. Cienc. Farm. 2007, 43, 465–469. [Google Scholar] [CrossRef]
- Amico-Roxas, M.; Caruso, A.; Trombadore, S.; Scifo, R.; Scapagnini, U. Gangliosides antinociceptive effects in rodents. Arch. Int. Pharmacodyn. Ther. 1984, 272, 103–117. [Google Scholar] [PubMed]
- Tjolsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Coderre, T.J.; Melzack, R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J. Neurosci. 1992, 12, 3665–3670. [Google Scholar] [PubMed]
- Souza, T.M.; Severi, J.A.; Silva, V.Y.A.; Santos, E.; Pietro, R.C.L.R. Bioprospecção de atividade antioxidante e antimicrobiana da casca de Stryphnodendron adstringens (Mart.) Coville (Leguminosae-Mimosoidae). Rev. Ciênc. Farm. Básica Apl. 2009, 28, 221–226. [Google Scholar]
- Sanches, A.C.C.; Lopes, G.C.; Nakamura, C.V.; Dias Filho, B.P.; Mello, J.C.P. Antioxidant and antifungal activities of extracts and condensed tannins from Stryphnodendron obovatum Benth. Rev. Bras. Cienc. Farm. 2005, 41, 101–107. [Google Scholar] [CrossRef]
- Min, K.J.; Kwon, T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr. Med. Res. 2014, 3, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Losada-Echeberria, M.; Herranz-Lopez, M.; Micol, V.; Barrajon-Catalan, E. Polyphenols as Promising Drugs against Main Breast Cancer Signatures. Antioxidants (Basel) 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.E.M.M.; Dias, A.P.M.; Capriles, P.V.S.Z.; Oliveira, M.B.N.; Amorim, E.L.C.; Lima, C.S.A.; Bernardo-Filho, M. Effect of barbatimão [Stryphnodendron adstringens (Mart.) Coville] infusion on the labling of blood elements with technetium-99m. Rev. Bras. Farmacogn. 2002, 12, 7–9. [Google Scholar] [CrossRef]
- Audi, E.A.; Toledo, C.E.M.; Santos, F.S.; Bellanda, P.R.; Alves-Do-Prado, W.; Ueda-Nakamura, T.; Nakamura, C.V.; Sakuragui, C.M.; Bersani-Amado, C.A.; Mello, J.C.P. Biological Activity and Quality Control of Extractand Stem Bark From Stryphnodendron adstringens. Acta Farm. Bonaerense 2004, 23, 328–333. [Google Scholar]
- Pinho, L.; Souza, P.N.S.; Macedo Sobrinho, E.; Almeida, A.C.; Martins, E.R. Antimicrobial activity of hydroalcoholic extracts from rosemary, peppertree, barbatimão and erva baleeira leaves and from pequi peel meal. Cienc. Rural 2012, 42, 326–331. [Google Scholar] [CrossRef]
- Eller, S.; Feitosa, V.A.; Arruda, T.A.; Antunes, R.M.P.; Catão, R.M.R. Interactive study of the antimicrobial activity of plant extracts. Rev. Ciênc. Farm. Básica Apl. 2015, 36, 131–136. [Google Scholar]
- Soares, S.P.; Vinholis, A.H.C.; Casemiro, L.A.; Silva, M.L.A.; Cunha, W.R.; Martins, C.H.G. Antibacterial activity of the crude hydroalcoholic extract of Stryphnodendron adstringens on dental caries microorganisms. Rev. Odonto Ciênc. 2008, 23, 141–144. [Google Scholar]
- Pereira, E.M.; Gomes, R.T.; Freire, N.R.; Aguiar, E.G.; Brandao, M.; Santos, V.R. In vitro antimicrobial activity of Brazilian medicinal plant extracts against pathogenic microorganisms of interest to dentistry. Planta Med. 2011, 77, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.R.; Castro, V.C.; Gracas Figueiredo Vilela, P.; Camargo, S.E.; Carvalho, C.A.; Jorge, A.O.; Oliveira, L.D. Cytotoxicity of Brazilian plant extracts against oral microorganisms of interest to dentistry. BMC Complement. Altern. Med. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.G.; Prince, K.A.; Higuchi, C.T.; Santos, A.C.B.; Lopes, L.M.X.; Simões, M.J.S.; Leite, C.Q.F. Antimycobacterial activity of some Brazilian indigenous medicinal drinks. Rev. Ciênc. Farm. Básica Apl. 2007, 28, 165–169. [Google Scholar]
- Morey, A.T.; Souza, F.C.; Santos, J.P.; Pereira, C.A.; Cardoso, J.D.; Almeida, R.S.; Costa, M.A.; Mello, J.C.P.; Nakamura, C.V.; Pinge-Filho, P.; et al. Antifungal Activity of Condensed Tannins from Stryphnodendron adstringens: Effect on Candida tropicalis Growth and Adhesion Properties. Curr. Pharm. Biotechnol. 2016, 17, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Rozental, S.; Mello, J.C.P.; Nakamura, C.V. Activity of tannins from Stryphnodendron adstringens on Cryptococcus neoformans: Effects on growth, capsule size and pigmentation. Ann. Clin. Microbiol. Antimicrob. 2009, 8. [Google Scholar] [CrossRef] [PubMed]
- Melo e Silva, F.; Paula, J.E.; Espindola, L.S. Evaluation of the antifungal potential of Brazilian Cerrado medicinal plants. Mycoses 2009, 52, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Trolezi, R.; Azanha, J.M.; Paschoal, N.R.; Chechi, J.L.; Dias Silva, M.J.; Fabris, V.E.; Vilegas, W.; Kaneno, R.; Fernandes Junior, A.; Bosco, S.M. Stryphnodendron adstringens and purified tannin on Pythium insidiosum: In vitro and in vivo studies. Ann. Clin. Microbiol. Antimicrob. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.R.; Brito, F.E., Jr.; Bento, E.B.; Matias, E.F.; Sousa, A.C.; Costa, J.G.; Coutinho, H.D.; Kerntopf, M.R.; Menezes, I.R. Antibacterial and modulatory effect of Stryphnodendron rotundifolium. Pharm. Biol. 2011, 49, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.C.A.; Rodovalho, N.C.M.; Pott, A.; Pott, V.J.; Ferreira, A.M.T.; Arruda, A.L.A.; Marques, M.C.S.; Castilho, R.O.; Bueno, N.R. Avaliação de atividades biológicas das sementes de Stryphnodendron obovatum Benth. (Leguminosae). Rev. Bras. Farmacogn. 2004, 14, 121–127. [Google Scholar] [CrossRef]
- Mayer, R.; Stecher, G.; Wuerzner, R.; Silva, R.C.; Sultana, T.; Trojer, L.; Feuerstein, I.; Krieg, C.; Abel, G.; Popp, M.; et al. Proanthocyanidins: Target Compounds as Antibacterial Agents. J. Agric. Food Chem. 2008, 56, 6959–6966. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ishiyama, K.; Sheng, H.; Ikai, H.; Kanno, T.; Niwano, Y. Bactericidal Activity and Mechanism of Photoirradiated Polyphenols against Gram-Positive and -Negative Bacteria. J. Agric. Food. Chem. 2015, 63, 7707–7713. [Google Scholar] [CrossRef] [PubMed]
- Glehn, E.A.V.; Rodrigues, G.P.S. Etest to confirm the action potential of plant hydroglycol extracts on Candida sp. (Berkhout). Rev. Bras. Plantas Med. 2012, 14, 435–438. [Google Scholar] [CrossRef]
- Oliveira, J.R.; Vilela, P.G.G.; Oliveira, F.E.; Belato, K.K.; Carvalho, C.A.T.; Jorge, A.O.C.; Oliveira, L.D. Antifungal effect of plant extracts on Candida albicans biofilm on acrylic resin. Braz. Dent. Sci. 2013, 16. [Google Scholar] [CrossRef]
- Luiz, R.L.; Vila, T.V.; Mello, J.C.P.; Nakamura, C.V.; Rozental, S.; Ishida, K. Proanthocyanidins polymeric tannin from Stryphnodendron adstringens are active against Candida albicans biofilms. BMC Complement. Altern. Med. 2015, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uppuluri, P.; Chaturvedi, A.K.; Srinivasan, A.; Banerjee, M.; Ramasubramaniam, A.K.; Kohler, J.R.; Kadosh, D.; Lopez-Ribot, J.L. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.; Sobel, J.D. Candida vulvovaginitis: A store with a buttery and a show window. Mycoses 2017, 60, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, C.; Bouchara, J.P.; Mignon, B. Updates on the epidemiology of dermatophyte infections. Mycopathologia 2008, 166, 335–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Heitman, J. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 2006, 60, 69–105. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.M.; Sales, P.M.; Simeoni, L.A.; Silva, E.C.; Silveira, D.; Magalhaes, P.O. Inhibitory activity of alpha-amylase and alpha-glucosidase by plant extracts from the Brazilian cerrado. Planta Med. 2012, 78, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Keske, M.A.; Ng, H.L.; Premilovac, D.; Rattigan, S.; Kim, J.A.; Munir, K.; Yang, P.; Quon, M.J. Vascular and Metabolic Actions of the Green Tea Polyphenol Epigallocatechin Gallate. Curr. Med. Chem. 2015, 22, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Silva Neto, C.R.; Lopes, R.A.; Contrera, M.G.D.; Pozetti, G.L. Efeitos antagonicos de plantas medicinais na diurese do rato. Pesqui. Homeopat. 1987, 4, 17–21. [Google Scholar]
- Silva Neto, C.R.; Lopes, R.A.; Rossi, E.; Contrera, M.G.D. Excrecao renal de agua, sodio e potassio em animais submetidos a sobrecarga aquosa de barbatimao: Stryphodendron obovatum. Pesqui. Homeopat. 1988, 5, 9–20. [Google Scholar]
- Vinaud, M.C.; Santos, S.C.; Ferri, P.H.; Lino Júnior, R.S.; Bezerra, J.C.B. Avaliação da atividade larvicida de plantas fitoterápicas do cerrado do gênero Stryphnodendron spp. sobre miracídios e cercárias de Schistosoma mansoni. Rev. Patol. Trop. 2005, 34, 137–143. [Google Scholar]
- Mendes, N.M.; Pereira, J.P.; Souza, C.P.; Oliveira, M.L.L. Ensaios preliminares em laboratório para verificar a ação moluscicida de algumas espécies da flora brasileira. Rev. Saúde Pública 1984, 18, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.C.; Silva, I.A.; Ferreira, H.D.; Ferri, P.H.; Santos, S.C. Molluscicidal activity against Biomphalaria glabrata of Brazilian Cerrado medicinal plants. Fitoterapia 2002, 73, 428–430. [Google Scholar] [CrossRef]
- Vinaud, M.C.; Lino Junior, R.S.; Bezerra, J.C.B. Activity of Stryphnodendron polyphyllum, a plant from the brazilian savannah, against hemocytes of Biomphalaria glabrata, an intermediate host of Schistosoma mansoni. J. Trop. Pathol. 2008, 37, 237–246. [Google Scholar]
- Holetz, F.B.; Ueda-Nakamura, T.; Dias Filho, B.P.; Cortez, D.A.G.; Mello, J.C.P.; Nakamura, C.V. Effect of plant extracts used in folk medicine on cell growth and differentiation of Herpetomonas samuelpessoai (Kinetoplastida, Trypanosomatidae) cultivated in defined medium. Acta Sci. 2002, 24, 657–662. [Google Scholar]
- Holetz, F.B.; Ueda-Nakamura, T.; Dias Filho, B.P.; Mello, J.C.P.; Morgado-Diaz, J.A.; Toledo, C.E.; Nakamura, C.V. Biological effects of extracts obtained from Stryphnodendron adstringens on Herpetomonas samuelpessoai. Mem. Inst. Oswaldo Cruz 2005, 100, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Luize, P.S.; Tiuman, T.S.; Morello, L.G.; Maza, P.K.; Ueda-Nakamura, T.; Dias Filho, B.P.; Cortez, D.A.G.; Mello, J.C.P.; Nakamura, C.V. Effects of medicinal plant extracts on growth of Leishmania (L.) amazonensis and Trypanosoma cruzi. Rev. Bras. Cienc. Farm. 2005, 41, 85–94. [Google Scholar] [CrossRef]
- Herzog-Soares, J.D.A.; Alves, R.K.; Isac, E.; Bezerra, J.C.B.; Gomes, M.H.; Santos, S.C.; Ferri, P.H. Atividade tripanocida in vivo de Stryphnodendron adstringens (barbatimão verdadeiro) e Caryocar brasiliensis (pequi). Rev. Bras. Farmacogn. 2002, 12, 1–2. [Google Scholar] [CrossRef]
- Herzog-Soares, J.D.A.; Isac, E.; Castro, A.M.; Bezerra, J.C.B. Bioactivity of Stryphnodendron adstringens, S. polyphyllum, Caryocar brasiliense, plants from brazilian’s savannah on the Trypanosoma cruzi in vivo. Biosci. J. 2006, 22, 113–118. [Google Scholar]
- Vandesmet, V.C.S.; Felipe, C.F.B.; Kerntopf, M.R.; Rolon, M.; Vega, C.; Coronel, C.; Barbosa, A.G.R.; Coutinho, H.D.M.; Menezes, I.R.A. The use of herbs against neglected diseases: Evaluation of in vitro leishmanicidal and trypanocidal activity of Stryphnodendron rotundifolium Mart. Saudi J. Biol. Sci. 2017, 24, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Lins Neto, M.A.F.; Caetano, L.C.; Peixoto Neto, P.A.S.; Silva, Z.P. Pharmaceutical Composition Using Stryphnodendron Extracts for Treating HPV Infections. U.S. Patent 9023405 B2, 5 May 2015. [Google Scholar]
- Lucena, M.N.; Mendes, M.M.; Brandeburgo, M.I.H. Avaliação da Estabilidade da Pomada à Base de Stryphnodendron adstringens (Mart.) Conville e Sua Eficacia na Neutralização dos Efeitos Locais Induzidos Pela Peçonha de Bothrops pauloensis. Horiz. Cient. 2009, 3, 1. Available online: https://ssl4799.websiteseguro.com/swge5/seg/cd2009/PDF/IC2009-0169.pdf (accessed on 5 November 2017).
- Valente, P.P.; Amorim, J.M.; Castilho, R.O.; Leite, R.C.; Ribeiro, M.F. In vitro acaricidal efficacy of plant extracts from Brazilian flora and isolated substances against Rhipicephalus microplus (Acari: Ixodidae). Parasitol. Res. 2014, 113, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Bichuette, M.E.; Varanda, E.M.; Barosela, J.R. Effects of ester fractions from leaf epicuticular waxes of Bauhinia rufa (Steud.) Bong. and Stryphnodendron adstringens (Mart.) Coville from cerrado on the aphid Rhopalosiphum maidis (Fitch.). Braz. J. Bot. 1998, 21, 101–104. [Google Scholar]
- Barbosa, F.S.; Leite, G.L.D.; Martins, E.R.; D’avila, V.A.; Cerqueira, V.M. Medicinal plant extracts on the control of Diabrotica speciosa (Coleoptera: Chrysomelidae). Rev. Bras. Plantas Med. 2013, 15, 142–149. [Google Scholar] [CrossRef]
- Vilar, J.B.; D’Oliveira, M.I.P.; Santos, S.C.; Chen, L.C. Cytotoxic and genotoxic investigation on barbatimão [Stryphnodendron adstringens (Mart:) Coville, 1910] extract. Braz. J. Pharm. Sci. 2010, 46, 687–694. [Google Scholar] [CrossRef]
- Sousa, N.C.; Carvalho, S.; Spano, M.A.; Graf, U. Absence of genotoxicity of a phytotherapeutic extract from Stryphnodendron adstringens (Mart.) Coville in somatic and germ cells of Drosophila melanogaster. Environ. Mol. Mutagen. 2003, 41, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Santos Filho, P.R.; Ferreira, L.A.; Gouvêa, C.M.C.P. Protective action against chemical-induced genotoxicity and free radical scavenging activities of Stryphnodendron adstringens (“barbatimão”) leaf extracts. Rev. Bras. Farmacogn. 2011, 21, 1000–1005. [Google Scholar] [CrossRef]
- Almeida, A.C.; Sobrinho, E.M.; Pinho, L.; Souza, P.N.S.; Martins, E.R.; Duarte, E.R.; Santos, H.O.; Brandi, I.V.; Cangussu, A.S.; Costa, J.P.R. Acute toxicity of leaf hydroalcoholic extracts of Lippia sidoides, Myracroduon urundeuva, Stryphnodendron adstringens and of Caryocar brasilliense administered by intraperitoneal route. Cienc. Rural 2010, 40, 200–203. [Google Scholar] [CrossRef]
- Rebecca, M.A.; Ishii-Iwamoto, E.L.; Grespan, R.; Cuman, R.K.; Caparroz-Assef, S.M.; Mello, J.C.P.; Bersani-Amado, C.A. Toxicological studies on Stryphnodendron adstringens. J. Ethnopharmacol. 2002, 83, 101–104. [Google Scholar] [CrossRef]
- Rebecca, M.A.; Ishii-Iwamoto, E.L.; Kelmer-Bracht, A.M.; Caparroz-Assef, S.M.; Cuman, R.K.; Pagadigorria, C.L.; Mello, J.C.P.; Bracht, A.; Bersani-Amado, C.A. Effect of Stryphnodendron adstringens (barbatimao) on energy metabolism in the rat liver. Toxicol. Lett. 2003, 143, 55–63. [Google Scholar] [CrossRef]
- Costa, M.A.; Ishida, K.; Kaplum, V.; Koslyk, E.D.; Mello, J.C.P.; Ueda-Nakamura, T.; Dias Filho, B.P.; Nakamura, C.V. Safety evaluation of proanthocyanidin polymer-rich fraction obtained from stem bark of Stryphnodendron adstringens (BARBATIMAO) for use as a pharmacological agent. Regul. Toxicol. Pharmacol. 2010, 58, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.; Mello, J.C.P.; Kaneshima, E.N.; Ueda-Nakamura, T.; Dias Filho, B.P.; Audi, E.A.; Nakamura, C.V. Acute and Chronic Toxicity of an Aqueous Fraction of the Stem Bark of Stryphnodendron adstringens (Barbatimao) in Rodents. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kucera, O.; Mezera, V.; Moravcova, A.; Endlicher, R.; Lotkova, H.; Drahota, Z.; Cervinkova, Z. In vitro toxicity of epigallocatechin gallate in rat liver mitochondria and hepatocytes. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.C.; Chan, W.H. Epigallocatechin gallate induces embryonic toxicity in mouse blastocysts through apoptosis. Drug Chem. Toxicol. 2014, 37, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Tokarnia, C.H.; Brito, M.F.; Driemeier, D.; Costa, J.B.D.; Camargo, A.J.R. Aborto em vacas na intoxicação experimental pelas favas de Stryphnodendron obovatum (Leg. Mimosoideae). Pesqui. Vet. Bras. 1998, 18, 35–38. [Google Scholar] [CrossRef]
- Brito, M.F.; Tokarnia, C.H.; Peixoto, P.V.; Silva, H.K.; Nogueira, M. Intoxicação experimental pelas favas de Stryphnodendron obovatum (Leg. Mimosoideae) em bovinos. 1. Caracterização do quadro clínico. Pesqui. Vet. Bras. 2001, 21, 9–17. [Google Scholar] [CrossRef]
- Brito, M.F.; Tokarnia, C.H.; Peixoto, P.V. Intoxicação experimental pelas favas de Stryphnodendron obovatum (Leg. Mimosoideae) em bovinos. 2. Achados anátomo e histopatológicos. Pesqui. Vet. Bras. 2001, 21, 61–71. [Google Scholar] [CrossRef]
| Species | Part of Plant | Medicinal Use | Form of Preparation and Administration | Reference |
|---|---|---|---|---|
| SA | Stem bark | Uterine infection, ovary inflammation, wound healing, ulcer, cicatrizing, anti-inflammatory, hygiene, sore throat and itch | Baths | [43] |
| SA | Stem bark | Ulcerous wounds | Macerated, used as bath | [44] |
| SA | Stem bark | Not mentioned | Topical use | [45] |
| SA | Stem bark | Wound healing | Decoction, infusion or macerated, for external or internal uses | [8] |
| SA | Stem bark | Wound, chilblain, diabetes, prostate problems, inflammation, gastritis, liver diseases, dental inflammation, pain in general | Not mentioned | [39] |
| SA | Stem bark | Leucorrhea, wound healing, ulcer and vaginal discharge | Not mentioned | [46] |
| SA | Stem bark | Urinary infection | Oral | [47] |
| SA | Stem bark | Wound healing | Tea, infusion, bottleful, powder | [48] |
| SR | Stem bark and seeds | Diuretic, anti-diarrheic, ulcer, cicatrizing, chilblain, astringent, for gums | Macerated in water | [49] |
| SR | Stem bark | Wounds, inflammation, gastritis and ulcer, vaginal inflammation, pain, infection, prostate disorders, sexually transmitted diseases, rheumatism, hypertension, dermatitis, burns, menopause, postpartum healing, renal calculi, influenza, lung diseases | Immersion in water for oral or topical administration | [50] |
| SR | Stem bark, roots and leaves | Inflammation, vaginal discharge, urinary infection, uterine lesions | Decoction and infusion | [51] |
| SR | Stem bark | General wound healing, ulcer, general inflammation, headache, gastritis, cancer, fever, leg, body, stomach and belly pain, cough, cuts, scabs, flu, sore throat, heart, childbirth inflammation, blood pressure, blood disorder, kidneys, lung inflammation, sinus and urinary infection, excessive menstruation, itch, vaginal discharge, stanch blood from cuts, skin allergy, swelling, tightening the vagina for sexual intercourse | Not mentioned | [36] |
| SR | Stem bark and roots | Backache | Macerated for oral administration | [52] |
| SR | Stem bark | Wound, uterus and skin inflammation, wound healing, genital disease and cancer | Immersion in water or decoction is prepared for oral and topical administration and baths | [40] |
| SR | Stem bark | Ulcer, wound healing, venereal disease, hemorrhage, diabetes, anthelmintic, high blood pressure, anemia, cancer, liver disease | Infusion and tincture | [24] |
| Number | Compound Name | Species | Reference |
|---|---|---|---|
| 1 | Gallic acid | SA, SP, SR | [54,56,60,62,64,65,66] |
| 2 | Catechin | SA, SR | [56,60,65,67] |
| 3 | Epicatechin | SA | [67] |
| 4 | Gallocatechin | SA, SP, SR | [54,56,62,64,65,67,68] |
| 5 | Epigallocatechin | SA, SP, SR | [54,56,62,64,65,66,67,68] |
| 6 | Epigallocatechin 3-O-gallate | SA, SR | [56,61,64,65,66,68] |
| 7 | Epigallocatechin 3-O-methylgallate | SA | [66] |
| 8 | Epigallocatechin 3-O-(3,5-dimethyl)gallate | SA | [54,68] |
| 9 | 4′-O-methylgallocatechin | SA, SP | [54,64,68] |
| 10 | 4′-O-methylepigallocatechin | SA | [66] |
| 11 | 4′-O-methylepigallocatechin-3-O-gallate | SA | [64] |
| 12 | Epigallocatechin 3-O-(3-methoxy-4-hydroxy)benzoate | SA | [54,68] |
| 13 | Gallocatechin-(4α→8)-epigallocatechin 3-O-(4-hydroxy)benzoate | SA | [54,68] |
| 14 | Epigallocatechin-(4β→8)-epigallocatechin 3-O-(4-hydroxy)benzoate | SA | [54,68] |
| 15 | Gallocatechin-(4β→8)-epigallocatechin 3-O-gallate | SA | [68] |
| 16 | Epigallocatechin-(4β→8)-gallocatechin | SA, SP | [54,68] |
| 17 | Epigallocatechin-(4β→8)-epigallocatechin | SA | [64,66,68] |
| 18 | Epigallocatechin-(4β→6)-epigallocatechin | SA | [68] |
| 19 | Epigallocatechin-(4β→8)-epigallocatechin3-O-gallate | SA | [68] |
| 20 | Epigallocatechin 3-O-gallate-(4β→8)-epigallocatechin 3-O-gallate | SA | [64,68] |
| 21 | 4′-O-methylepigallocatechin 3-O-gallate-epigallocatechin 3-O-gallate | SA | [64] |
| 22 | Epigallocatechin-epigallocatechin 3-O-gallate | SA | [64,66,68] |
| 23 | 4′-O-methylepigallocatechin-epigallocatechin | SA | [64] |
| 24 | 4′-O-methylepigallocatechin-4′-O-methylepigallocatechin | SA | [66] |
| 25 | Robinetinidol | SA | [66] |
| 26 | Robinetinidol-(4α→8)-epigallocatechin | SA | [66,69] |
| 27 | Robinetinidol-(4β→8)-epigallocatechin | SA | [69] |
| 28 | Robinetinidol-4′-O-methylepigallocatechin | SA | [66,69] |
| 29 | Robinetinidol-(4β→8)-epigallocatechin-3-O-gallate | SA | [69] |
| 30 | Robinetinidol-(4α→8)-epigallocatechin-3-O-gallate | SA | [69] |
| 31 | Robinetinidol-(4α→6)-gallocatechin | SA | [69] |
| 32 | Robinetinidol-(4α→6)-epigallocatechin | SA | [69] |
| 33 | Robinetinidol-[4β→6(8)]-gallocatechin | SA | [69] |
| 34 | Robinetinidol-(4α→8)-gallocatechin | SA | [69] |
| 35 | 4′-O-methylrobinetinidol-(4α→8)-4′-O-methylgallocatechin | SA | [54] |
| 36 | 4′-O-methylrobinetinidol-(4α→8)-4′-O-methylepigallocatechin | SA | [54] |
| 37 | 4′-O-methylgallocatechin-(4α→8)-4′-O- methylgallocatechin | SA | [54,70] |
| 38 | 4′-O-methylrobinetinidol-(4β→6)-4′-O-methylgallocatechin | SP | [54] |
| 39 | Fisetinidol-(4α→8)-gallocatechin | SP | [54] |
| 40 | Fisetinidol-(4β→8)-gallocatechin | SP | [54] |
| 41 | Polymer of 2114 Da of molecular weight with 6 monomers of flavan-3-ols and one galoil group consisting of prodelphinidin and prorobinetinidin units with configuration 2,3-cis and 2,3-trans | SA | [42] |
| 42 | Caffeic acid | SR | [60] |
| 43 | Rutin | SR | [60] |
| Ethnopharmacological Use | Scientifically Observed? | Related Compound |
|---|---|---|
| Wound healing | Yes | 1, 5–7, 9, 17, 22, 24–28 |
| Gastric ulcer | Yes, but toxicity was observed | 1, 5–7, 9, 17, 22, 24–28 |
| Anti-inflammatory | Yes | 1, 4–6, 9, 11, 17, 20–23 |
| Against pain | Yes—peripheral antinociception | 6 and 41 |
| Cancer | Antioxidant activity has been extensively evaluated, but anticancer activity is not conclusive | 1, 2, 6, 42 and 43 |
| Antimicrobial-oral and genitourinary infections | Yes—against gram positive bacteria and Candida species | 1 and 41 |
| Species | Extracts/Fraction | Microorganisms | Reference |
|---|---|---|---|
| SA | Hydroalcoholic and acetone:water extracts from the bark | Staphylococcus aureus | [41,102,107,108,109] |
| SA | Hydroalcoholic bark extract | Staphylococcus epidermidis; Enterococcus faecalis; Streptococcus salivarius; Streptococcus sanguinis; Streptococcus mitis; Streptococcus mutans; Streptococcus sobrinus; Lactobacillus casei | [41,102,110] |
| SA | Ethanolic and hexanic bark extracts | Candida albicans; Streptococcus mutans; Aggregatibacter actinomycetemcomitans | [111] |
| SA | Propylene glycol | S. aureus; S. epidermidis; S.mutans; C. albicans; C. tropicalis; C. glabrata | [112] |
| SA | Hydroalcoholic bark extract | Mycobacterium tuberculosis | [113] |
| SP | Ethyl acetate fraction | S. aureus; Bacillus subtilis | [87] |
| SR | Ethyl acetate fraction | S. aureus | [87] |
| SA | Polymer-rich subfraction | C. albicans; C. tropicalis, Cryptococcus neoformans | [42,114,115] |
| SA | Hexanic leaf extract | Trychophyton rubrum | [116] |
| SA | Methanolic extract and tannin fraction from the bark | Pythium insidiosum | [117] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza-Moreira, T.M.; Queiroz-Fernandes, G.M.; Pietro, R.C.L.R. Stryphnodendron Species Known as “Barbatimão”: A Comprehensive Report. Molecules 2018, 23, 910. https://doi.org/10.3390/molecules23040910
Souza-Moreira TM, Queiroz-Fernandes GM, Pietro RCLR. Stryphnodendron Species Known as “Barbatimão”: A Comprehensive Report. Molecules. 2018; 23(4):910. https://doi.org/10.3390/molecules23040910
Chicago/Turabian StyleSouza-Moreira, Tatiana M., Geisiany M. Queiroz-Fernandes, and Rosemeire C. L. R. Pietro. 2018. "Stryphnodendron Species Known as “Barbatimão”: A Comprehensive Report" Molecules 23, no. 4: 910. https://doi.org/10.3390/molecules23040910
APA StyleSouza-Moreira, T. M., Queiroz-Fernandes, G. M., & Pietro, R. C. L. R. (2018). Stryphnodendron Species Known as “Barbatimão”: A Comprehensive Report. Molecules, 23(4), 910. https://doi.org/10.3390/molecules23040910






