Preliminary Characterization and Bioactivities of Some Impatiens L. Water-Soluble Polysaccharides
Abstract
:1. Introduction
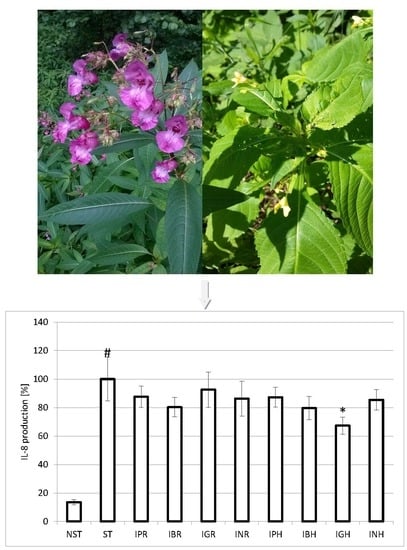
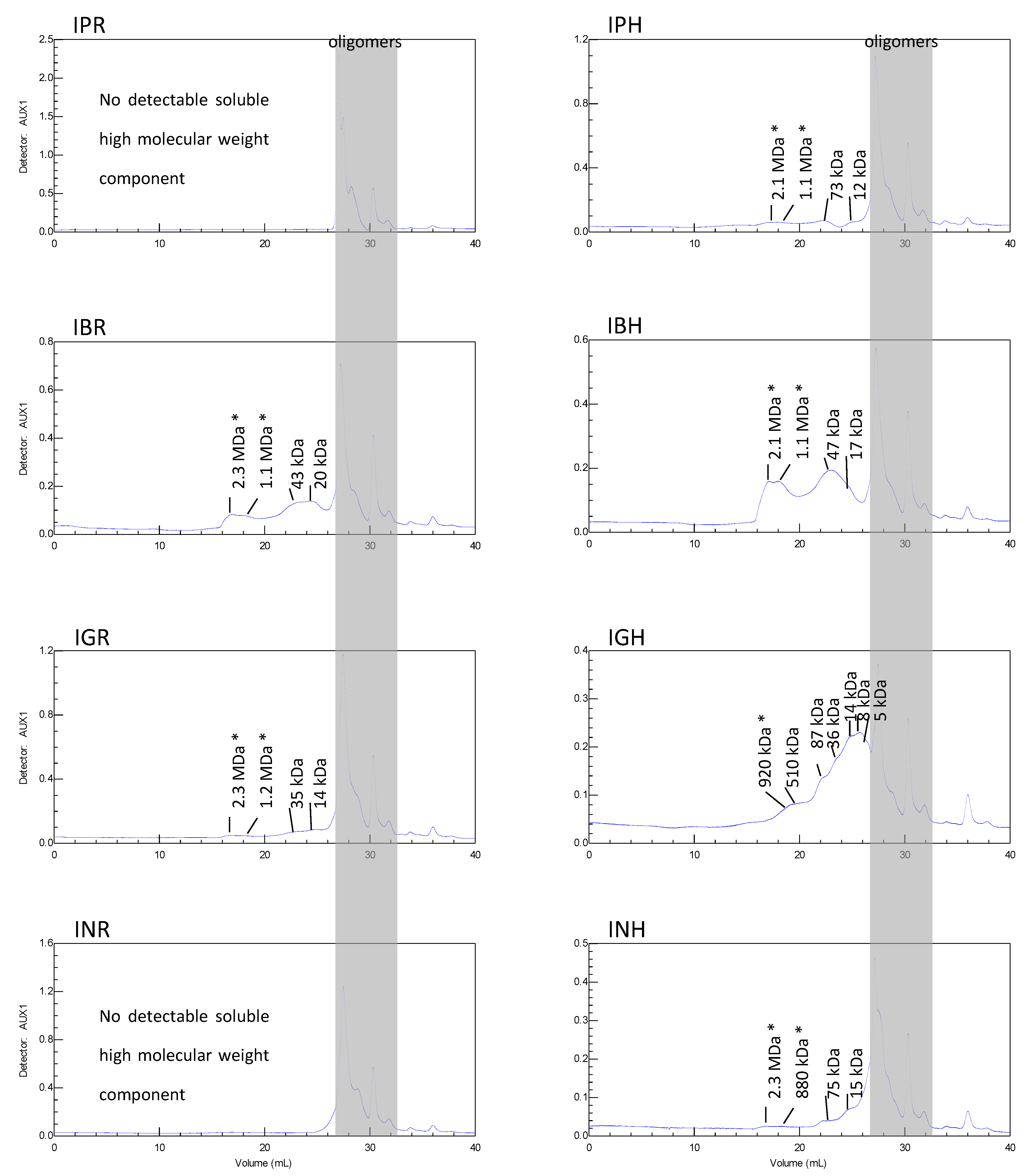
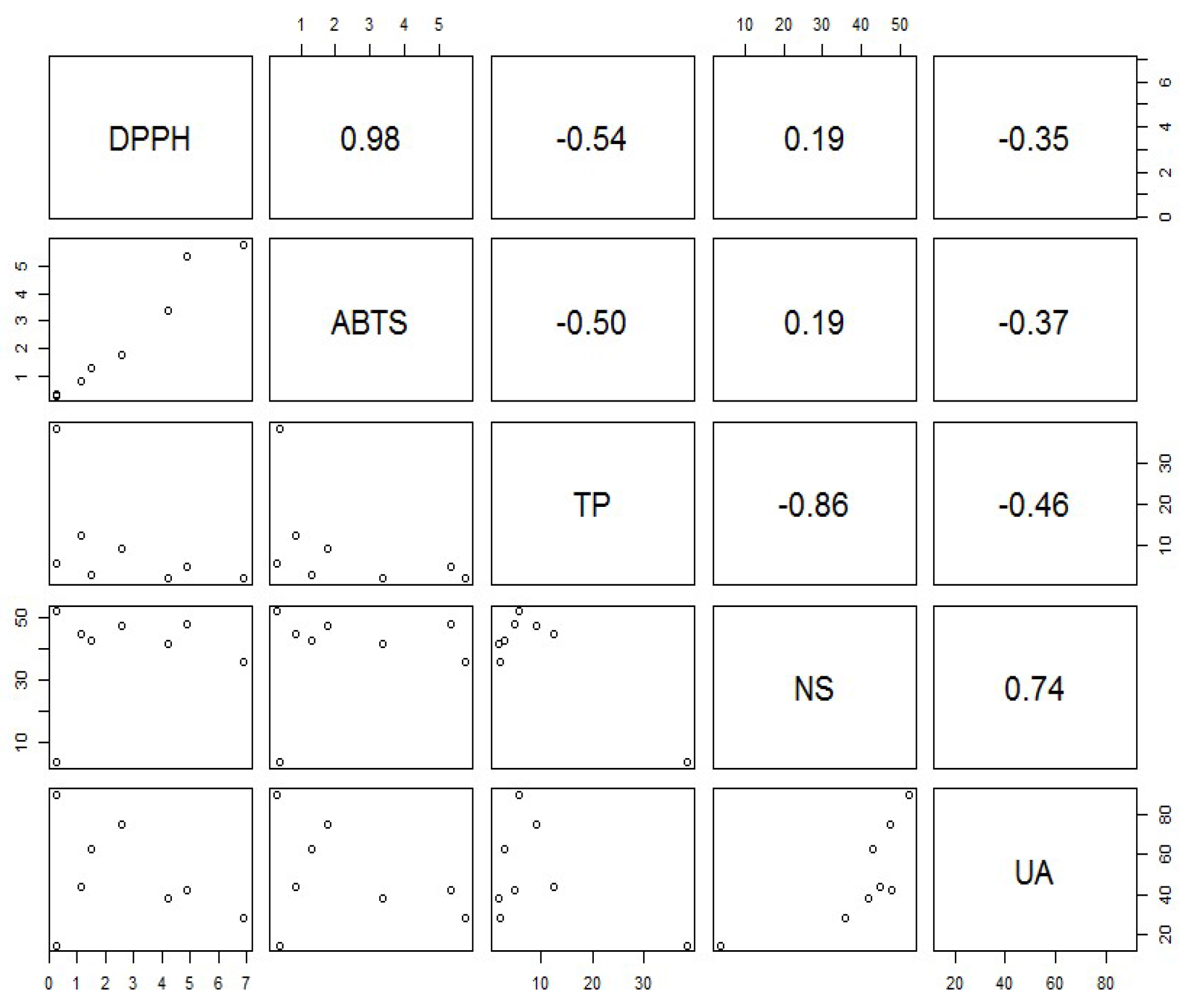
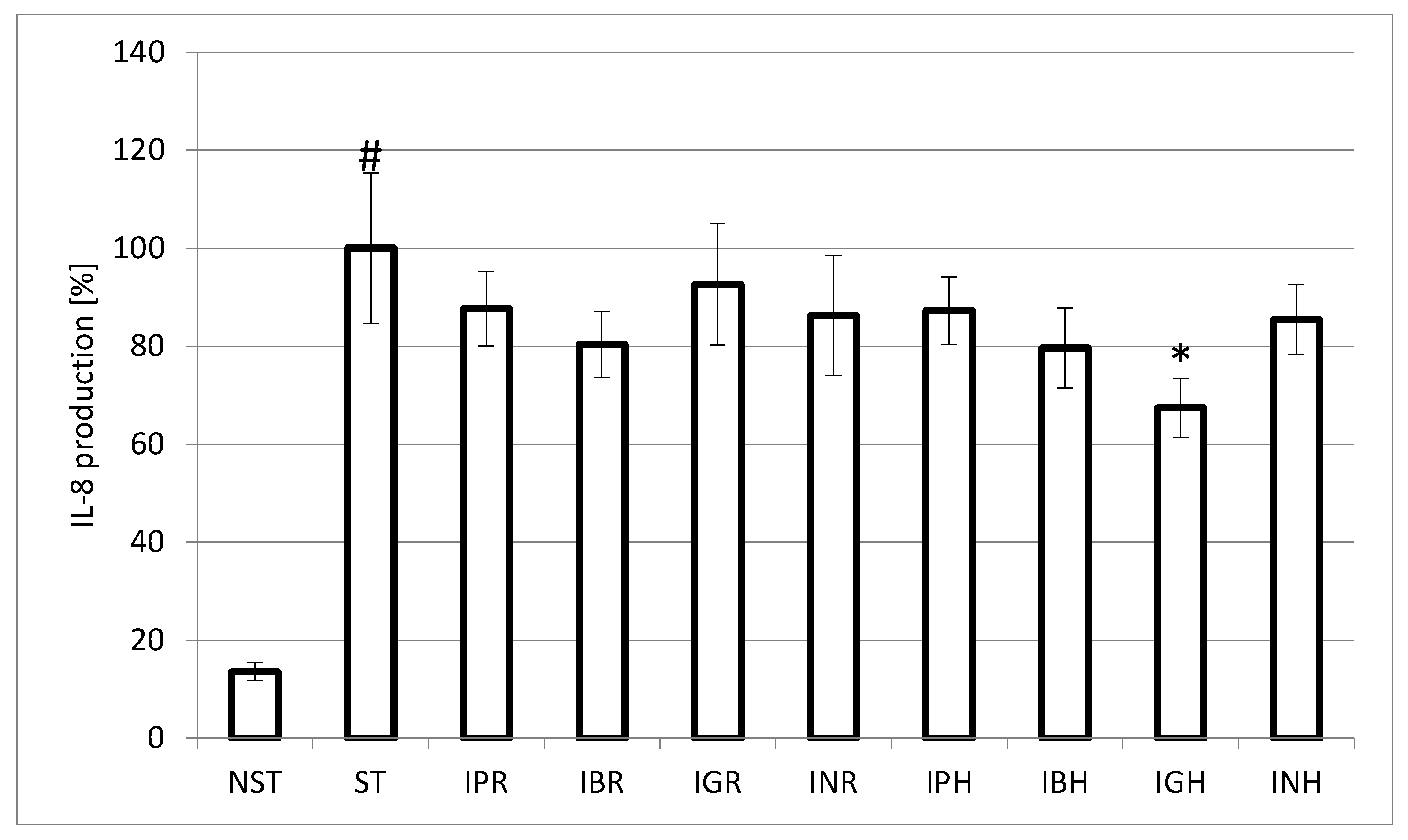
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction of Polysaccharides
3.3. Chemical Composition Analysis
3.4. Monosaccharide Composition Analysis
3.5. Size Exclusion Chromatography (SEC)
3.6. Reactivity with Yariv Reagent
3.7. Antioxidant Activity
3.8. Neutrophils Isolation
3.9. Cytotoxicity
3.10. IL-8 Production
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Ghildyal, P.; Grønhaug, T.E.; Rusten, A.; Skogsrud, M.; Rolstad, B.; Diallo, D.; Michaelsen, T.E.; Inngjerdingen, M.; Paulsen, B.S. Chemical composition and immunological activities of polysaccharides isolated from the Malian medicinal plant Syzygium guineense. J. Pharmacogn. Phytother. 2010, 2, 76–85. [Google Scholar]
- Schepetkin, A.B.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Xie, G.; Kirpotina, L.N.; Klein, R.A.; Jutila, M.A.; Quinn, M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. Int. Immunopharmacol. 2008, 8, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Beta-glucan recognition by the innate immune system. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Dietary Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Ma, L.; Zhang, Z.; Cao, L.; Liu, J.; Zeng, X. Preparation, preliminary characterization and immunostimulatory activity of polysaccharide fractions from the peduncles of Hovenia dulcis. Food Chem. 2013, 138, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L. The Complexity of Membrane-Bound Glycans in Health and Disease and the Beneficial Properties of Glyconutrients. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2013. [Google Scholar]
- Szewczyk, K.; Olech, M. Optimization of extraction method for LC-MS based determination of phenolic acid profiles in different Impatiens species. Phytochem. Lett. 2017, 20, 322–330. [Google Scholar] [CrossRef]
- Szewczyk, K.; Zidorn, C.; Biernasiuk, A.; Komsta, Ł.; Granica, S. Polyphenols from Impatiens (Balsaminaceae) and their antioxidantand antimicrobial activities. Ind. Crops Prod. 2016, 86, 262–272. [Google Scholar] [CrossRef]
- Vieira, M.N.; Winterhalter, P.; Jerz, G. Flavonoids from the flowers of Impatiens glandulifera Royle isolated by high performance countercurrent chromatography. Phytochem. Anal. 2016, 27, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Kalemba, D.; Komsta, Ł.; Nowak, R. Comparison of the essential oil composition of selected Impatiens species and its antioxidant activities. Molecules 2016, 21, 1162. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, A.; Brenne, X.; Feist, E.; Metz, N.; Weniger, B.; Anton, R. Quantitative determination of naphthoquinones of Impatiens species. Phytochem. Anal. 2001, 12, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Ortin, Y.; Evans, P. Trans-tetradec-2-enoic acid in Impatiens glandulifera. Synth. Commun. 2013, 43, 1404–1412. [Google Scholar] [CrossRef]
- Pavela, R.; Vrchotová, N.; Sera, B. Repellency and toxicity of three Impatiens species (Balsaminaceae) extracts on Myzus persicae Sulzer (Homoptera: Aphididae). J. Biopestic. 2009, 2, 48–51. [Google Scholar]
- Cimmino, A.; Mathieu, V.; Evidente, M.; Ferderin, M.; Banuls, L.M.Y.; Masi, M.; De Carvalho, A.; Kiss, R.; Evidente, A. Glanduliferins A and B, two new glucosylated steroids from Impatiens glandulifera, with in vitro growth inhibitory activity in human cancer cells. Fitoterapia 2016, 109, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, K.; Podolak, I.; Galanty, A.; Żmudzki, P.; Koczurkiewicz, P.; Piska, K.; Pękala, E.; Janeczko, Z. Two new triterpenoid saponins from the leaves of Impatiens parviflora DC. and their cytotoxic activity. Ind. Crops Prod. 2017, 96, 71–79. [Google Scholar] [CrossRef]
- Li, H.J.; Yu, J.J.; Li, P. Simultaneous qualification and quantification of baccharane glycosides in Impatientis Semen by HPLC-ESI-MSD and HPLC-ELSD. J. Pharm. Biomed. Anal. 2011, 54, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Shoji, N.; Umeyama, A.; Yoshikawa, K.; Nagai, M.; Arihara, S. Baccharane glycosides from seeds of Impatiens balsamina. Phytochemistry 1994, 37, 1437–1441. [Google Scholar] [CrossRef]
- Shoji, N.; Umeyama, A.; Saitou, N.; Yoshikawa, K.; Kan, Y.; Arihara, S. Hosenkosides A, B, C, D, and E, novel baccharane glycosides from the seeds of Impatiens balsamina. Tetrahedron 1994, 50, 4973–4986. [Google Scholar] [CrossRef]
- Panichayupakaranant, P.; Noguchi, H.; De-Eknamhul, W.; Sankawa, U. Naphthoquinones and coumarins from Impatiens balsamina root cultures. Phytochemistry 1995, 40, 1141–1143. [Google Scholar] [CrossRef]
- Klein, A.O.; Hagen, C.W. Anthocyanin production in detached petals of Impatiens balsamina L. Plant Physiol. 1961, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hromádková, Z.; Košťálová, Z.; Vrchotová, N.; Ebringerová, A. Non-cellulosic polysaccharides from the leaves of small balsam (Impatiens parviflora DC.). Carbohydr. Res. 2014, 389, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, T.V.; Scott, R.; Whittaker, H.A. The Yield, Development and Chemical Composition of Some Fast-Growing Indigenous and Naturalised British Plant Species in Relation to Management as Energy Crops; ITE0640; Institute of Terrestrial Ecology: Cumbria, UK, 1981; p. 89, Unpublished; Available online: http://nora.nerc.ac.uk/id/eprint/8196 (accessed on 30 January 2018).
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Van Holst, G.J.; Clarke, A.E. Quantification of arabinogalactanprotein in plant extracts by single radial gel diffusion. Anal. Biochem. 1985, 148, 446–450. [Google Scholar] [CrossRef]
- Seifert, G.J.; Roberts, K. The biology of arabinogalactan proteins. Annu. Rev. Plant Biol. 2007, 58, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Serpe, M.D.; Nothnagel, E.A. Effect of Yariv phenylglycosides on Rosa cell suspensions: Evidence for the involvement of arabinogalactan-proteins in cell proliferation. Planta 1994, 193, 542–551. [Google Scholar] [CrossRef]
- Bossy, A.; Blaschek, W.; Classen, B. Characterization and immunolocalization of arabinogalactan-proteins in roots of Echinacea purpurea. Planta Med. 2009, 75, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, X.; Wang, Y.; Liu, G.; Zhang, Z.; Zhao, Z.; Cheng, H. In vitro antioxidative and immunological activities of polysaccharides from Zizyphus jujuba cv. Muzao. Int. J. Biol. Macromol. 2017, 95, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, Y.; Jiang, Z.; Zhong, S.; Chen, W.; Zheng, F.; Shi, G. Purification, partial characterization and bioactivity of sulfated polysaccharides from Grateloupia livida. Int. J. Biol. Macromol. 2017, 94, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yang, X.; Huang, W.W.; Zhao, H.T.; Wang, J.; Xu, R.B.; Hu, X.L.; Shen, S.Y.; Qin, D. Characterization and bioactivity of polysaccharides obtained from pine cones of Pinus koraiensis by graded ethanol precipitation. Molecules 2013, 18, 9933–9948. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, J. Structural characterization of a water-soluble polysaccharide from the roots of Codonopsis pilosula and its immunity activity. Int. J. Biol. Macromol. 2008, 43, 279–282. [Google Scholar]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xyu, X.; Zhu, Y. Optimization of hydroxyl radical scavenging activity of exopolysaccharides from Inonotus obliquus in submerged fermentation using response surface methodology. J. Microbiol. Biotechnol. 2010, 20, 835–843. [Google Scholar] [PubMed]
- Wang, J.H.; Luo, J.P.; Yang, X.F.; Zha, X.Q. Structural analysis of a rhamnoarabinogalactan from the stems of Dendrobium nobile Lindl. Food Chem. 2010, 122, 572–576. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Li, H.; Ni, W.; Han, H.; Li, N.; Wan, B.; Zhou, Y.; Tai, G. Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng C.A. Meyer. Carbohydr. Polym. 2009, 77, 544–552. [Google Scholar] [CrossRef]
- Sharma, H.K.; Chhangte, L.; Dolui, A.K. Traditional medicinal plants in Mizoram, India. Fitoterapia 2001, 72, 146–161. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Göllner, E.M.; Blaschek, W.; Classen, B. Structural investigations on arabinogalactan-protein from wheat, isolated with Yariv reagent. J. Agric. Food Chem. 2010, 58, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
- Bleton, J.; Mejanelle, P.; Sansoulet, J.; Goursaud, S.; Tchapla, A. Characterisation of neutral sugars and uronic acids after methanolysis and trimethylsilylation for recognition of plant gums. J. Chromatogr. A 1996, 270, 27–49. [Google Scholar] [CrossRef]
- Kim, J.S.; Laskowich, E.R.; Arumugham, R.G.; Kaiser, R.E.; MacMichael, G.J. Determination of saccharide content in pneumococcal polysaccharides and conjugate vaccines by GC-MSD. Anal. Biochem. 2005, 347, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, E.; Berset, C.M. Use of free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. 1968, 19, 125–131. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

| IPR | IPH | IBR | IBH | IGR | IGH | INR | INH | ||
|---|---|---|---|---|---|---|---|---|---|
| Yield (% w/w) | 1.97 | 4.30 | 6.73 | 18.63 | 7.74 | 8.32 | 4.30 | 4.72 | |
| Neutral sugars (%) | 3.68 ± 0.03 | 44.96 ± 0.13 | 47.42 ± 0.31 | 52.17 ± 0.82 | 41.96 ± 0.15 | 43.06 ± 1.04 | 36.03 ± 0.10 | 47.96 ± 0.15 | |
| Total phenolics (mg GAE/g DE) | 38.27 ± 0.54 | 12.53 ± 0.42 | 9.21 ± 0.13 | 5.81 ± 0.11 | 1.93 ± 0.06 | 3.02 ± 0.08 | 2.14 ± 0.11 | 5.03 ± 0.24 | |
| Uronic acids (%) | 1.48 ± 0.02 | 4.38 ± 0.06 | 7.52 ± 0.23 | 8.94 ± 0.62 | 3.78 ± 0.15 | 6.83 ± 0.11 | 2.81 ± 0.06 | 4.23 ± 0.57 | |
| Contents of the sugar (mol %) | Gal | 0.15 | 7.80 | 10.19 | 12.31 | 6.54 | 11.86 | 2.28 | 9.73 |
| GalA | n.d. | 10.97 | 37.60 | 52.14 | 9.92 | 46.92 | 5.74 | 5.22 | |
| Ara | n.d. | 2.53 | 3.29 | 2.91 | 2.53 | 5.70 | 0.89 | 5.19 | |
| GlcA | n.d. | 7.17 | 15.29 | 17.71 | 6.69 | 10.03 | n.d. | 6.69 | |
| Man | n.d. | 3.04 | 3.80 | 5.62 | 2.74 | 2.43 | 1.22 | 3.34 | |
| Glc | 0.30 | 0.61 | 1.06 | 0.91 | 1.22 | 3.19 | 6.69 | 1.52 | |
| Rha | n.d. | 0.42 | 0.83 | 0.83 | 0.42 | 1.39 | 0.42 | 0.42 | |
| Xyl | n.d. | 0.13 | 0.13 | 0.25 | 0.25 | 0.25 | 0.38 | 0.25 | |
| Fuc | n.d. | 0.30 | 0.30 | 0.61 | 0.15 | 0.15 | n.d. | 0.30 | |
| IPR | IBR | IGR | INR | IPH | IBH | IGH | INH | AA | Trolox | |
|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | 0.27 ± 0.02 | 2.55 ± 0.05 | 4.22 ± 0.06 | 6.89 ± 0.18 | 1.13 ± 0.02 | 0.24 ± 0.01 | 1.49 ± 0.01 | 4.85 ± 0.13 | 0.11 ± 0.01 | 0.08 ± 0.01 |
| ABTS | 0.38 ± 0.02 | 1.78 ± 0.03 | 3.37 ± 0.10 | 5.75 ± 0.19 | 0.84 ± 0.03 | 0.32 ± 0.02 | 1.30 ± 0.01 | 5.31 ± 0.13 | 0.21 ± 0.01 | 0.24 ± 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczyk, K.; Heise, E.M.; Piwowarski, J.P. Preliminary Characterization and Bioactivities of Some Impatiens L. Water-Soluble Polysaccharides. Molecules 2018, 23, 631. https://doi.org/10.3390/molecules23030631
Szewczyk K, Heise EM, Piwowarski JP. Preliminary Characterization and Bioactivities of Some Impatiens L. Water-Soluble Polysaccharides. Molecules. 2018; 23(3):631. https://doi.org/10.3390/molecules23030631
Chicago/Turabian StyleSzewczyk, Katarzyna, Esther Marie Heise, and Jakub P. Piwowarski. 2018. "Preliminary Characterization and Bioactivities of Some Impatiens L. Water-Soluble Polysaccharides" Molecules 23, no. 3: 631. https://doi.org/10.3390/molecules23030631
APA StyleSzewczyk, K., Heise, E. M., & Piwowarski, J. P. (2018). Preliminary Characterization and Bioactivities of Some Impatiens L. Water-Soluble Polysaccharides. Molecules, 23(3), 631. https://doi.org/10.3390/molecules23030631