Optimization of the Ultrasonic-Assisted Extraction of Phenolic Compounds from Oryza Sativa L. ‘Violet Nori’ and Determination of the Antioxidant Properties of its Caryopses and Leaves
Abstract
:1. Introduction
- developing an efficient and environmental friendly method for the extraction of anthocyanins and other phenolic compounds from the caryopses, flour, and leaves of ‘Violet Nori’;
- evaluating the antioxidant properties and the anthocyanin content of the extracted samples and their changes in leaves throughout plant growth;
- singling out the best and most economically handled raw material for the sustainable industrial extraction of its anthocyanins.
2. Results
2.1. Preliminary Experiments
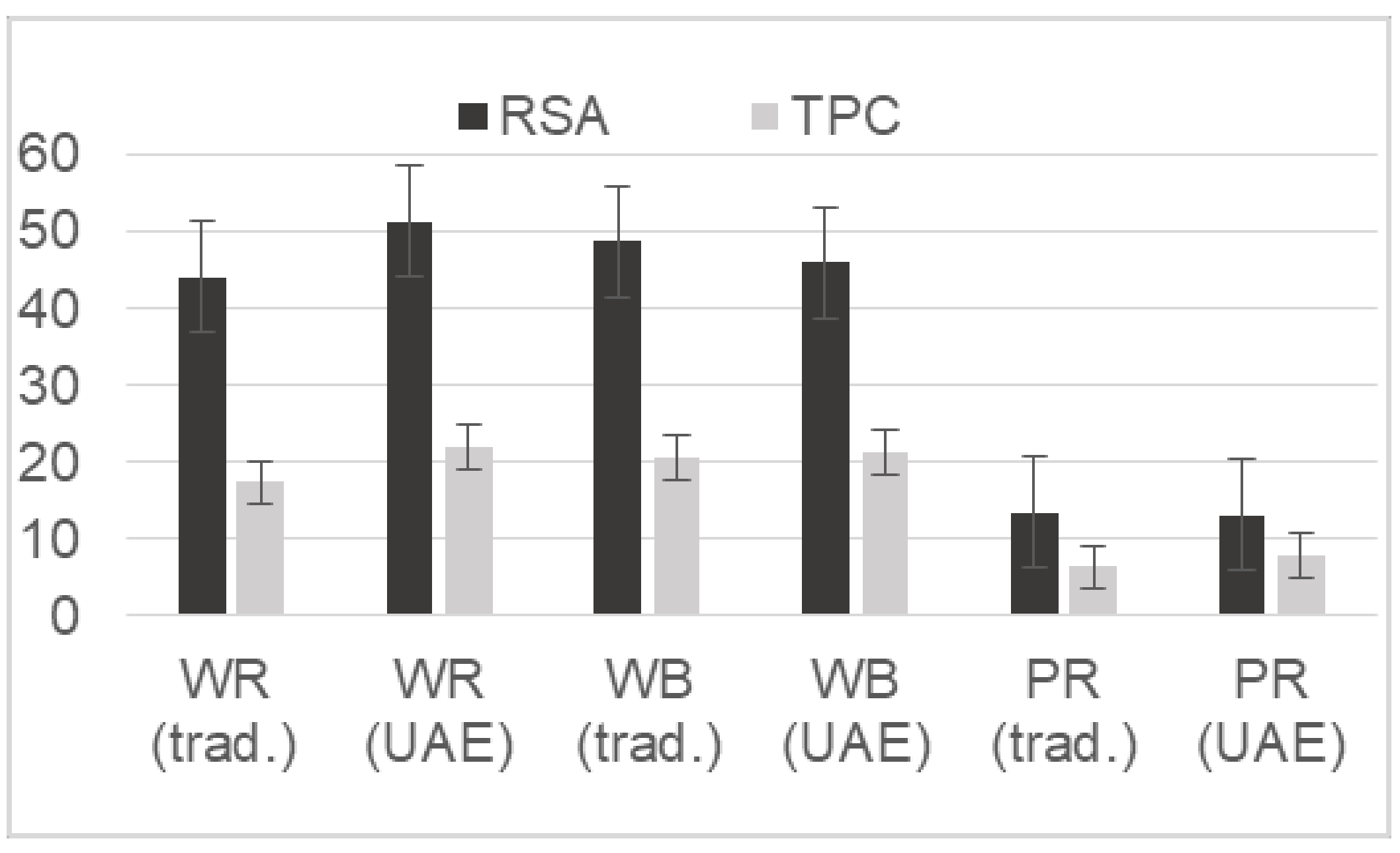
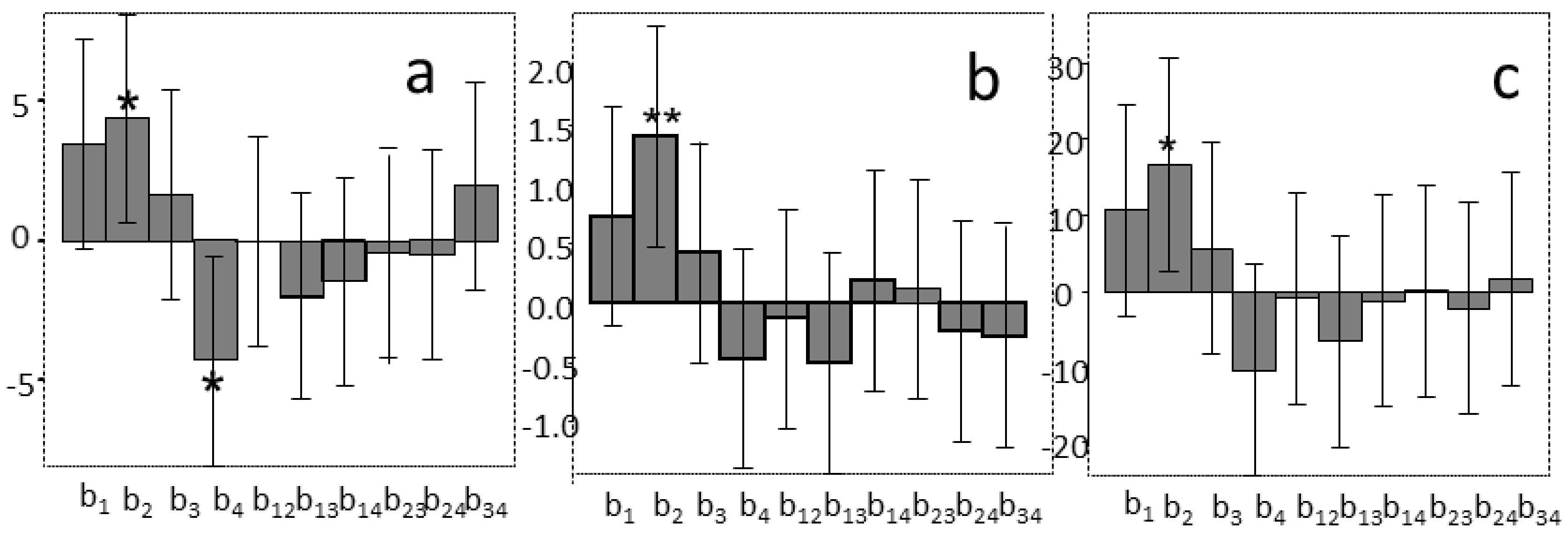
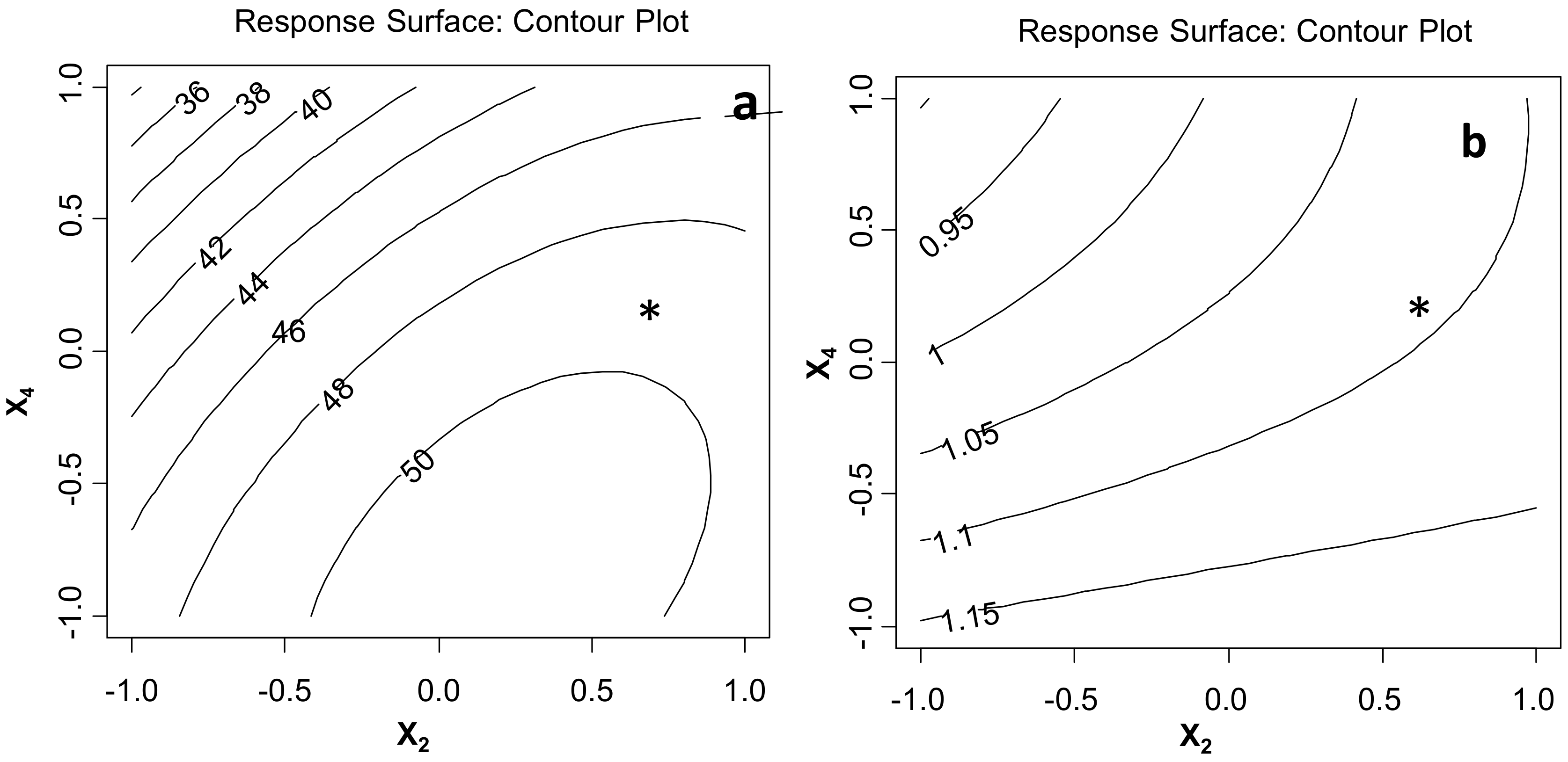
2.2. The First Tentative Direct UAE
2.3. The Selected UAE Conditions
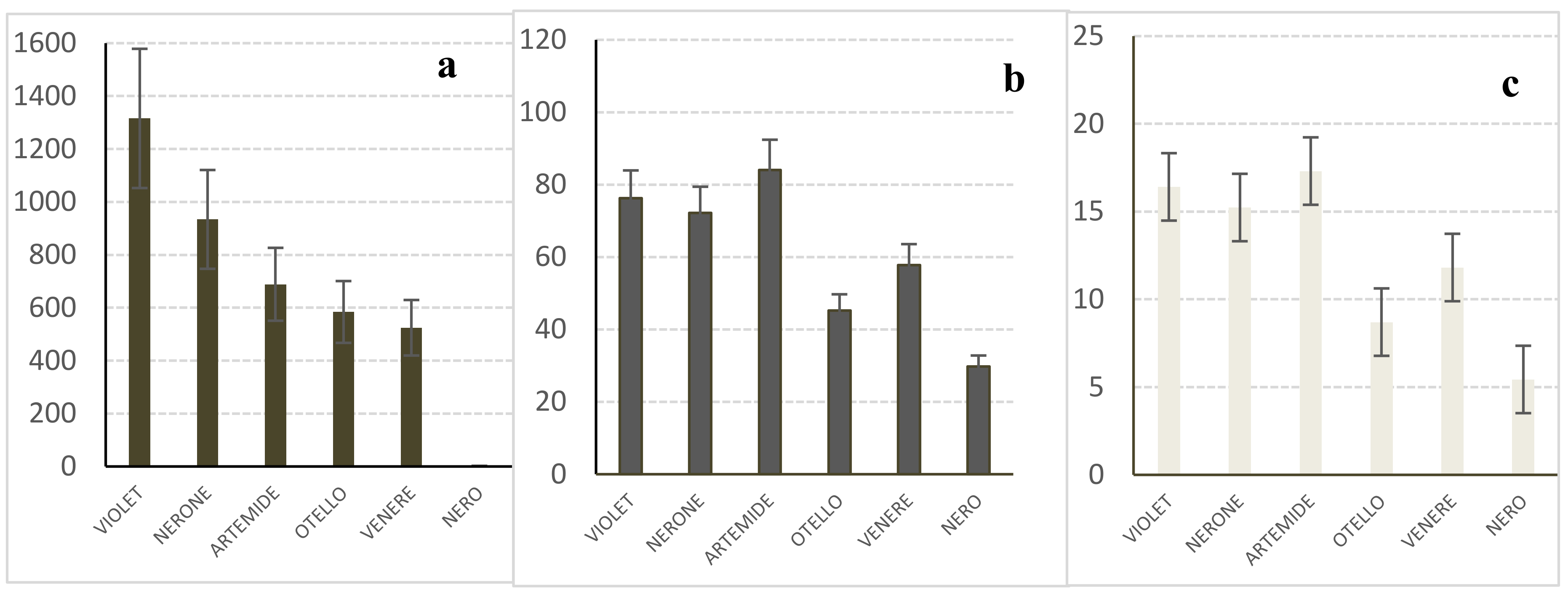
2.4. Analyses of the Caryopses and Flour of ‘Violet Nori’ and Comparison with the Caryopses of Other Black Cultivars
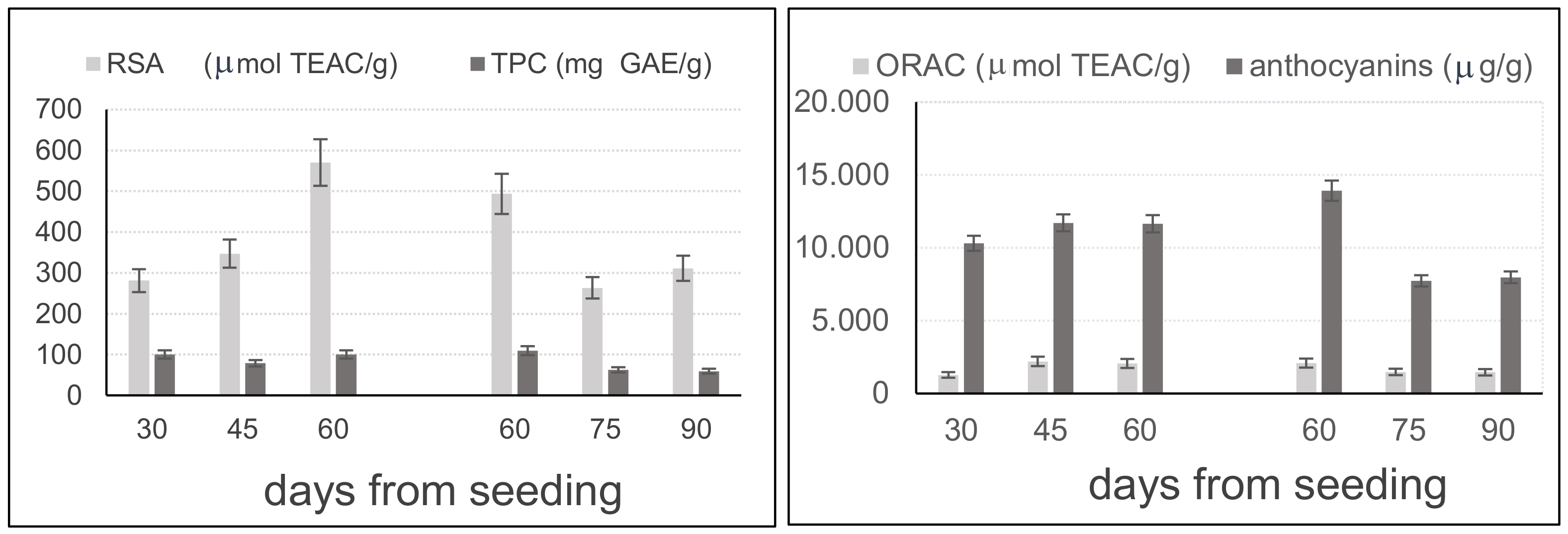
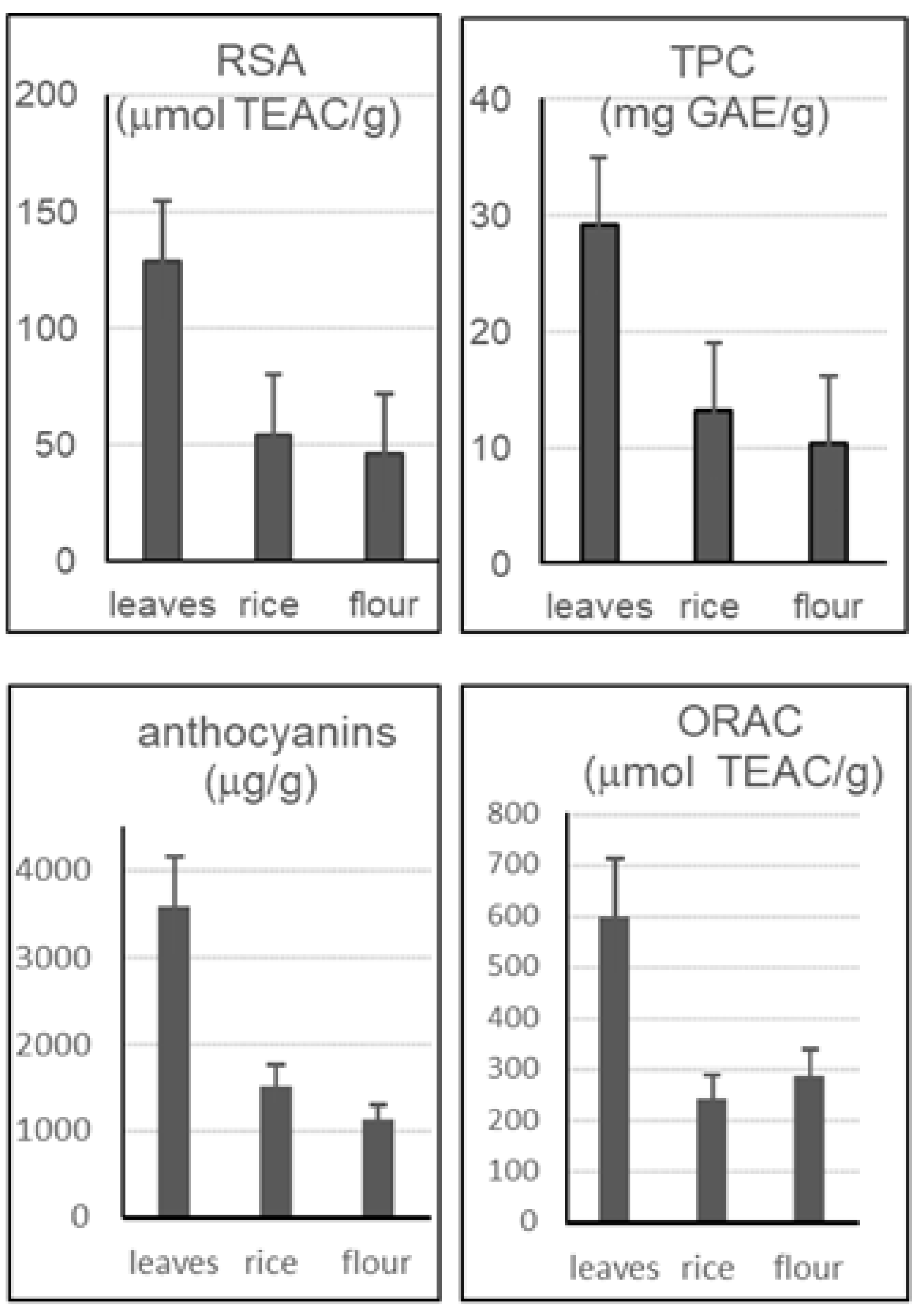
2.5. Analyses of the Leaves of ‘Violet Nori’
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Equipment
4.3. Plant Materials
4.4. Experimental Design for Improving UAE Yields
4.5. The Final UAE Conditions
4.6. Determination of TPC
4.7. Determination of RSA
4.8. Determination of the Oxygen Radical Absorbing Capacity (ORAC)
4.9. HPLC Determination of the Anthocyanin Content
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Italian Ministry of agricultural food and forestry policies. Available online: http://www.enterisi.it/servizi/Menu/dinamica.aspx?idSezione=17505&idArea=17548&idCat=17552&ID=17552&TipoElemento=categoria (accessed on 28 November 2017).
- Kim, M.-K.; Koh, H.-A.; Koh, K.; Kim, H.-S.; Lee, Y.-S.; Kim, Y.-H. Identification and quantification of anthocyanin pigments in colored rice. Nutr. Res. Pract. 2008, 2, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Gao, L. Blue and purple grains. In Specialty Grains for Food and Feed; Abdel-Aal, E., Wood, P., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 2005; pp. 45–67. ISBN 1891127411. [Google Scholar]
- Goufo, P.; Trindade, H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. & Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Abdel Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.N.; Wu, H.L.; Yeh, H.I.; Chu, C.S.; Lin, H.C.; Lee, W.C. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids 2006, 41, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Choi, S.P.; Kang, M.Y.; Koh, H.J.; Kozukue, N.; Friedman, M. Antioxidative activities of bran from twenty-one pigmented rice cultivars. Food Chem. 2006, 94, 613–620. [Google Scholar] [CrossRef]
- Zhang, M.W.; Zhang, R.F.; Guo, B.J.; Chi, J.W.; Wei, Z.C.; Xu, Z.H. The hypolipidemic and antioxidative effects of black rice pericarp anthocyanin in rats. Acta Nutr. Sinica 2006, 28, 404–408. [Google Scholar]
- Shen, Y.; Jin, L.; Xiao, P.; Lu, Y.; Bao, J.S. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J. of Cereal Sci. 2009, 49, 106–111. [Google Scholar] [CrossRef]
- Hyun, J.W.; Chung, H.S. Cyanidin and malvidin from Oryza sativa cv. Heugjinjubyeo mediate cytotoxicity against human monocytes leukemia cells by arrest of G 2 /M phase and induction of apoptosis. J. Agric. Food Chem. 2004, 52, 2213–2217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Giusti, M.M.; Malik, M.; Moyer, M.P.; Magnuson, B.A. Effects of commercial anthocyanin-rich extracts on colonic cancer and nontumorigenic colonic cell growth. J. Agric. Food Chem. 2004, 52, 6122–6128. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Osawa, T. Cyanidin 3-O-β-glucoside suppresses nitric oxide production during a zymosan treatment in rats. J. Nutr. Sci. Vitamin. 2002, 48, 305–310. [Google Scholar] [CrossRef]
- Chen, X.Q.; Nagao, N.; Itani, T.; Irifune, K. Anti-oxidative analysis and identification and quantification of anthocyanin pigments in different coloured rice. Food Chem. 2012, 135, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Gomez Alonso, A.; Locatelli, M.; Travaglia, F.; Coïsson, J.D.; Hermosin Gutierrez, I.; Arlorio, M. Phenolics characterization and antioxidant activity of six different Oryza sativa cultivars grown in Piedmont (Italy). Food Res. Int. 2014, 65, 282–290. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Monograph; WHO Press: Geneva, Switzerland, 2012; Volume 96, Available online: http://monographs.iarc.fr/ENG/Monographs/vol96/index.php (accessed on 4 January 2018).
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Juraimi, A.S.; Tayeb Meigooni, A. Comparative Evaluation of Different Extraction Techniques and Solvents for the Assay of Phytochemicals and Antioxidant Activity of Hashemi Rice Bran. Molecules 2015, 20, 10822–10838. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Marchesan, E.; Sachet Massoni, P.F.; Picolli da Silva, L.; Meneghetti Sarzi Sartori, G.; Bruck Ferreira, R. Antioxidant properties of rice grains with light brown, red and black pericarp colors and the effect of processing. Food Res. Int. 2013, 50, 698–703. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bartnik, D.D.; Mohler, C.M.; Houlihan, M. Methods for the production of food grade extracts. U.S. Patent 20060088627, 27 April 2006. [Google Scholar]
- U.S. Food and Drug Administration. CFR—Code of Federal Regulations Title 21, B, 184, 2018. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=08d7b882ac7656d40f2f422caee53d25&mc=true&node=pt21.3.184&rgn=div5 (accessed on 4 April 2018).
- Tabaraki, R.; Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ult. Sonochem. 2011, 18, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.H.; Prior, R.L. Measurement of oxygen radical absorbance capacity in biological samples. Oxidants and anti-oxidants. Meth. Enzym. 1999, 299, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical oxygen assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of “Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays”. J. Funct. Food 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Italian Chemical Society. Division of Analytical Chemistry-Group of Chemometrics. R-Based Chemometric Software. Available online: http://gruppochemiometria.it/index.php/software/ (accessed on 4 January 2018).
- Santos, H.M.; Lodeiro, C. The Power of Ultrasound. In Ultrasound in Chemistry: Analytical Applications; Capelo-Martínez, J.L., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 1–16. ISBN 978352731934. [Google Scholar]
- Ieri, F.; Innocenti, M.; Possieri, L.; Gallori, S.; Mulinacci, N. Phenolic composition of “bud extracts” of Ribes nigrum L., Rosa canina L., and Tilia Tomentosa M. J. Pharm. Biomedic. Anal. 2015, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all the compounds reported in Material and methods are available from the authors. |
| Experiment | X1 a | X2 | X3 | X4 | Y1 | Y2 |
|---|---|---|---|---|---|---|
| 1 | 30% | 20% | 5 min | 1:40 | 35.8 | 7.4 |
| 2 | 70% | 20% | 5 min | 1:40 | 52.3 | 12.7 |
| 3 | 30% | 80% | 5 min | 1:40 | 47.7 | 12.0 |
| 4 | 70% | 80% | 5 min | 1:40 | 54.5 | 11.7 |
| 5 | 30% | 20% | 15 min | 1:40 | 33.1 | 10.0 |
| 6 | 70% | 20% | 15 min | 1:40 | 48.2 | 10.0 |
| 7 | 30% | 80% | 15 min | 1:40 | 52.7 | 15.1 |
| 8 | 70% | 80% | 15 min | 1:40 | 53.7 | 14.4 |
| 9 | 30% | 20% | 5 min | 1:32 | 25.5 | 8.1 |
| 10 | 70% | 20% | 5 min | 1:32 | 32.5 | 9.7 |
| 11 | 30% | 80% | 5 min | 1:32 | 34.5 | 10.6 |
| 12 | 70% | 80% | 5 min | 1:32 | 47.7 | 13.9 |
| 13 | 30% | 20% | 15 min | 1:32 | 46.0 | 10.2 |
| 14 | 70% | 20% | 15 min | 1:32 | 35.2 | 10.2 |
| 15 | 30% | 80% | 15 min | 1:32 | 40.7 | 10.3 |
| 16 | 70% | 80% | 15 min | 1:32 | 47.2 | 12.8 |
| 17 | 50% | 50% | 10 min | 1:36 | 47.1 | 11.2 |
| 18 | 50% | 50% | 10 min | 1:36 | 49.5 | 11.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turrini, F.; Boggia, R.; Leardi, R.; Borriello, M.; Zunin, P. Optimization of the Ultrasonic-Assisted Extraction of Phenolic Compounds from Oryza Sativa L. ‘Violet Nori’ and Determination of the Antioxidant Properties of its Caryopses and Leaves. Molecules 2018, 23, 844. https://doi.org/10.3390/molecules23040844
Turrini F, Boggia R, Leardi R, Borriello M, Zunin P. Optimization of the Ultrasonic-Assisted Extraction of Phenolic Compounds from Oryza Sativa L. ‘Violet Nori’ and Determination of the Antioxidant Properties of its Caryopses and Leaves. Molecules. 2018; 23(4):844. https://doi.org/10.3390/molecules23040844
Chicago/Turabian StyleTurrini, Federica, Raffaella Boggia, Riccardo Leardi, Matilde Borriello, and Paola Zunin. 2018. "Optimization of the Ultrasonic-Assisted Extraction of Phenolic Compounds from Oryza Sativa L. ‘Violet Nori’ and Determination of the Antioxidant Properties of its Caryopses and Leaves" Molecules 23, no. 4: 844. https://doi.org/10.3390/molecules23040844
APA StyleTurrini, F., Boggia, R., Leardi, R., Borriello, M., & Zunin, P. (2018). Optimization of the Ultrasonic-Assisted Extraction of Phenolic Compounds from Oryza Sativa L. ‘Violet Nori’ and Determination of the Antioxidant Properties of its Caryopses and Leaves. Molecules, 23(4), 844. https://doi.org/10.3390/molecules23040844








