Antioxidant Activities of Phenolic Metabolites from Flemingia philippinensis Merr. et Rolfe and Their Application to DNA Damage Protection
Abstract
1. Introduction
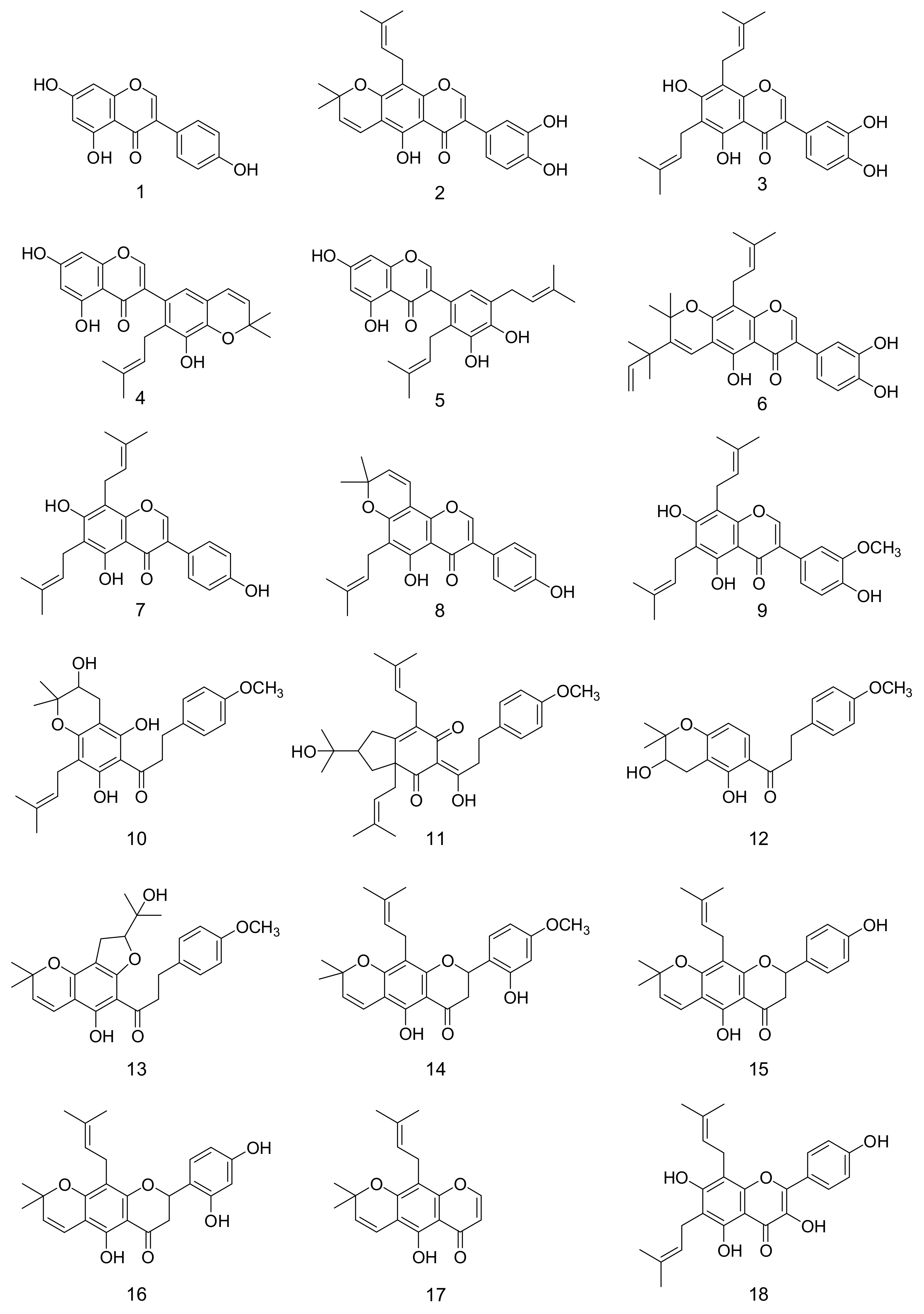
2. Results and Discussion
2.1. Total Content of Phenolics and Flavonoids
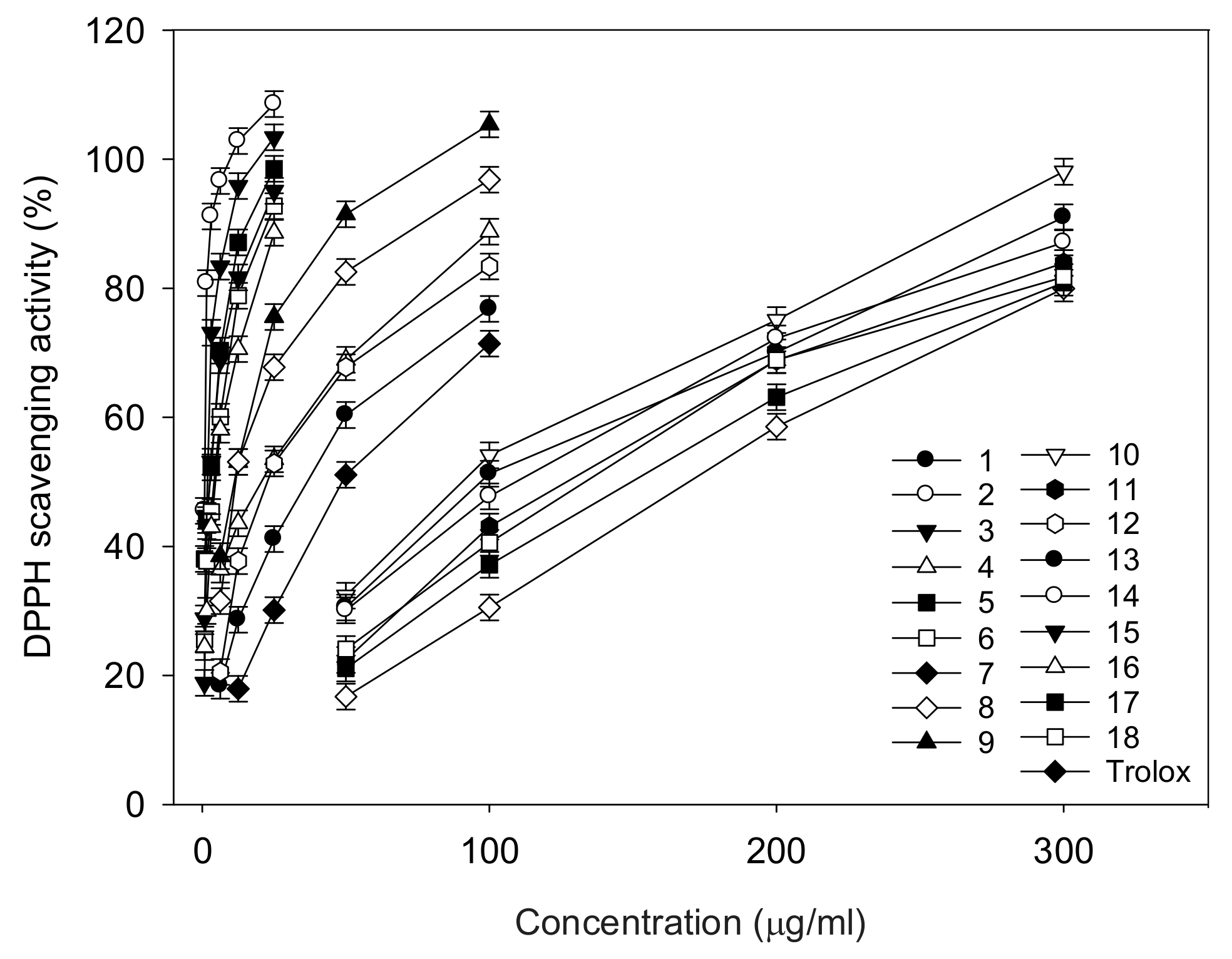
2.2. DPPH Radical Scavenging Activity by ESR
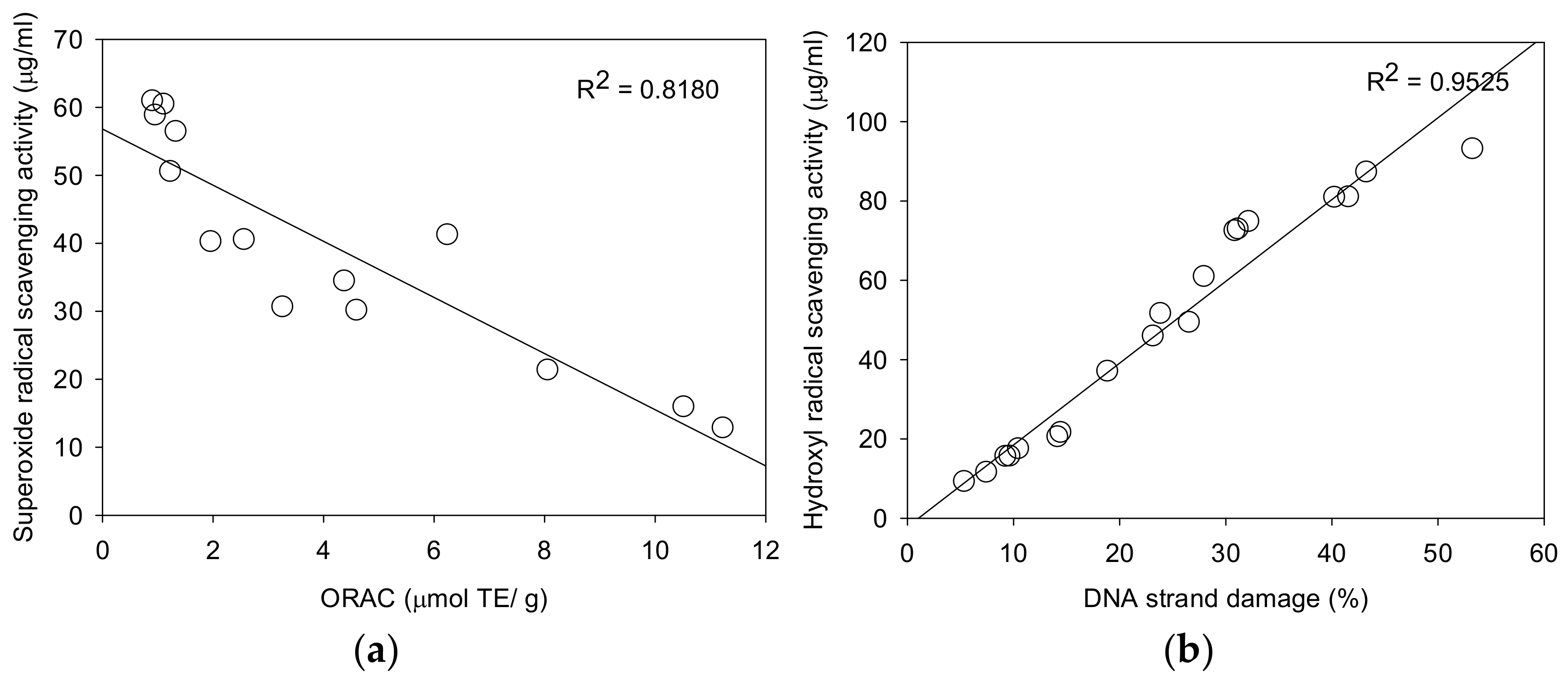
2.3. Peroxyl Radical Scavenging Activity by ORAC
2.4. Superoxide Anion Radical Scavenging Activity by ESR
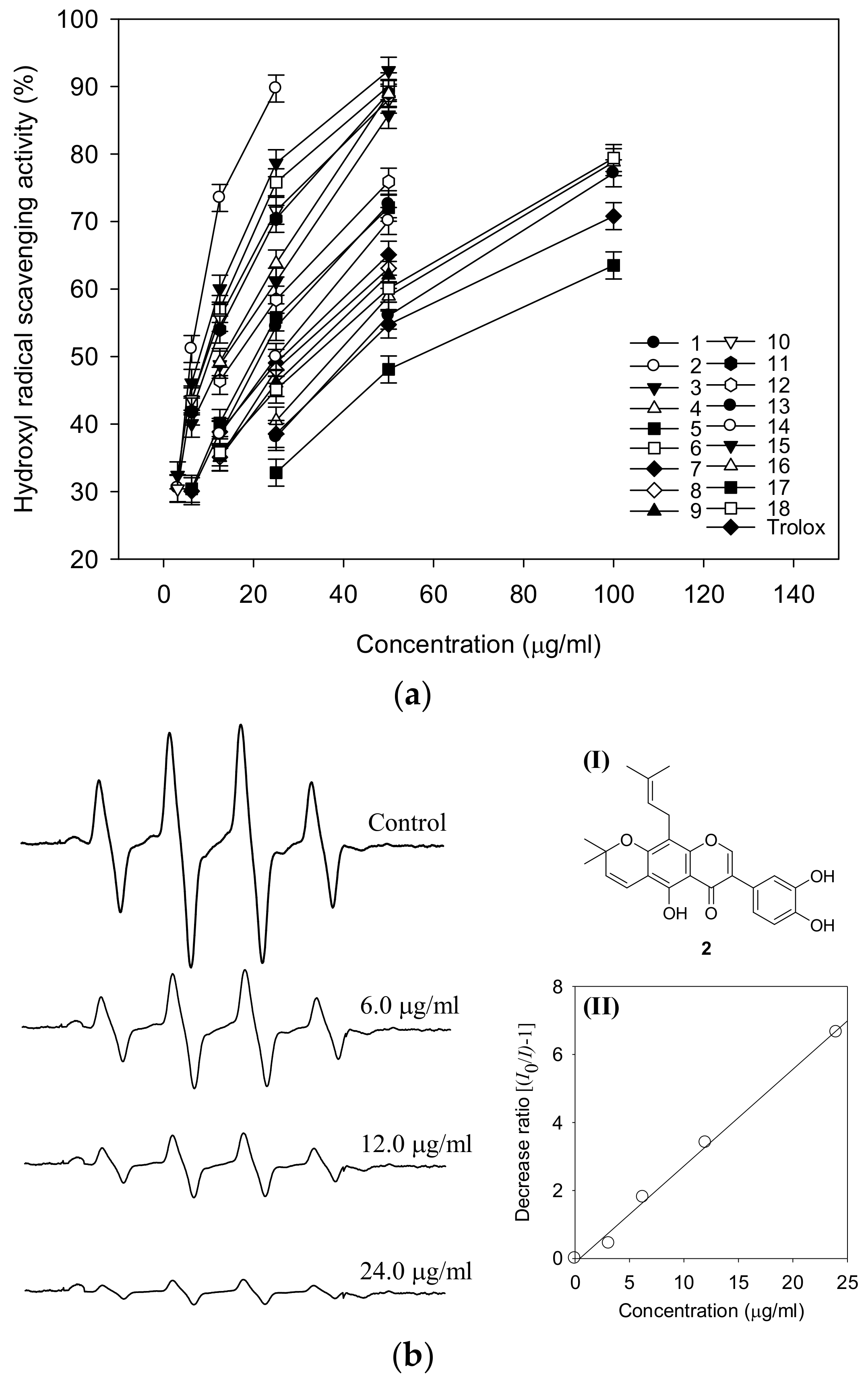
2.5. Hydroxyl Radical Scavenging Activity by ESR
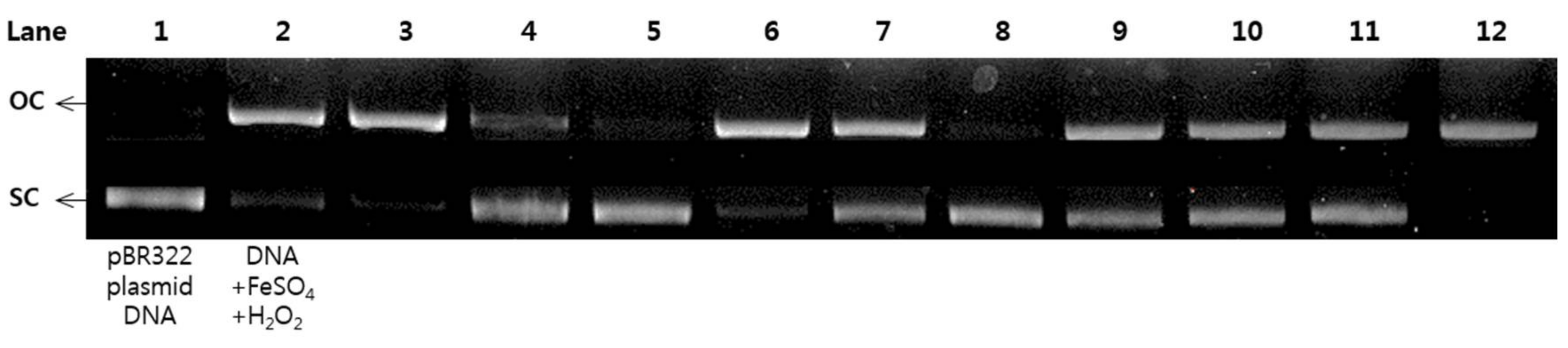
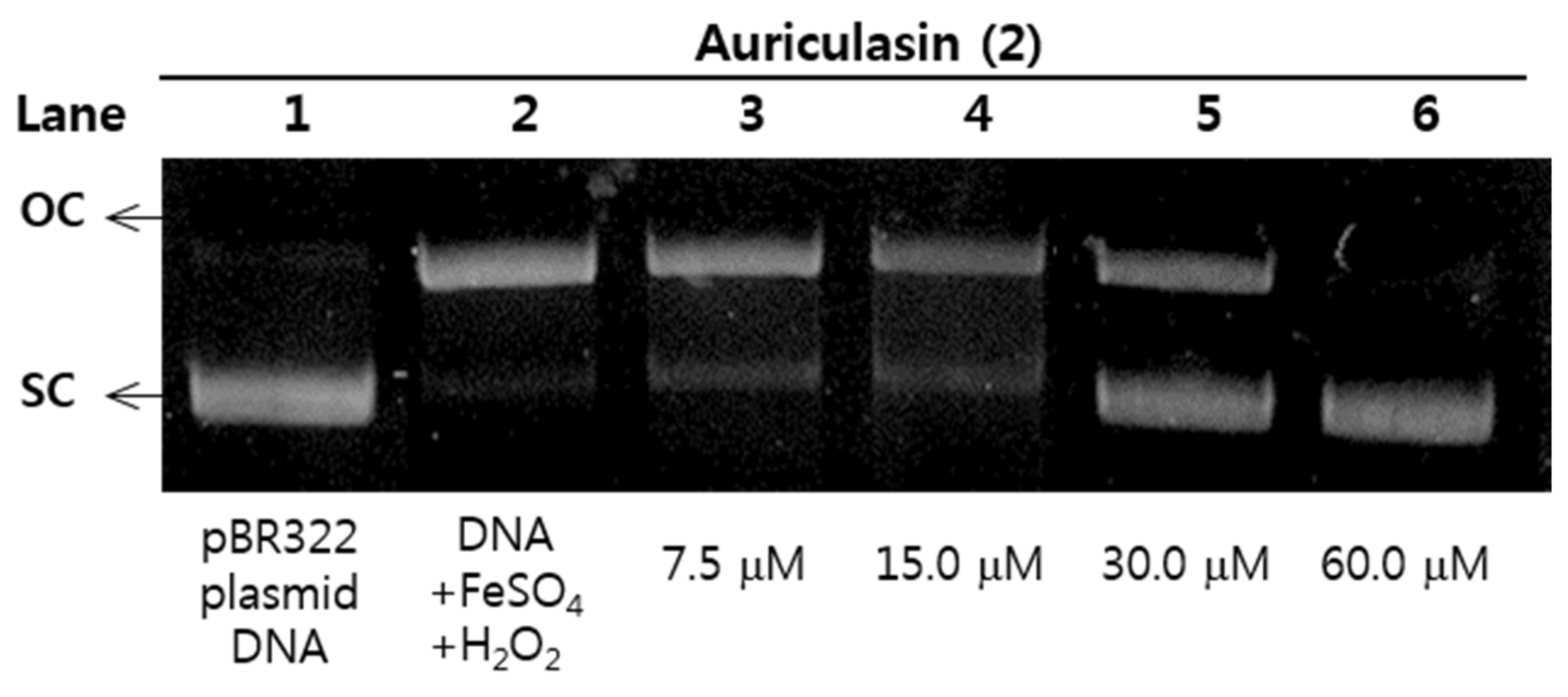
2.6. Protective Effects of pBR322 Plasmid DNA Damage
3. Materials and Methods
3.1. Reagents and Materials
3.2. Equipment
3.3. Extraction and Isolation of F. philippinensis
3.4. Total Phenolic Content (TPC)
3.5. Total Flavonoid Content (TFC)
3.6. Electron Spin Resonance Spectroscopy (ESR)
3.6.1. DPPH Radical Scavenging Assay by ESR
3.6.2. Hydroxyl Radical Scavenging Assay by ESR
3.6.3. Superoxide Radical Scavenging Assay by ESR
3.7. ORAC Scavenging Activity Assay
3.8. DNA Damage Protective Effect Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med. 1991, 91, 14S–22S. [Google Scholar] [CrossRef]
- Reilly, P.M.; Schiller, H.J.; Bulkley, G.B. Pharmacologic approach to tissue injury mediated by free radicals and other reactive oxygen metabolites. Am. J. Surg. 1991, 161, 488–503. [Google Scholar] [CrossRef]
- Imlay, J.A. Cellular Defenses against Superoxide and Hydrogen Peroxide. Annu. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Mena, S.; Ortega, A.; Estrela, J.M. Oxidative stress in environmental-induced carcinogenesis. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 674, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.K.; Brzezinska-Slebodzinska, E.; Madsen, F.C. Oxidative Stress, Antioxidants, and Animal Function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Rahman, T.; Hosen, I.; Islam, M.M.T.; Shekhar, H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012, 3, 997–1019. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Dinstel, R.R.; Cascio, J.; Koukel, S. The antioxidant level of Alaska’s wild berries: High, higher and highest. Int. J. Circumpolar Health 2013, 72. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; Sridhar, C.; Reddy, Y.S.R.; De, B. Free radicals, antioxidants, diseases and phytomedicines: Current status and future prospect. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 91–100. [Google Scholar] [CrossRef]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Cadet, J.; Delatour, T.; Douki, T.; Gasparutto, D.; Pouget, J.P.; Ravanat, J.L.; Sauvaigo, S. Hydroxyl radicals and DNA base damage. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1999, 424, 9–21. [Google Scholar] [CrossRef]
- Hsu, C.S.; Li, Y. Aspirin potently inhibits oxidative DNA strand breaks: Implications for cancer chemoprevention. Biochem. Biophys. Res. Commun. 2002, 293, 705–709. [Google Scholar] [PubMed]
- Moraes, M.C.S. DNA repair mechanisms protect our genome from carcinogenesis. Front. Biosci. 2012, 17, 1362–1388. [Google Scholar] [CrossRef]
- De Bont, R. Endogenous DNA damage in humans: A review of quantitative data. Mutagenesis 2004, 19, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.M.; Chiu, H.H.; Liao, S.T.; Chen, Y.T.; Chang, H.C.; Wen, K.C. Isoflavonoid-rich Flemingia macrophylla extract attenuates UVB-induced skin damage by scavenging reactive oxygen species and inhibiting MAP kinase and MMP expression. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, Q.; Bian, K. Ultrasonic-assisted extraction and evaluation of biological activities of flavonoids from Flemingia philippinensis Merr. et Rolfe. Trop. J. Pharm. Res. 2015, 14, 1365–1371. [Google Scholar] [CrossRef][Green Version]
- Chen, M.; Luo, S.Q.; Chen, J.H. Studies on the chemical constituents of Flemingia philippinensis. Acta Pharm. Sin. 1991, 26, 42–48. [Google Scholar]
- Li, H.; Yang, M.; Miao, J.; Ma, X. Prenylated isoflavones from Flemingia philippinensis. Magn. Reson. Chem. 2008, 46, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Curtis-Long, M.J.; Yuk, H.J.; Kim, D.W.; Tan, X.F.; Park, K.H. Bacterial neuraminidase inhibitory effects of prenylated isoflavones from roots of Flemingia philippinensis. Bioorg. Med. Chem. 2013, 21, 6398–6404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuk, H.J.; Kim, J.Y.; Kim, D.W.; Song, Y.H.; Tan, X.F.; Curtis-Long, M.J.; Park, K.H. Novel chromenedione derivatives displaying inhibition of protein tyrosine phosphatase 1B (PTP1B) from Flemingia philippinensis. Bioorg. Med. Chem. Lett. 2016, 26, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Curtis-Long, M.J.; Lee, B.W.; Yuk, H.J.; Kim, D.W.; Tan, X.F.; Park, K.H. Inhibition of tyrosinase activity by polyphenol compounds from Flemingia philippinensis roots. Bioorg. Med. Chem. 2014, 22, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.-M.; Nakamura, N.; Akao, T.; Nishihara, T.; Hattori, M. Estrogenic and antiestrogenic activities of the roots of Moghania philippinensis and their constituents. Biol. Pharm. Bull. 2004, 27, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, G.S.; Noskov, S.M. Antioxidant systems of erythrocytes in rheumatism. Ter. Arkhiv 1985, 57, 121–123. [Google Scholar]
- Man-qin, F.; Dun, D.; Shi-xiu, F.; Ri-ming, H.; Shuai, T.; Sheng-xiang, Q. Chemical Constituents from Roots of Flemingia philippinensis. Chin. Herb. Med. 2012, 4, 8–11. [Google Scholar] [CrossRef]
- Das, K.; Samanta, L.; Chainy, G.B.N. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J. Biochem. Biophys. 2000, 37, 201–204. [Google Scholar]
- Pohl, F.; Goua, M.; Bermano, G.; Russell, W.R.; Scobbie, L.; Maciel, P.; Kong Thoo Lin, P. Revalorisation of rapeseed pomace extracts: An in vitro study into its anti-oxidant and DNA protective properties. Food Chem. 2018, 239, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Atanassova, M.; Georgieva, S.; Ivancheva, K. Total Phenolic and Total Flavonoid Contents, Antioxidant Capacity and Biological Contaminants in Medicinal Herbs. J. Univ. Chem. Technol. Metall. 2011, 46, 81–88. [Google Scholar]
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free radical scavenging properties of wheat extracts. J. Agric. Food Chem. 2002, 50, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Lee, J.H.; Lee, S.T.; Lee, H.S.; Lee, W.S.; Jeong, T.S.; Park, K.H. Antioxidant and cytotoxic activities of xanthones from Cudrania tricuspidata. Bioorg. Med. Chem. Lett. 2005, 15, 5548–5552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, X.; He, R.; Cheng, S.; Wenjuan, X. Scavenging effect of extracts of green tea and natural antioxidants on active oxygen radicals. Cell Biophys. 1989, 14, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.J.; Ambigaipalan, P.; Shahidi, F. Biological Activities of Camelina and Sophia Seeds Phenolics: Inhibition of LDL Oxidation, DNA Damage, and Pancreatic Lipase and α-Glucosidase Activities. J. Food Sci. 2018, 83, 237–245. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–18 are available from the authors. |

| Extractant | Total Phenolics | Total Flavonoids | DPPH |
|---|---|---|---|
| (GAE mg/100 g) | (QE mg/100 g) | (% at 100 μg/mL) | |
| 0% EtOH | 3300 ± 104.5 | 285 ± 13.5 | 37.2 ± 1.3 |
| 20% EtOH | 3411 ± 185.0 | 892 ± 22.7 | 49.7 ± 0.8 |
| 50% EtOH | 4285 ± 98.7 | 2862 ± 56.4 | 53.6 ± 0.4 |
| 80% EtOH | 4947 ± 100.3 | 3058 ± 10.5 | 78.0 ± 1.2 |
| Compounds | DPPH 1 | ORAC 2 | O2•− 1 | •OH 1 |
|---|---|---|---|---|
| (μg/mL) | (μmol TE/g) | (μg/mL) | (μg/mL) | |
| 1 | 37.3 ± 1.5 | 6.2 ± 0.5 | 41.2 ± 1.8 | 43.3 ± 0.1 |
| 2 | 0.9 ± 0.05 | 11.2 ± 0.6 | 12.8 ± 1.1 | 5.4 ± 0.03 |
| 3 | 2.0 ± 0.03 | 10.5 ± 0.3 | 15.9 ± 0.7 | 7.5 ± 0.4 |
| 4 | 21.8 ± 0.5 | 32.3 ± 1.2 | 21.5 ± 0.8 | 40.3 ± 1.2 |
| 5 | 2.8 ± 0.1 | 15.6 ± 0.7 | 32.4 ± 0.3 | 23.2 ± 0.9 |
| 6 | 4.5 ± 0.7 | 2.5 ± 0.3 | 40.5 ± 0.2 | 9.3 ± 0.05 |
| 7 | 50.6 ± 2.4 | 1.3 ± 0.1 | 56.4 ± 0.4 | 28.0 ± 1.4 |
| 8 | 164.5 ± 2.8 | 2.0 ± 0.4 | 40.2 ± 0.3 | 30.9 ± 0.1 |
| 9 | 10.4 ± 0.4 | 8.1 ± 0.9 | 21.3 ± 0.6 | 31.2 ± 0.9 |
| 10 | 85.3 ± 1.6 | 1.2 ± 0.5 | 50.5 ± 0.7 | 9.7 ± 0.3 |
| 11 | 125.4 ± 2.8 | 0.9 ± 0.03 | 60.9 ± 1.8 | 10.5 ± 0.4 |
| 12 | 58.2 ± 0.6 | 0.3 ± 0.02 | 92.3 ± 4.4 | 18.9 ± 1.0 |
| 13 | 96.8 ± 2.0 | 1.0 ± 0.1 | 58.8 ± 2.0 | 23.9 ± 0.8 |
| 14 | 110.3 ± 3.8 | 1.1 ± 0.2 | 60.4 ± 1.3 | 26.6 ± 0.4 |
| 15 | 22.8 ± 0.2 | 3.3 ± 0.7 | 30.6 ± 0.5 | 11.4 ± 0.3 |
| 16 | 4.7 ± 0.9 | 4.6 ± 0.5 | 30.1 ± 0.8 | 12.5 ± 0.7 |
| 17 | 148.2 ± 2.2 | 0.5 ± 0.03 | 92.8 ± 2.4 | 53.3 ± 0.2 |
| 18 | 18.4 ± 3.5 | 4.4 ± 0.8 | 34.4 ± 1.6 | 32.2 ± 0.1 |
| Trolox | 3.7 ± 0.06 | - | 8.8 ± 0.3 | 21.8 ± 0.7 |
| Compounds | •OH Scavenging Activity (%) | ||
|---|---|---|---|
| 6.0 mg/mL | 12.0 mg/mL | 24.0 mg/mL | |
| 2 | 73.50 | 89.71 | 98.74 |
| 3 | 60.05 | 78.65 | 92.35 |
| 4 | 28.40 | 40.50 | 58.94 |
| 5 | 40.15 | 55.88 | 72.05 |
| Trolox | 22.70 | 38.54 | 54.75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Wang, Y.; Song, Y.H.; Uddin, Z.; Li, Z.P.; Ban, Y.J.; Park, K.H. Antioxidant Activities of Phenolic Metabolites from Flemingia philippinensis Merr. et Rolfe and Their Application to DNA Damage Protection. Molecules 2018, 23, 816. https://doi.org/10.3390/molecules23040816
Kim JY, Wang Y, Song YH, Uddin Z, Li ZP, Ban YJ, Park KH. Antioxidant Activities of Phenolic Metabolites from Flemingia philippinensis Merr. et Rolfe and Their Application to DNA Damage Protection. Molecules. 2018; 23(4):816. https://doi.org/10.3390/molecules23040816
Chicago/Turabian StyleKim, Jeong Yoon, Yan Wang, Yeong Hun Song, Zia Uddin, Zuo Peng Li, Yeong Jun Ban, and Ki Hun Park. 2018. "Antioxidant Activities of Phenolic Metabolites from Flemingia philippinensis Merr. et Rolfe and Their Application to DNA Damage Protection" Molecules 23, no. 4: 816. https://doi.org/10.3390/molecules23040816
APA StyleKim, J. Y., Wang, Y., Song, Y. H., Uddin, Z., Li, Z. P., Ban, Y. J., & Park, K. H. (2018). Antioxidant Activities of Phenolic Metabolites from Flemingia philippinensis Merr. et Rolfe and Their Application to DNA Damage Protection. Molecules, 23(4), 816. https://doi.org/10.3390/molecules23040816





