Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators
Abstract
1. Introduction
2. Production of Alpha Emitters
3. General Radiopharmaceutical Issues
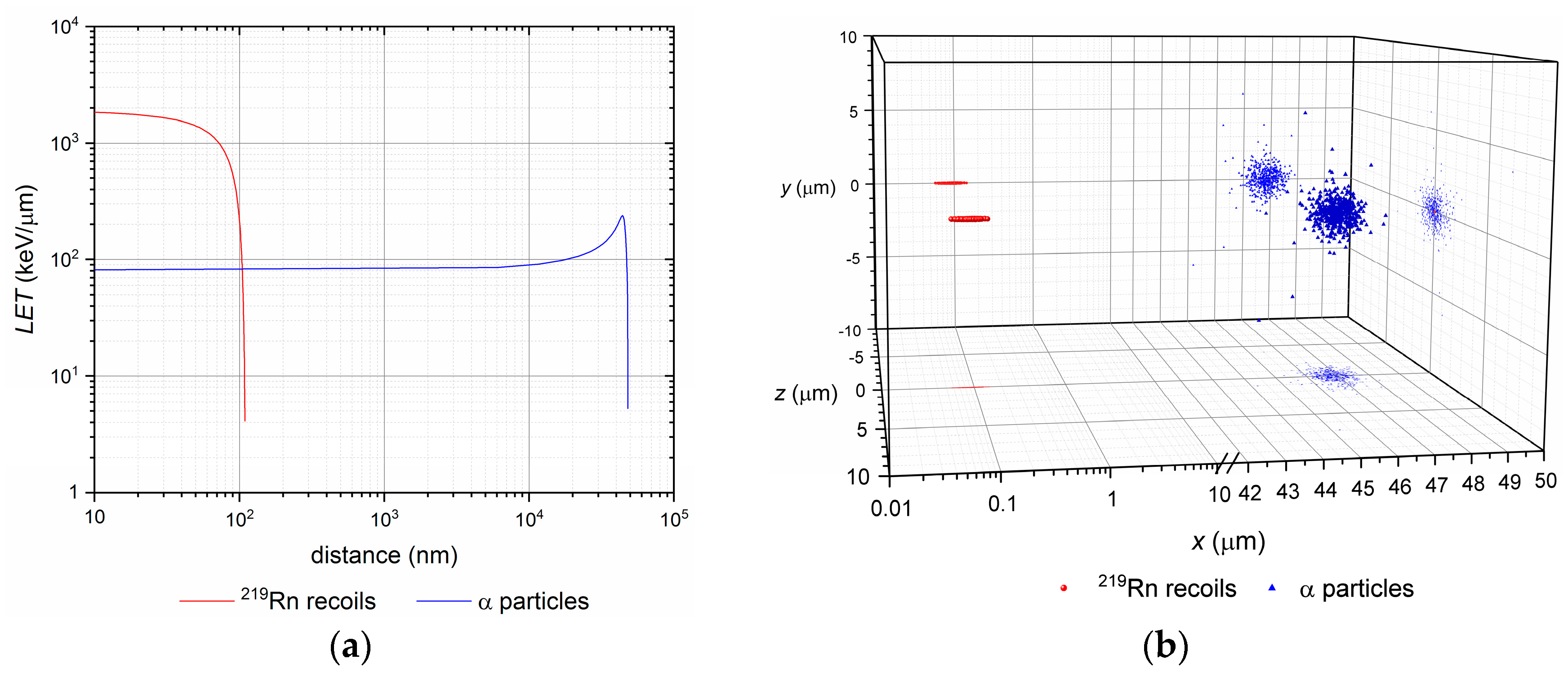
3.1. Nuclear Recoil Effect and the Release of Daughter Nuclei
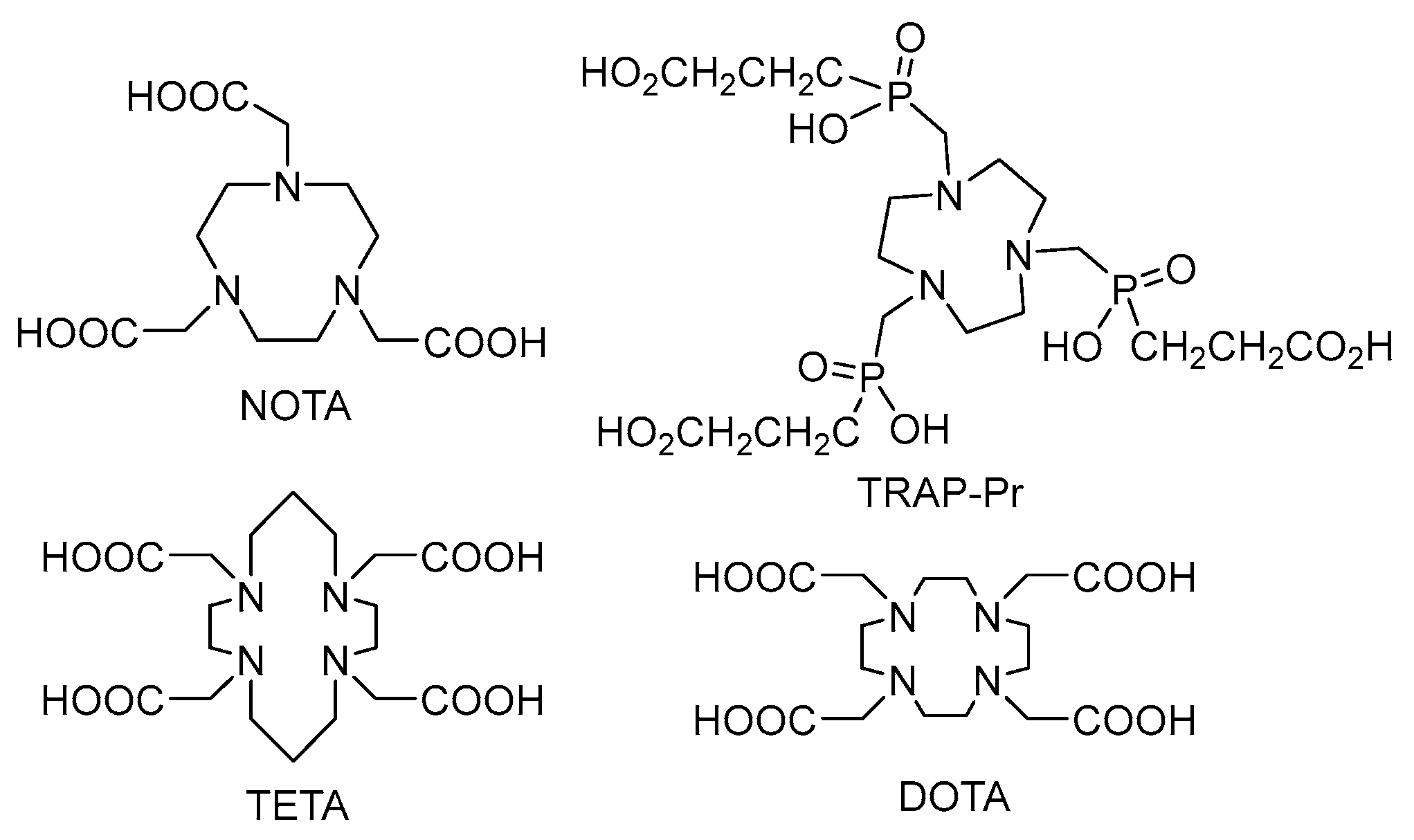
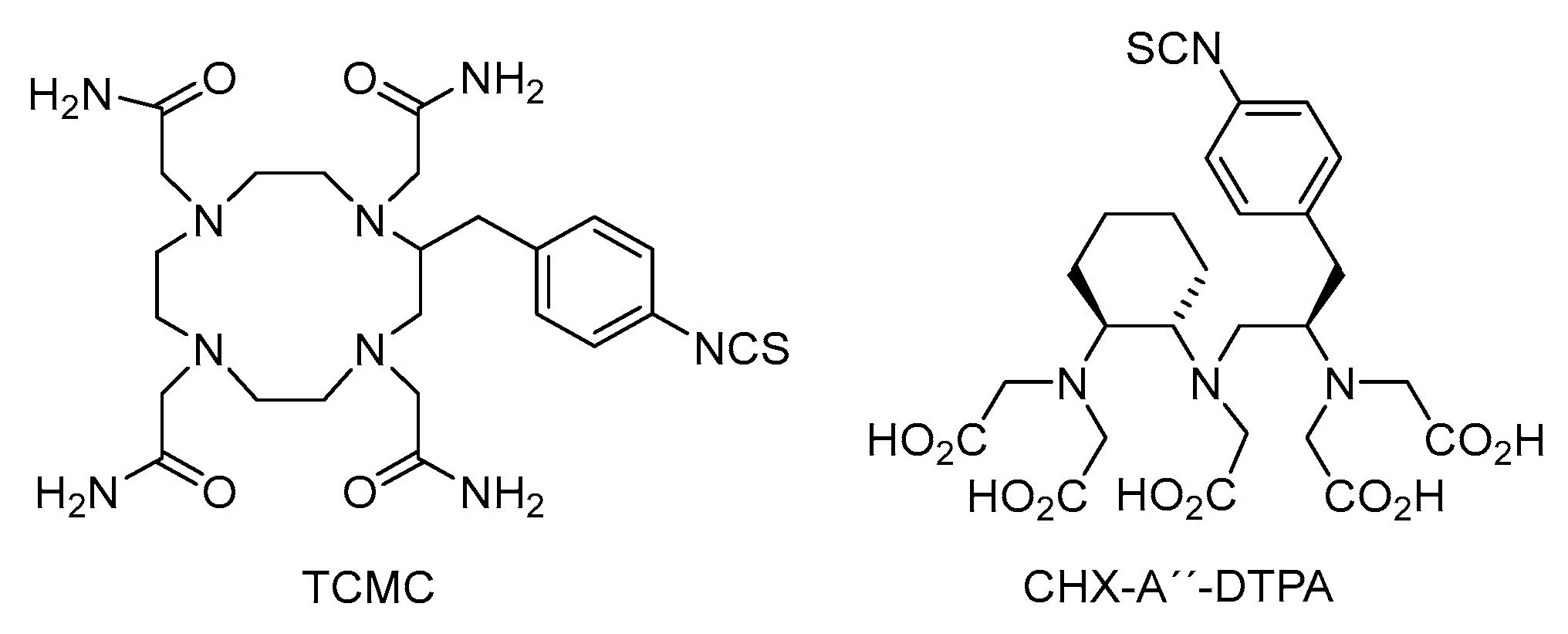
3.2. Labelling Chemistry
3.3. Targeting and Clearance
- “self-targeting” based on physiological affinity of the radioisotope to a given tissue; thus radium tends to accumulate in bones or pertechnetate, astatine or iodide in the thyroid;
- “passive targeting” or “blood circulation and extravasation” is based on accumulation of nanoparticles in the areas around the tumors with leaky vasculature; commonly referred to as the enhanced permeation and retention (EPR) effect [59];
- “active induced targeting” based on specific ligand-receptor interactions between labelled small molecules, peptides, mAbs and their fragments and target cells; externally activated exposure is also possible (temperature, magnetic field or other activators) [60].
3.4. Dosimetry
3.4.1. Body Level
3.4.2. Organ and Sub-Organ Levels
3.4.3. Cellular and Subcellular Level
4. Vectors for Targeted Alpha Particle Therapy
4.1. Small Molecules
4.1.1. MABG
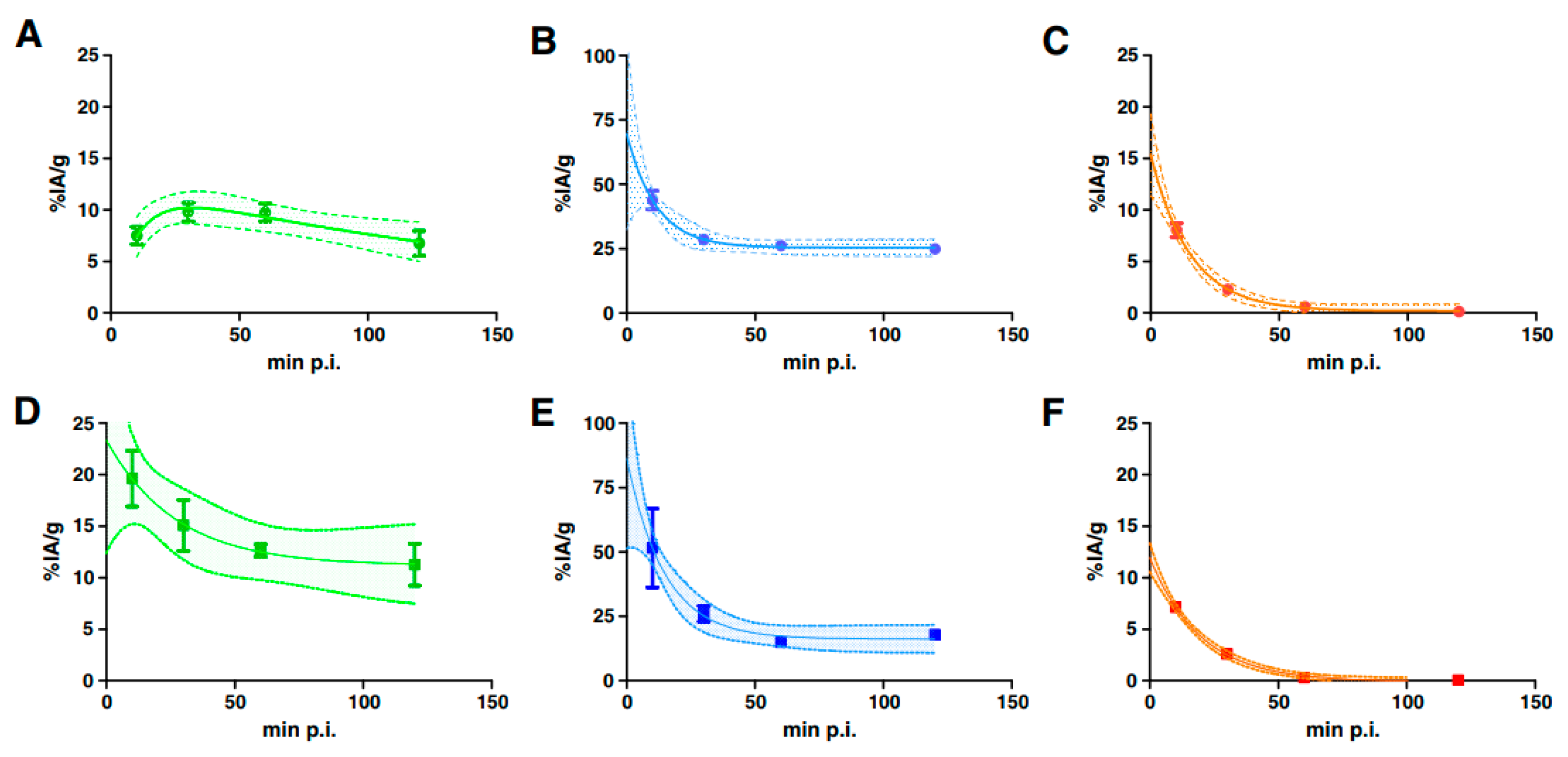
4.1.2. Prostate-Specific Membrane Antigen (PSMA)
4.1.3. Substance P
4.2. Biomolecules—Antibodies
4.3. Macromolecules and Nanoconstructs
5. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, H.; Senthamizhchelvan, S.; Hobbs, R.F.; Sgouros, G. Alpha Particle Emitter Radiolabeled Antibody for Metastatic Cancer: What Can We Learn from Heavy Ion Beam Radiobiology? Antibodies 2012, 1, 124–148. [Google Scholar] [CrossRef]
- Borchardt, P.E.; Yuan, R.R.; Miederer, M.; McDevitt, M.R.; Scheinberg, D.A. Targeted Actinium-225 In Vivo Generators for Therapy of Ovarian Cancer. Cancer Res. 2003, 63, 5084–5090. [Google Scholar] [PubMed]
- Jaggi, J.S.; Kappel, B.J.; McDevitt, M.R.; Sgouros, G.; Flombaum, C.D.; Cabassa, C.; Scheinberg, D.A. Efforts to control the errant products of a targeted in vivo generator. Cancer Res. 2005, 65, 4888–4895. [Google Scholar] [CrossRef] [PubMed]
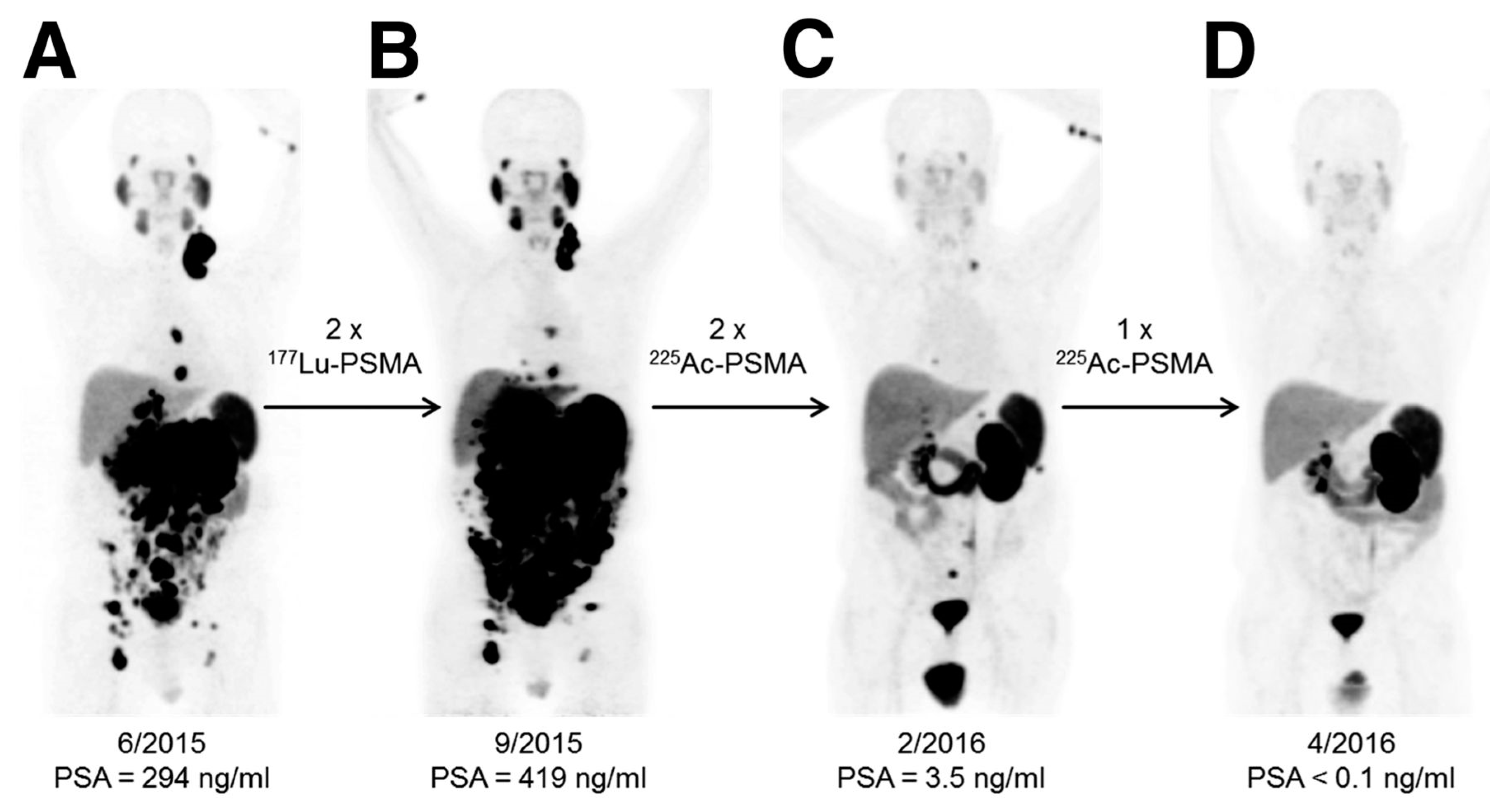
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Habekorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.; Kennel, S.J.; Stuckey, A.; Osborne, D.; Wall, J.; Rondinone, A.J.; Standaert, R.F.; Mirzadeh, S. LaPO4 nanoparticles doped with actinium-225 that partially sequester daughter radionuclides. Bioconj. Chem. 2011, 22, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Kozempel, J.; Vlk, M. Nanoconstructs in Targeted Alpha-Therapy. Rec. Pat. Nanomed. 2014, 4, 71–76. [Google Scholar] [CrossRef]
- Mokhodoeva, O.; Vlk, M.; Málková, E.; Kukleva, E.; Mičolová, P.; Štamberg, K.; Šlouf, M.; Dzhenloda, R.; Kozempel, J. Study of Ra-223 uptake mechanism by Fe3O4 nanoparticles: Towards new prospective theranostic SPIONs. J. Nanopart. Res. 2016, 18, 301. [Google Scholar] [CrossRef]
- Piotrowska, A.; Leszczuk, E.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Functionalized NaA nanozeolites labeled with Ra-224,Ra-225 for targeted alpha therapy. J. Nanopart. Res. 2013, 15, 2082. [Google Scholar] [CrossRef] [PubMed]
- Máthé, D.; Szigeti, K.; Hegedűs, N.; Horváth, I.; Veres, D.S.; Kovács, B.; Szűcs, Z. Production and in vivo imaging of 203Pb as a surrogate isotope for in vivo 212Pb internal absorbed dose studies. Appl. Radiat. Isot. 2016, 114, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R. History and current uses of 224Ra in ankylosing spondylitis and other diseases. Environ. Int. 1993, 19, 467–473. [Google Scholar] [CrossRef]
- Nedrow, J.R.; Josefsson, A.; Park, S.; Back, T.; Hobbs, R.F.; Brayton, C.; Bruchertseifer, F.; Morgenstern, A.; Sgouros, G. Pharmacokinetics, microscale distribution, and dosimetry of alpha-emitter-labeled anti-PD-L1 antibodies in an immune competent transgenic breast cancer model. EJNMI Res. 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Al Darwish, R.; Staudacher, A.H.; Li, Y.; Brown, M.P.; Bezak, E. Development of a transmission alpha particle dosimetry technique using A549 cells and a Ra-223 source for targeted alpha therapy. Med. Phys. 2016, 43, 6145–6153. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, N.L.; Graves, E.E. The Potential for Cerenkov luminescence imaging of alpha emitting isotopes. Phys. Med. Biol. 2012, 57, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, J.S.; Seshan, S.V.; McDevitt, M.R.; Sgouros, G.; Hyjek, E.; Scheinberg, D.A. Mitigation of radiation nephropathy after internal α-particle irradiation of kidneys. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Dekempeneer, Y.; Keyaerts, M.; Krasniqi, A.; Puttemans, J.; Muyldermans, S.; Lahoutte, T.; D’huyvetter, M.; Devoogdt, N. Targeted alpha therapy using short-lived alpha-particles and the promise of nanobodies as targeting vehicle. Expert Opin. Biol. Ther. 2016, 16, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Kozempel, J.; Vlk, M.; Malková, E.; Bajzíková, A.; Bárta, J.; Santos-Oliveira, R.; Malta Rossi, A. Prospective carriers of 223Ra for targeted alpha particle therapy. J. Radioanal. Nucl. Chem. 2015, 304, 443–447. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Holzwarth, U.; Haberl, N.; Kozempel, J.; Hirn, S.; Wenk, A.; Schleh, C.; Schäffler, M.; Lipka, J.; Semmler-Behnke, M.; et al. Quantitative biokinetics of titanium dioxide nanoparticles after intravenous injection in rats: Part 1. Nanotoxicology 2017, 11, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Jekunen, A.; Kairemo, K.; Karnani, P. In Vivo Modulators of Antibody Kinetics. Acta Oncol. 1996, 35, 267–271. [Google Scholar] [CrossRef] [PubMed]
- McAlister, D.R.; Horwitz, E.P. Chromatographic generator systems for the actinides and natural decay series elements. Radiochim. Acta 2017, 99, 151–159. [Google Scholar] [CrossRef]
- Sobolev, A.S.; Aliev, R.A.; Kalmykov, S.N. Radionuclides emitting short-range particles and modular nanotransporters for their delivery to target cancer cells. Russ. Chem. Rev. 2016, 85, 1011–1032. [Google Scholar] [CrossRef]
- Steinber, E.P.; Stehney, A.F.; Stearns, C.; Spaletto, I. Production of 149Tb in gold by high-energy protons and its use as an intensity monitor. Nucl. Phys. A 1968, 113, 265–271. [Google Scholar] [CrossRef]
- Beyer, G.J.; Čomor, J.J.; Daković, M.; Soloviev, D.; Tamburella, C.; Hagebo, E.; Allan, B.; Dmitriev, S.N.; Zaitseva, N.G.; Starodub, G.Y.; et al. Production routes of the alpha emitting 149Tb for medical application. Radiochim. Acta 2002, 90, 247–252. [Google Scholar] [CrossRef]
- Lebeda, O.; Jiran, R.; Ráliš, J.; Štursa, J. A new internal target system for production of At-211 on the cyclotron U-120M. Appl. Radiat. Isot. 2005, 63, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Zalutsky, M.R.; Pruszynski, M. Astatine-211: Production and Availability. Curr. Radiopharm. 2011, 4, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Apostolidis, C.; Molinet, R.; Luetzenkirchen, K. Method for Producing Actinium-225. EP1610346 A1. 28 December 2005. [Google Scholar]
- Apostolidis, C.; Molinet, R.; Rasmussen, G.; Morgenstern, A. Production of Ac-225 from Th-229 for targeted alpha therapy. Anal. Chem. 2005, 77, 6288–6291. [Google Scholar] [CrossRef] [PubMed]
- Griswold, J.R.; Medvedev, D.G.; Engle, J.W.; Copping, R.; Fitzsimmons, J.M.; Radchenko, V.; Cooley, J.C.; Fassbender, M.E.; Denton, D.L.; Murphy, K.E.; et al. Large scale accelerator production of 225Ac: Effective cross sections for 78-192 MeV protons incident on Th-232 targets. Appl. Radiat. Isot. 2016, 118, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.; Henriksen, G. The Preparation and Use of Radium-223 to Target Calcified Tissues for Pain Palliation, Bone Cancer Therapy, and Bone Surface Conditioning. WO 2000/40275. 13 July 2000. [Google Scholar]
- Henriksen, G.; Hoff, P.; Alstad, J.; Larsen, R.H. 223Ra for endoradiotherapeutic applications prepared from an immobilized 227Ac/227Th source. Radiochim. Acta 2001, 89, 661–666. [Google Scholar] [CrossRef]
- Guseva, L.I.; Tikhomirova, G.S.; Dogadkin, N.N. Anion-exchange separation of radium from alkaline-earth metals and actinides in aqueous-methanol solutions of HNO3. 227Ac-223Ra generator. Radiochemistry 2004, 46, 58–62. [Google Scholar] [CrossRef]
- Shishkin, D.N.; Kupitskii, S.V.; Kuznetsov, S.A. Extraction generator of 223Ra for nuclear medicine. Radiochemistry 2011, 53, 343–345. [Google Scholar] [CrossRef]
- Schwarz, U.; Daniels, R. Novel Radiotherapeutic Formulations Containing 224Ra and a Method for Their Production. WO 2002/015943. 28 February 2002. [Google Scholar]
- Šebesta, F.; Starý, J. A generator for preparation of carrier-free 224Ra. J. Radioanal. Chem. 1974, 21, 151–155. [Google Scholar] [CrossRef]
- Larsen, R.H. Radiopharmaceutical Solutions with Advantageous Properties. WO 2016/135200. 1 September 2016. [Google Scholar]
- Ziegler, J.F. SRIM-2013 Code. Available online: http://www.srim.org/ (accessed on 11 November 2017).
- Frenvik, J.O.; Dyrstad, K.; Kristensen, S.; Ryan, O.B. Development of separation technology for the removal of radium-223 from targeted thorium conjugate formulations. Part I: Purification of decayed thorium-227 on cation exchange columns. Drug Dev. Ind. Pharm. 2017, 43, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Frenvik, J.O.; Dyrstad, K.; Kristensen, S.; Ryan, O.B. Development of separation technology for the removal of radium-223 from targeted thorium conjugate formulations. Part II: Purification of targeted thorium conjugates on cation exchange columns. Drug Dev. Ind. Pharm. 2017, 43, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.P.; Kalinina, M.K.; Levkovich, Y.I. Blood flow velocity in capillaries of brain and muscles and its physiological significance. Microvasc. Res. 1981, 22, 143–155. [Google Scholar] [CrossRef]
- Maheshwari, V.; Dearling, J.L.J.; Treves, S.T.; Packard, A.B. Measurement of the rate of copper(II) exchange for 64Cu complexes of bifunctional chelators. Inorg. Chim. Acta 2012, 393, 318–323. [Google Scholar] [CrossRef]
- Chakravarty, R.; Chakraborty, S.; Ram, R.; Vatsa, R.; Bhusari, P.; Shukla, J.; Mittal, B.R.; Dash, A. Detailed evaluation of different 68Ge/68Ga generators: An attempt toward achieving efficient 68Ga radiopharmacy. J. Label. Compd. Radiopharm. 2016, 59, 87. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Plutnar, J.; Wester, H.J. Bone-seeking TRAP conjugates: Surprising observations and their implications on the development of gallium-68-labeled bisphosphonates. EJNMMI Res. 2012, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Holub, J.; Meckel, M.; Kubíček, V.; Rösch, F.; Hermann, P. Gallium(III) complexes of NOTA-bis (phosphonate) conjugates as PET radiotracers for bone imaging. Contrast Media Mol. Imaging 2015, 10, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.A.; Liu, Y.L.; Chen, C.Y.; Chou, X.M. Ligand Preorganization in Metal Ion Complexation: Molecular Mechanics/Dynamics, Kinetics, and Laser-Excited Luminescence Studies of Trivalent Lanthanide Complex Formation with Macrocyclic Ligands TETA and DOTA. Inorg. Chem. 2001, 40, 3448–3455. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.; de Blois, E.; Konijnenberg, M.; Morgenstern, A.; Bruchertseifer, F.; Breeman, W.; de Jong, M. Optimizing labeling conditions of 213Bi-somatostatin analogs for receptor-mediated processes in preclinical models. J. Nucl. Med. 2014, 55 (Suppl. 1), 1179. [Google Scholar]
- Ryan, O.B.; Cuthbertson, A.; Herstad, G.; Grant, D.; Bjerke, R.M. Development of effective chelators for Th-227 to be used in targeted thorium conjugates. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017; p. 57. [Google Scholar]
- Notni, J.; Pohle, K.; Wester, H.J. Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: Practical consequences for the future of gallium68-PET. EJNMMI Res. 2012, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Simeček, J.; Hermann, P.; Wester, H.J.; Notni, J. How is 68Ga-labeling of macrocyclic chelators influenced by metal ion contaminants in 68Ge/68Ga generator eluates? ChemMedChem 2013, 8, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkom, U.; Morgenstern, A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Knoesen, O.; Meckel, M.; Modiselle, M.; Vorster, M.; Marx, S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1099–1100. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Reber, J.; Haller, S.; Dorrer, H.; Köster, U.; Johnston, K.; Zhernosekov, K.; Türler, A.; Schibli, R. Folate Receptor Targeted Alpha-Therapy Using Terbium-149. Pharmaceuticals 2014, 7, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Vermeulen, C.; Köster, U.; Johnston, K.; Türler, A.; Schibli, R.; Van der Meulen, N.P. Alpha-PET with terbium-149: Evidence and perspectives for radiotheragnostics. EJNMMI Radiopharm. Chem. 2016, 1, 5. [Google Scholar] [CrossRef]
- Beyer, G.J.; Miederer, M.; Vranješ-Durić, S.; Čomor, J.J.; Künzi, G.; Hartley, O.; Senekowitsch-Schmidtke, R.; Soloviev, D.; Buchegger, F. The ISOLDE Collaboration. Targeted alpha therapy in vivo: Direct evidence for single cancer cell kill using 149Tb-rituximab. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.B.; Hamlin, D.K.; Wilbur, D.S.; Wilson, L.J. 211AtCl@US-Tube Nanocapsules: A New Concept in Radiotherapeutic-Agent Design. Small 2007, 3, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Kučka, J.; Hrubý, M.; Koňák, Č.; Kozempel, J.; Lebeda, O. Astatination of nanoparticles containing silver as possible carriers of 211At. Appl. Radiat. Isot. 2006, 64, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Leszczuk, E.; Piotrowska, A.; Bilewicz, A. Modified TiO2 nanoparticles as carries for At-211. J. Label. Compd. Radiopharm. 2013, 56, S242. [Google Scholar]
- Dziawer, L.; Koźmiński, P.; Męczyńska-Wielgosz, S.; Pruszyński, M.; Łyczko, M.; Wąs, B.; Celichowski, G.; Grobeny, J.; Jastrzębsky, J.; Bilewicz, A. Gold nanoparticle bioconjugates labelled with 211At for targeted alpha therapy. RSC Adv. 2017, 7, 41024–41032. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Seideman, J.; Sofou, S. Enhanced Loading Efficiency and Retention of 225Ac in Rigid Liposomes for Potential Targeted Therapy of Micrometastases. Bioconj. Chem. 2008, 19, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Baidoo, K.E.; Yong, K.; Brechbiel, M.W. Molecular Pathways: Targeted α-Particle Radiation Therapy. Clin. Cancer Res. 2013, 19, 530–537. [Google Scholar] [CrossRef] [PubMed]
- De Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A Critical Review of Alpha Radionuclide Therapy—How to Deal with Recoiling Daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.; Konijnenberg, M.W.; de Blois, E.; Koelewijn, S.; Baum, R.P.; Morgenstern, A.; Bruchertseifer, F.; Breeman, W.A.; de Jong, M. Influence of tumour size on the efficacy of targeted alpha therapy with 213Bi-[DOTA0,Tyr3]-octreotate. EJNMMI Res. 2016, 6, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Song, E.Y.; Abbas Rizvi, S.M.; Qu, C.F.; Raja, C.; Brechbiel, M.W.; Morgenstern, A.; Apostolidis, C.; Allen, B.J. Pharmacokinetics and toxicity of 213Bi-labeled PAI2 in preclinical targeted alpha therapy for cancer. Cancer Biol. Ther. 2007, 6, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Nedrow, J.R.; Josefsson, A.; Park, S.; Hobbs, R.F.; Bruchertseifer, F.; Morgenstern, A.; Sgouros, G. Reducing renal uptake of free 213Bi associated with the decay of 225Ac-labeled radiopharmaceuticals. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017; p. 67. [Google Scholar]
- Hanadate, S.; Washiyama, K.; Yoshimoto, M.; Matsumoto, H.; Tsuji, A.; Higashi, T.; Yoshii, Y. Oral administration of barium sulfate reduces radiation exposure to the large intestine during alpha therapy with radium-223 dichloride. J. Nucl. Med. 2017, 58 (Suppl. l), 1030. [Google Scholar]
- Edem, P.E.; Fonslet, J.; Kjaer, A.; Herth, M.; Severin, G. In Vivo Radionuclide Generators for Diagnostics and Therapy. Bioinorg. Chem. Appl. 2016, 6148357. [Google Scholar] [CrossRef] [PubMed]
- Hindorf, C.; Chittenden, S.; Aksnes, A.K.; Parker, C.; Flux, G.D. Quantitative imaging of 223Ra-chloride (Alpharadin) for targeted alpha-emitting radionuclide therapy of bone metastases. Nucl. Med. Commun. 2012, 33, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.K.H.; Ramogida, C.F.; Rodriguez-Rodriguez, C.; Blinder, S.; Kunz, P.; Sossi, V.; Schaffer, P. Multi-isotope SPECT imaging of the Ac-225 decay chain: Feasibility studies. Phys. Med. Biol. 2017, 62, 4406–4420. [Google Scholar] [CrossRef] [PubMed]
- Bäck, T.; Jacobsson, L. The α-Camera: A Quantitative Digital Autoradiography Technique Using a Charge-Coupled Device for Ex Vivo High-Resolution Bioimaging of α-Particles. J. Nucl. Med. 2010, 51, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Altman, M.B.; Wang, S.J.; Whitlock, J.L.; Roeske, J.C. Cell detection in phase-contrast images used for alpha-particle track-etch dosimetry: A semi-automated approach. Phys. Med. Biol. 2005, 50, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Muggiolu, G.; Pomorski, M.; Claverie, G.; Berthet, G.; Mer-Calfati, C.; Saada, S.; Devès, G.; Simon, M.; Seznec, H.; Barberet, P. Single α-particle irradiation permits real-time visualization of RNF8 accumulation at DNA damaged sites. Sci. Rep. 2017, 7, 41764. [Google Scholar] [CrossRef] [PubMed]
- Gholami, Y.; Zhu, X.; Fulton, R.; Meikle, S.; El-Fakhri, G.; Kuncic, Z. Stochastic simulation of radium-223 dichloride therapy at the sub-cellular level. Phys. Med. Biol. 2015, 60, 6087–6096. [Google Scholar] [CrossRef] [PubMed]
- Roeske, J.C.; Stinchcomb, T.G. The average number of alpha-particle hits to the cell nucleus required to eradicate a tumour cell population. Phys. Med. Biol. 2006, 51, N179–N186. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, E.; Arazi, L.; Efrati, M.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. Comparative in vitro microdosimetric study of murine- and human-derived cancer cells exposed to alpha particles. Radiat. Res. 2012, 177, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Batra, V.; Ranieri, P.; Makvandi, M.; Tsang, M.; Hou, C.; Li, Y.; Vaidyanathan, G.; Pryma, D.A.; Maris, J.M. Development of meta-[211At]astatobenzylguanidine ([211At]MABG) as an alpha particle emitting systemic targeted radiotherapeutic for neuroblastoma. Cancer Res. 2015, 75 (Suppl. 15), 1610. [Google Scholar] [CrossRef]
- Ohshima, Y.; Watanabe, S.; Tsuji, A.; Nagatsu, K.; Sakashima, T.; Sugiyama, A.; Harada, Y.; Waki, A.; Yoshinaga, K.; Ishioka, N. Therapeutic efficacy of α-emitter meta-211At-astato-benzylguanidine (MABG) in a pheochromocytoma model. J. Nucl. Med. 2016, 57 (Suppl. 2), 468. [Google Scholar]
- Vaidyanathan, G.; Affleck, D.J.; Alston, K.L.; Zhao, X.-G.; Hens, M.; Hunter, D.H.; Babich, J.; Zalutsky, M.R. A Kit Method for the High Level Synthesis of [211At]MABG. Bioorg. Med. Chem. 2007, 15, 3430–3436. [Google Scholar] [CrossRef] [PubMed]
- Nonnekens, J.; Chatalic, K.L.S.; Molkenboer-Kuenen, J.D.M.; Beerens, C.E.M.T.; Bruchertseifer, F.; Morgenstern, A.; Veldhoven-Zweistra, J.; Schottelius, M.; Wester, H.-J.; van Gent, D.C.; et al. 213Bi-Labeled Prostate-Specific Membrane Antigen-Targeting Agents Induce DNA Double-Strand Breaks in Prostate Cancer Xenografts. Cancer Biother. Radiopharm. 2017, 32, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Krolicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Rola, R.; Merlo, A.; et al. Targeted alpha therapy of glioblastoma multiforme: Clinical experience with 213Bi- and 225Ac-Substance P. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017; p. 24. [Google Scholar]
- Cordier, D.; Krolicki, L.; Morgenstern, A.; Merlo, A. Targeted Radiolabeled Compounds in Glioma Therapy. Semin. Nucl. Med. 2016, 46, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.; Bezak, E.; Allen, B.J. Global comparison of targeted alpha vs targeted beta therapy for cancer: In vitro, in vivo and clinical trials. Crit. Rev. Oncol. Hematol. 2018, 123, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Aghevlian, S.; Boyle, A.J.; Reilly, R.M. Radioimmunotherapy of cancer with high linear energy transfer (LET) radiation delivered by radionuclides emitting α-particles or Auger electrons. Adv. Drug Deliv. Rev. 2017, 109, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Jurcic, J.; Scheinberg, D. Efficacy of Ac-225-labeled anti-CD33 antibody in acute myeloid leukemia (AML) correlates with peripheral blast count. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017; p. 22. [Google Scholar]
- Actinium Pharmaceuticals Provides Update on Actimab-A Phase 2 Clinical Trial for Patients with Acute Myeloid Leukemia. Available online: https://ir.actiniumpharma.com/press-releases/detail/247 (accessed on 31 December 2017).
- Autenrieth, M.E.; Horn, T.; Kurtz, F.; Nguyen, K.; Morgenstern, A.; Bruchertseifer, F.; Schwaiger, M.; Blechert, M.; Seidl, C.; Senekowitsch-Schmidtke, R.; et al. Intravesical radioimmunotherapy of carcinoma in situ of the urinary bladder after BCG failure. Urol. A 2017, 56, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Tworowska, I.; Stallons, T.; Saidi, A.; Wagh, N.; Rojas-Quijano, F.; Jurek, P.; Kiefer, G.; Torgue, J.; Delpassand, E. Pb203-AR-RMX conjugates for image-guided TAT of neuroendocrine tumors (NETs). In Proceedings of the American Association for Cancer Research Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar] [CrossRef]
- RadioMedix and AREVA Med Announce Initiation of Phase 1 Clinical Trial of AlphaMedixTM, a Targeted Alpha Therapy for Patients with Neuroendocrine Tumors. Available online: http://radiomedix.com/news/radiomedix-and-areva-med-announce-initiation-of-phase-1-clinical-trial-of-alphamedixtm-a-targeted-alpha-therapy-for-patients-with-neuroendocrine-tumors (accessed on 31 December 2017).
- Dadachova, E.; Morgenstern, A.; Bruchertseifer, F.; Rickles., D.J. Radioimmunotherapy with novel IgG to melanin and its comparison with immunotherapy. J. Nucl. Med. 2017, 58 (Suppl. 1), 1036. [Google Scholar]
- Boudousq, V.; Bobyk, L.; Busson, M.; Garambois, V.; Jarlier, M.; Charalambatou, P.; Pèlegrin, A.; Paillas, S.; Chouin, N.; Quenet, F.; et al. Comparison between Internalizing Anti-HER2 mAbs and Non-Internalizing Anti-CEA mAbs in Alpha-Radioimmunotherapy of Small Volume Peritoneal Carcinomatosis Using 212Pb. PLoS ONE 2013, 8, e69613. [Google Scholar] [CrossRef] [PubMed]
- Ballangrud, Å.M.; Yang, W.-H.; Palm, S.; Enmon, R.; Borchardt, P.E.; Pellegrini, V.A.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Alpha-Particle Emitting Atomic Generator (Actinium-225)-Labeled Trastuzumab (Herceptin) Targeting of Breast Cancer Spheroids. Clin. Cancer Res. 2004, 10, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Boskovitz, A.; McLendon, R.E.; Okamura, T.; Sampson, J.H.; Bigner, D.D.; Zalutsky, M.R. Treatment of HER2-positive breast carcinomatous meningitis with intrathecal administration of α-particle-emitting 211At-labeled trastuzumab. Nucl. Med. Biol. 2009, 36, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Pruszyński, M.; D’Huyvetter, M.; Cędrowska, E.; Lahoutte, T.; Bruchertseifer, F.; Morgenstern, A. Preclinical evaluation of anti-HER2 2Rs15d nanobody labeled with 225Ac. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017; p. 34. [Google Scholar]
- Dekempeneer, Y.; D’Huyvetter, M.; Aneheim, E.; Xavier, C.; Lahoutte, T.; Bäck, T.; Jensen, H.; Caveliers, V.; Lindegren, S. Preclinical evaluation of astatinated nanobodies for targeted alpha therapy. In Proceedings of the 10th International Symposium on Targeted Alpha Therapy, Kanazawa, Japan, 30 May–1 June 2017; p. 35. [Google Scholar]
- Puentes, L.; Xu, K.; Hou, C.; Mach, R.H.; Maris, J.M.; Pryma, D.A.; Makvandi, M. Targeting PARP-1 to deliver alpha-particles to cancer chromatin. In Proceedings of the American Association for Cancer Research Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar] [CrossRef]
- Carrasquillo, J.A. Alpha Radionuclide Therapy: Principles and Applications to NETs. In Diagnostic and Therapeutic Nuclear Medicine for Neuroendocrine Tumors; Pacak, K., Taïeb, D., Eds.; Humana Press: Cham, Switzerland, 2017; pp. 429–445. [Google Scholar]
- Norain, A.; Dadachova, E. Targeted Radionuclide Therapy of Melanoma. Semin. Nucl. Med. 2016, 46, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Banerjee, S. Envisaging an alpha therapy programme in the atomic energy establishments: The priorities and the nuances. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- Koziorowski, J.; Stanciu, A.E.; Gomez-Vallejo, V.; Llop, J. Radiolabeled nanoparticles for cancer diagnosis and therapy. Anticancer Agents Med. Chem. 2017, 17, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Beeler, E.; Gabani, P.; Singh, O.M. Implementation of nanoparticles in therapeutic radiation oncology. J. Nanopart. Res. 2017, 19, 179. [Google Scholar] [CrossRef]
- Drude, N.; Tienken, L.; Mottaghy, F.M. Theranostic and nanotheranostic probes in nuclear medicine. Methods 2017, 130, 14–22. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.F.; Robertson, D.; Pevsner, P.H.; Wall, J.S.; Mirzadeh, S.; Kennel, S.J. LnPO4 Nanoparticles Doped with Ac-225 and Sequestered Daughters for Targeted Alpha Therapy. Cancer Biother. Radiopharm. 2014, 29, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.V.; Woodward, J.D.; Chen, N.; Rondinone, A.J.; Castano, C.H.; Mirzadeh, S. Synthesis and characterization of lanthanum phosphate nanoparticles as carriers for Ra-223 and Ra-225 for targeted alpha therapy. Nucl. Med. Biol. 2015, 42, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Salberg, G.; Larsen, R. Alpha-Emitting Hydroxyapatite Particles. WO 2005/079867. 1 September 2015. [Google Scholar]
- Westrøm, S.; Bønsdorff, T.B.; Bruland, Ø.; Larsen, R. Therapeutic Effect of α-Emitting Ra-Labeled Calcium Carbonate Microparticles in Mice with Intraperitoneal Ovarian Cancer. Transl. Oncol. 2018, 11, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.; Majkowska-Pilip, A.; Bilewicz, A.; Dobrowolski, J.C. On AunAt clusters as potential astatine carriers. RSC Adv. 2017, 7, 35854–35857. [Google Scholar] [CrossRef]
- Piotrowska, A.; Męczyńska-Wielgosz, S.; Majkowska-Pilip, A.; Koźmiński, P.; Wójciuk, G.; Cędrowska, E.; Bruchertseifer, F.; Morgenstern, A.; Kruszewski, M.; Bilewicz, A. Nanozeolite bioconjugates labeled with 223Ra for targeted alpha therapy. Nucl. Med. Biol. 2017, 47, 10–18. [Google Scholar] [CrossRef] [PubMed]
- De Kruijff, R.M.; Drost, K.; Thijssen, L.; Morgenstern, A.; Bruchertseifer, F.; Lathouwers, D.; Wolterbeek, H.T.; Denkova, A.G. Improved 225Ac daughter retention in LnPO4 containing polymersomes. Appl. Radiat. Isot. 2017, 128, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Bandekar, A.; Sempkowski, M.; Banerjee, S.R.; Pomper, M.G.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Nanoconjugation of PSMA-targeting ligands enhances perinuclear localization and improves efficacy of delivered alpha-particle emitters against tumor endothelial analogues. Mol. Cancer Ther. 2016, 15, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Sempkowski, M.; Holleran, T.; Linz, T.; Bertalan, T.; Josefsson, A.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Alpha-particle radiotherapy: For large solid tumors diffusion trumps targeting. Biomaterials 2017, 130, 67–75. [Google Scholar] [CrossRef] [PubMed]
| Radionuclide System * | Half-Life | Eαmax/Echain [MeV] | Production | Status | References |
|---|---|---|---|---|---|
| 149Tb | 4.12 h | 3.97 | 152Gd(p, 4n)149Tb | Research | [22,23] |
| 211At | 7.2 h | 5.87 | 209Bi(α, 3n)211At | Clinical trials | [24,25] |
| 229Th/ | 7340 years | 5.83/27.62 | 229Th decay | Clinical trials | [26,27,28] |
| 225Ac// | 10 days | 226Ra(p, 2n)225Ac | |||
| 213Bi | 46 min | 232Th(p, x)225Ac | |||
| 227Ac/ | 27 years | 5.87/26.70 | 227Ac/227Th/223Ra decay | Clinical praxis | [29,30,31,32] |
| 227Th/ | 18 days | ||||
| 223Ra | 11 days | ||||
| 228Th/ | 1.9 years | 5.69/27.54 | 228Th/224Ra decay | Formerly in c.p., Research | [33,34,35] |
| 224Ra/ | 3.7 days | ||||
| 212Pb | 10.6 h |
| Material | Range (nm) |
|---|---|
| Au | 11 |
| ZrO2 ICRU-712 | 26 |
| Al2O3 ICRU-106 | 27 |
| TiO2 ICRU-652 | 28 |
| SiO2 ICRU-245 | 46 |
| adult cortical bone | 53 |
| human blood | 85 |
| prostate tissue | 87 |
| water | 89 |
| nitrogen gas | 76,000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozempel, J.; Mokhodoeva, O.; Vlk, M. Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators. Molecules 2018, 23, 581. https://doi.org/10.3390/molecules23030581
Kozempel J, Mokhodoeva O, Vlk M. Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators. Molecules. 2018; 23(3):581. https://doi.org/10.3390/molecules23030581
Chicago/Turabian StyleKozempel, Ján, Olga Mokhodoeva, and Martin Vlk. 2018. "Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators" Molecules 23, no. 3: 581. https://doi.org/10.3390/molecules23030581
APA StyleKozempel, J., Mokhodoeva, O., & Vlk, M. (2018). Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators. Molecules, 23(3), 581. https://doi.org/10.3390/molecules23030581