Synergistic Effects of Salvianolic Acid B and Puerarin on Cerebral Ischemia Reperfusion Injury
Abstract
1. Introduction
2. Results
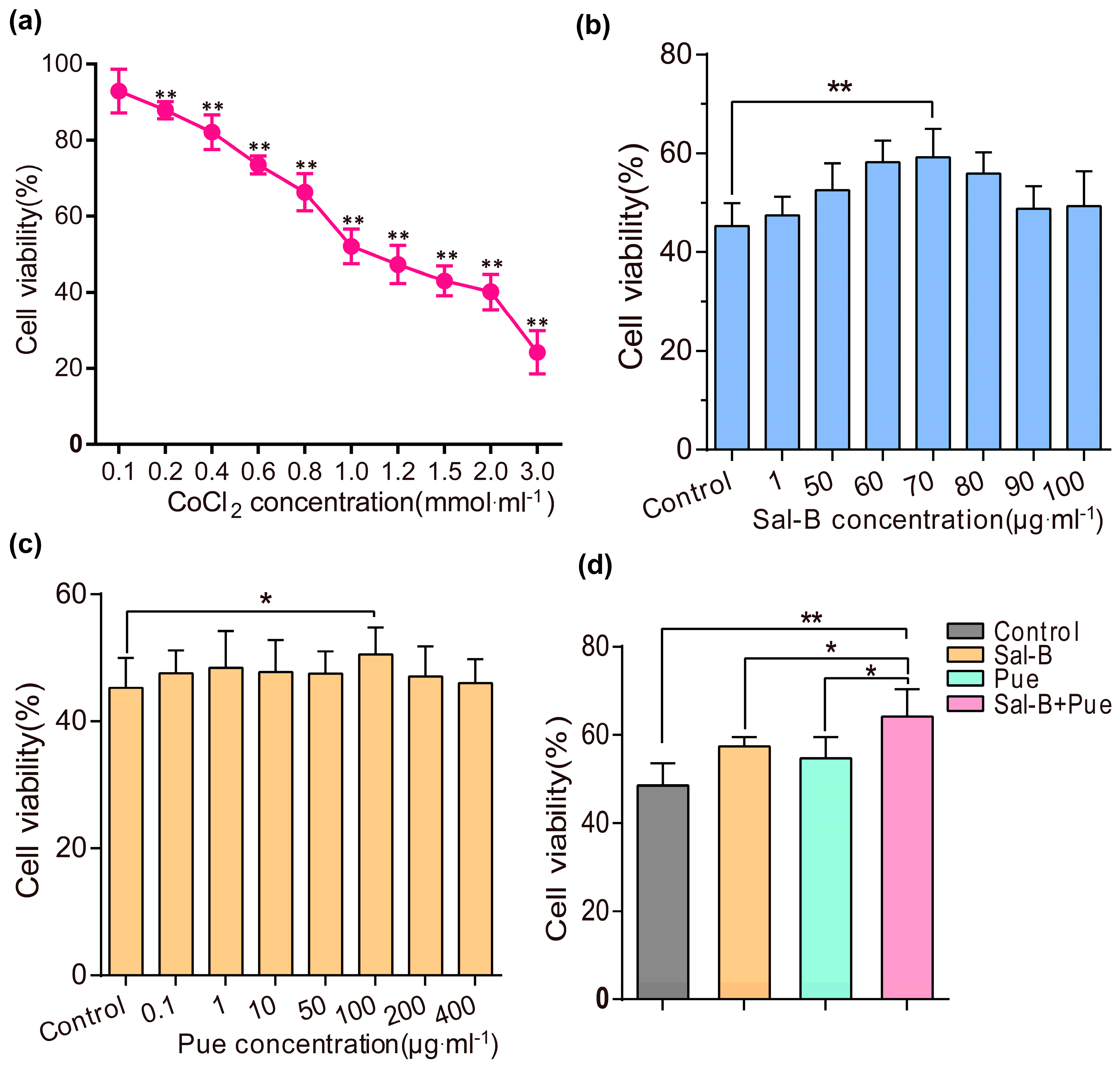
2.1. Sal-B, Pue and Sal-B + Pue Increased Cells Viabilities on Cobalt Chloride (CoCl2) Induced PC12 Cells Injury
2.2. Combination of Sal-B and Pue Could Reduce ROS Level, Inhibit Apoptosis and Increase Mitochondrial Membrane Potential of CoCl2 Induced PC12 Cells
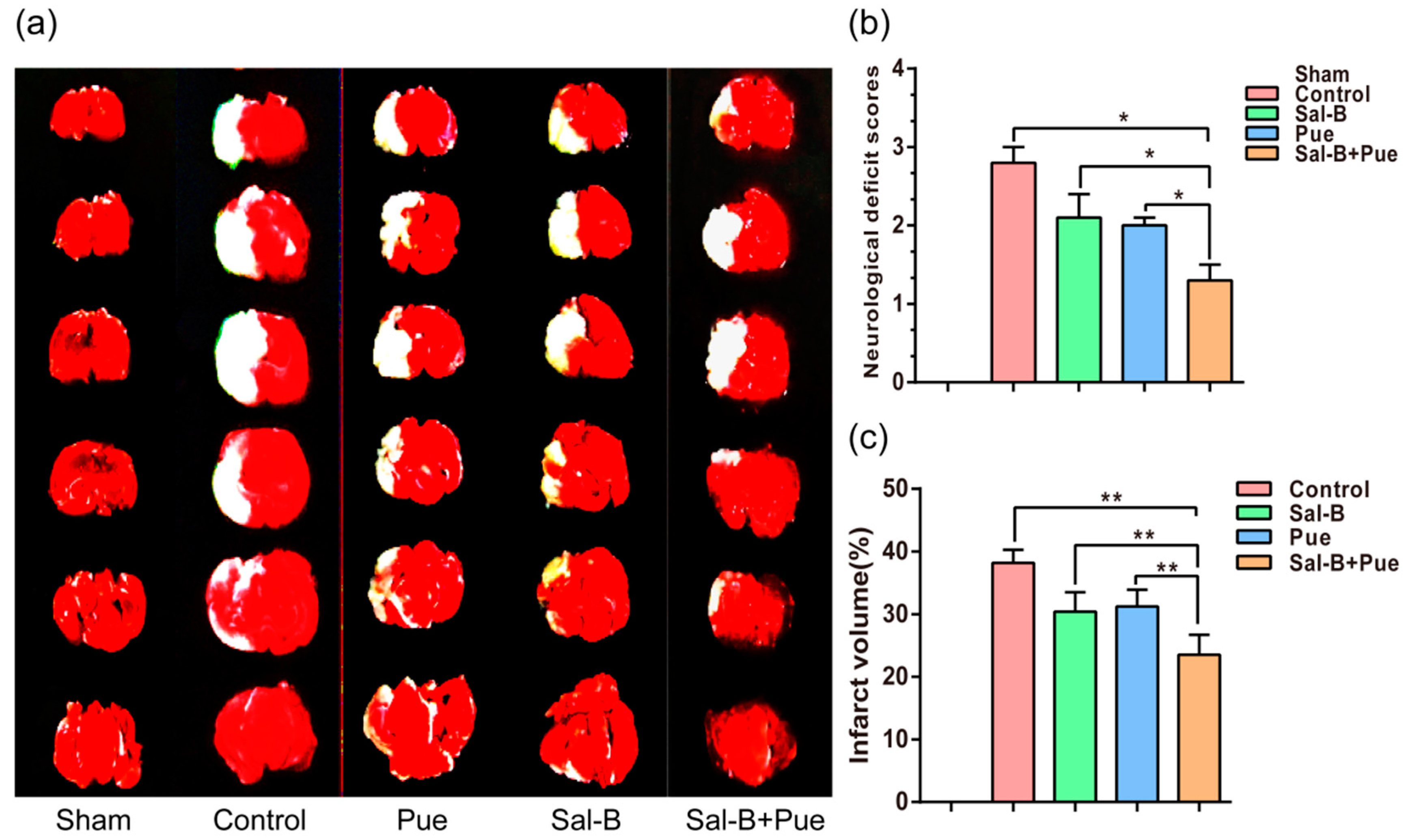
2.3. Combination of Sal-B and Pue Decreased the Volume of Cerebral Infarction in Rats
2.4. Combination of Sal-B and Pue Inhibited Neuronal Apoptosis and Relieved Injury of Ischemia Tissue in Rats
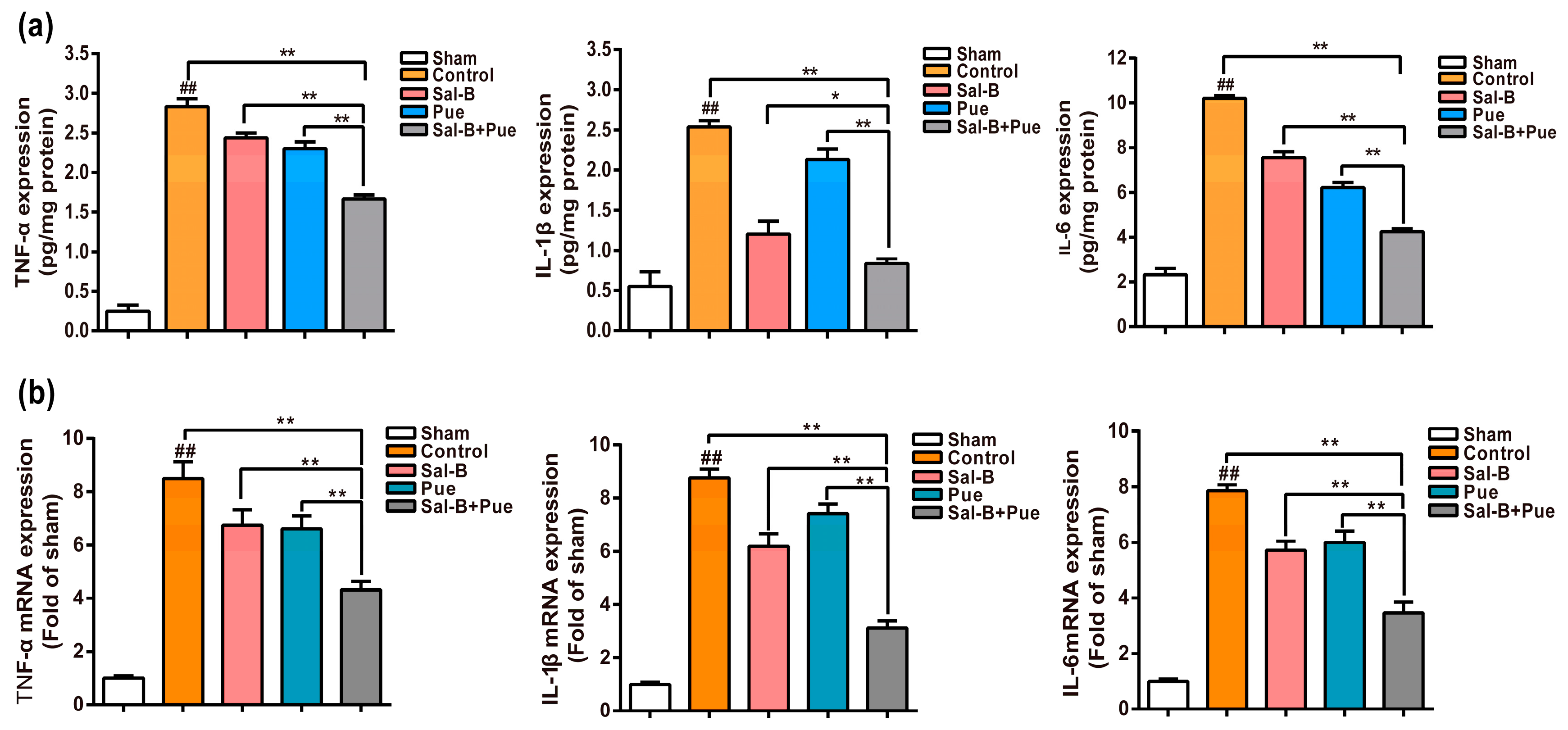
2.5. Combination of Sal-B and Pue Attenuated Pro-Inflammatory Mediators mRNA and Protein Expression in Penumbra Region
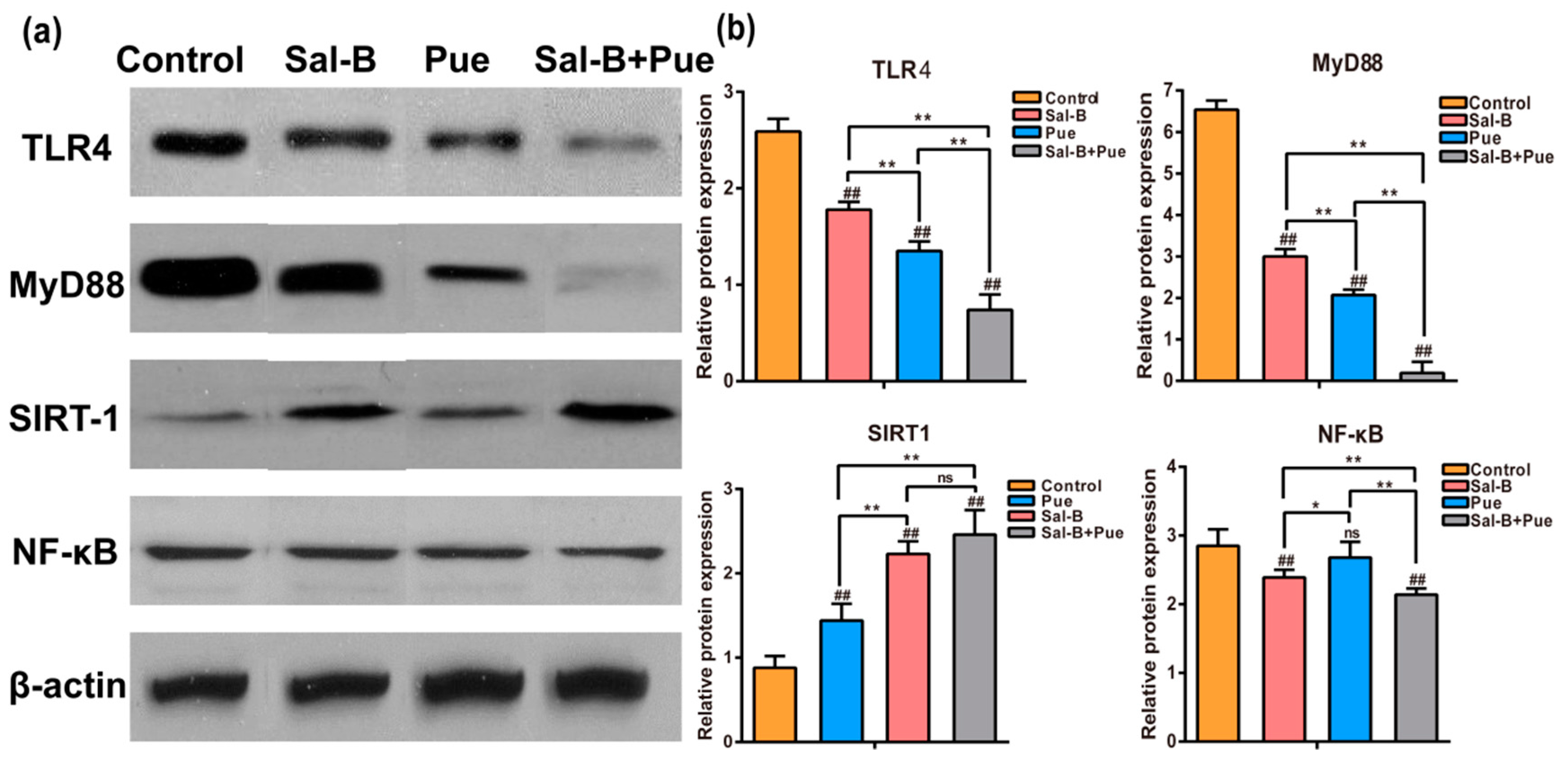
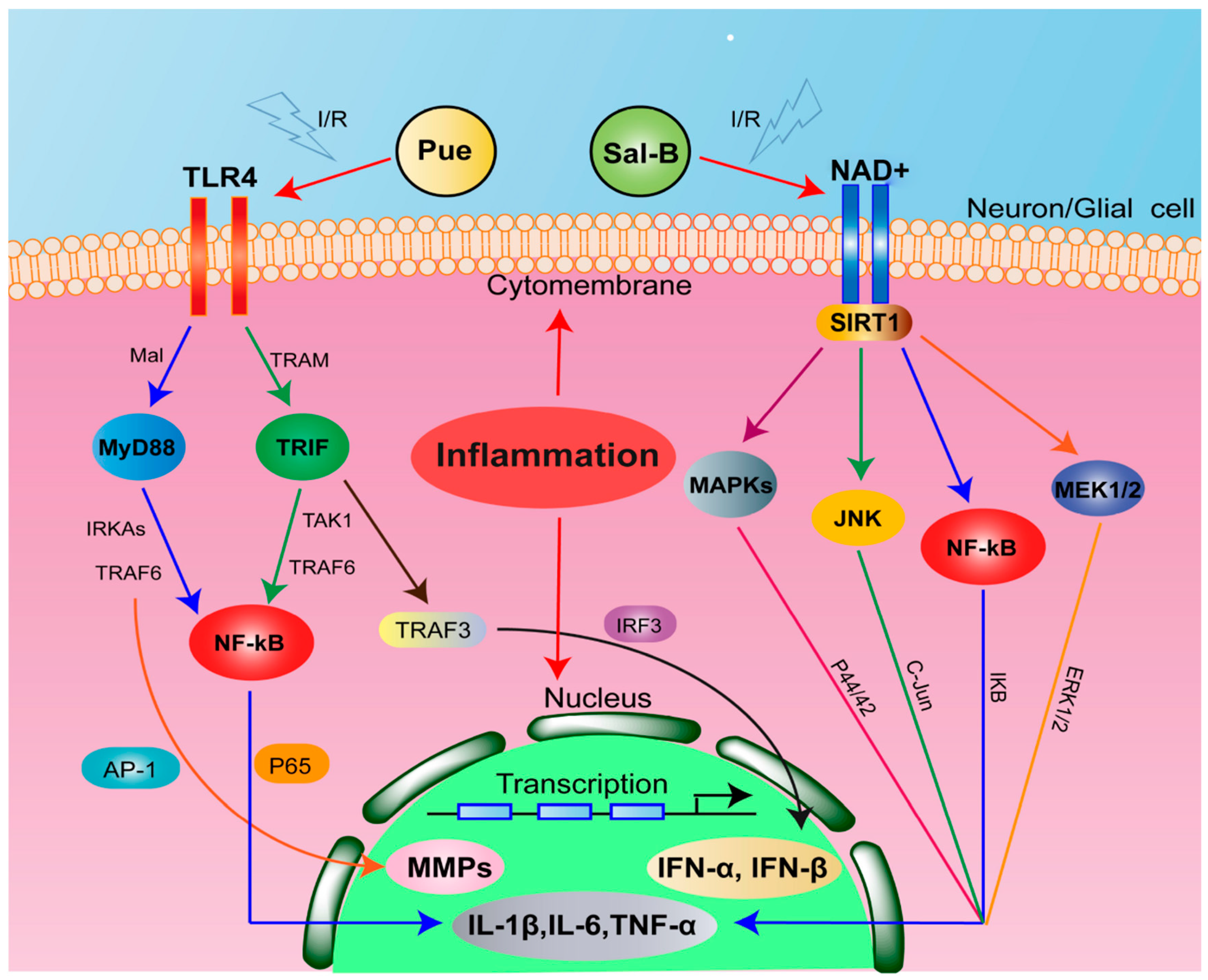
2.6. Combination of Sal-B and Pue Inhibited TLR4/MyD88 and SIRT1 Activation Signaling Pathway in Penumbra Tissue
3. Discussion
4. Materials and Methods
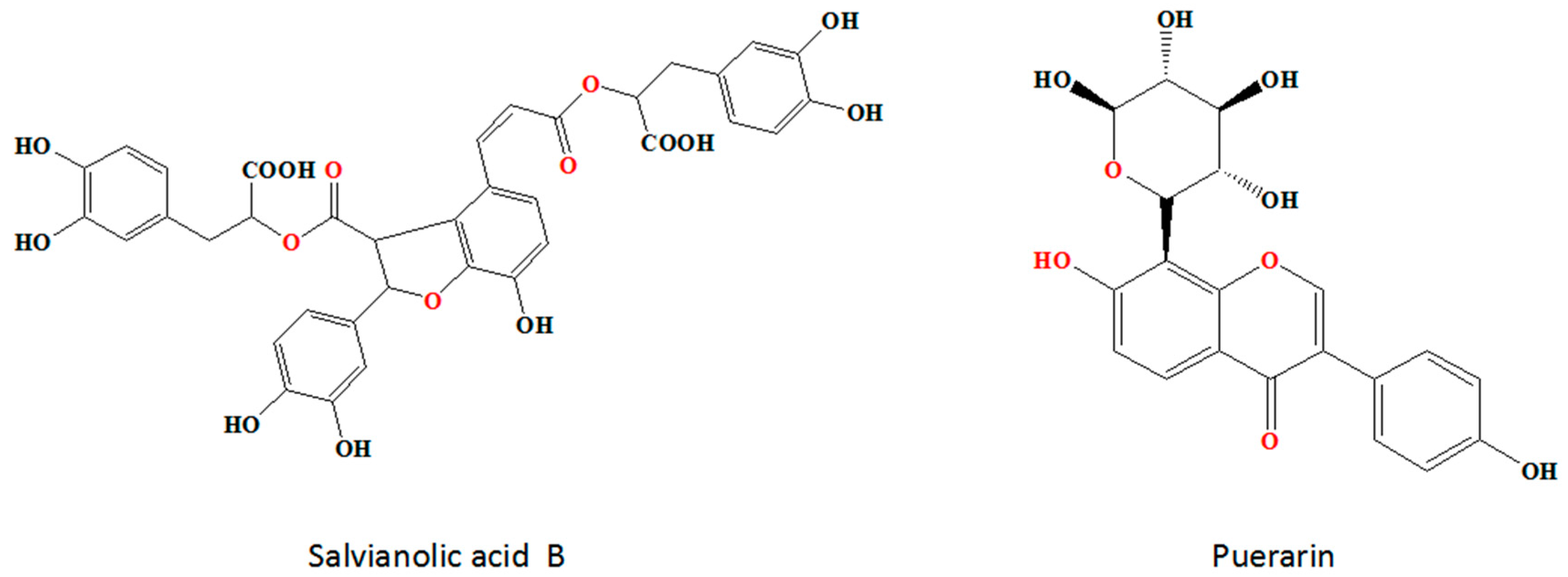
4.1. Materials
4.2. Cytotoxicity of Sal-B and Pue
4.3. Effect of Sal-B and Pue against CoCl2 Induced Cell Injury
4.4. Effect of Sal-B and Pue against ROS, Apoptosis and MMP In Vitro
4.5. Rat Model of Middle Cerebral Artery Occlusion (MCAO)
4.6. Effect of Sal-B and Pue against IS In Vivo
4.7. Effect of Sal-B and Pue on Inflammatory Cytokines In Vivo
4.8. Effect of Sal-B and Pue on mRNA Expression of TLR4, MyD88, NF-κB and TNF-α in Penumbra
4.9. Effect of Sal-B and Pue on Protein Expression of TLR4, MyD88, NF-κB and TNF-α in Penumbra
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Q.; Zhao, Y.H. Therapeutic Angiogenesis after ischemic stroke: Chinese medicines, Bone marrow stromal cells (BMSCs) and their combinational treatment. Am. J. Chin. Med. 2014, 42, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Sahota, P.; Savitz, S.I. Investigational therapies for ischemic stroke: Neuroprotection and neurorecovery. Neurotherapeutics 2011, 8, 434–451. [Google Scholar] [CrossRef] [PubMed]
- Mattiasson, G.; Shamloo, M.; Gido, G.; Mathi, K.; Tomasevic, G.; Yi, S.; Warden, C.H.; Castilho, R.F.; Melcher, T.; Gonzalez-Zulueta, M.; et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat. Med. 2003, 9, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Sucher, N.J. Stroke therapy in traditional Chinese medicine (TCM): Prospects for drug discovery and development. Phytomedcine 2002, 9, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.R.; Yang, X.W.; Zhang, Y.; Liu, J.-X.; Yang, X.-B.; Liu, Y.; Shi, R.-B. Three new isoflavone glycosides from Tongmai granules. J. Asian Nat. Prod. Res. 2011, 13, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Wu, M.J. Energy theory of drug and cooperation mechanism of energy for active ingredients of traditional Chinese medicine. Chin. Tradit. Herb. Drugs 2003, 34, 865–872. [Google Scholar]
- Wu, S.; Wang, J.M.; Zhang, C.B.; Zhou, L.N. Effect of Gegen plus danshen intravenous injections regimen on vascular endothelial functioning. J. Postgrad. Med. Chin. 2004, 27, 19–22. [Google Scholar]
- Li, X.Y.; Tang, H.J.; Zhang, L.; Yang, L.; Li, P.; Chen, J. A selective knockout method for discovery of minor active components from plant extracts: Feasibility and challenges as illustrated by an application to Salvia miltiorrhiza. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1068, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dang, L.; Zhang, X.; Fang, W.; Hou, M.; Liu, T.; Wang, Z. Physicochemical properties and micro-structural characteristics in starch from kudzu root as affected by cross-linking. Food Chem. 2017, 219, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Du, G.Y.; Xiong, Y.L.; Zhao, Y.; Cui, H.F.; Cao, C.Y.L.; Liu, S. Protective effects of 3’-methoxy-puerarin on rat brain suffering from ischemia. Zhongguo Zhong Yao Za Zhi 2008, 33, 537–540. [Google Scholar] [PubMed]
- He, H.Y.; Guo, T.; Zhang, P.Y.; Yang, L.Q.; Deng, Y.H. Puerarin provides a neuroprotection against transient cerebral ischemia by attenuating autophagy at the ischemic penumbra in neurons but not in astrocytes. Neurosci. Lett. 2017, 643, 45–51. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chen, G.; Yu, X.D.; Li, Y.C.; Zhang, L.; He, Z.Z.; Zhang, N.N.; Yang, X.P.; Zhao, Y.S.; Li, N.; et al. Salvianolic Acid B Ameliorates Cerebral Ischemia/Reperfusion Injury Through Inhibiting TLR4/MyD88 Signaling Pathway. Inflammation 2016, 39, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.X.; Zhong, A.Q.; Ma, H.N.; Li, D.; Hu, Y.; Xu, Y.Z.; Zhang, J.P. Neuroprote ctive effect of salvianolic acid B against cerebral ischemic injury in rats via the C D40/NF-κB pathway associated with suppression of platelets activation and neur oinflammation. Brain Res. 2017, 1661, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.R.; Lehar, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Hu, Y.Y.; Tan, W.; Wu, X.; Chen, R.; Cao, J.; Chen, M.; Wang, Y. Compatibility art of traditional Chinese medicine: From the perspective of herb pairs. J. Ethnopharmacol. 2012, 143, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Observation on the effect of combination of Puerarin injection and Danshen injection in the treatment of acute cerebral infarction. Chin. Commun. Dr. Chin. 2005, 15, 35. [Google Scholar]
- Ding, C.Y.; Wang, R.X.; Guo, L.X. Effect of puerarin combined with compound salvia injection on neurological function of patients with cerebral infarction. Strait. Pharmaceutical. J. Chin. 2013, 25, 41–43. [Google Scholar]
- Eskes, C.; Juillerat-Jeanneret, L.; Leuba, G.; Honegger, P.; Monnet-Tschudi, F. Involvement of microglia-neuron interactions in the tumor necrosis factor-alpha release, microglial activation and neurodegeneration induced by trimethyltin. J. Neurosci. Res. 2003, 71, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Hanke, M.L.; Kielian, T. Toll-like receptors in health and disease in the brain: Mechanisms and therapeutic potential. Clin. Sci. (Lond) 2011, 121, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, X.; Tong, Y.; Liu, Y.; Zhang, B. TLR4 mediated MyD88-dependent signaling pathway is activated by cerebral ischemia-reperfusion in cortex in mice. Biomed. Pharmacother. 2009, 63, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.X.; Yang, Q.W.; Lv, F.L.; Cui, J.; Fu, H.B.; Wang, J.Z. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem. Biophys. Res. Commun. 2007, 353, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.S.; Arumugam, T.V.; Xu, X.G.; Cheng, A.W.; Mughal, M.R.; Dong, G.J.; Lathia, J.D.; Ou-yang, X.; Chigurupati, S.; Ouyang, X. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc. Natl. Acad. Sci. USA 2007, 104, 13798–13803. [Google Scholar] [CrossRef] [PubMed]
- Herskovits, A.Z.; Guarente, L. SIRT1 in neurodevelopment and brain senescence. Neuron 2014, 81, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, L.; Liu, P.P.; Hu, W.W.; Zhu, X.D.; Shen, H.; Yao, Y. Puerarin protects brain tissue against cerebral ischemia reperfusion injury by inhibiting the inflammatory response. Neural Regen. Res. 2014, 9, 2074–2080. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, Y.; Hu, Y.; Zhai, X.; Xu, W.; Jing, H.; Tian, X.; Lin, Y.; Gao, D.; Yao, J. Salvianolic acid B protects against acute ethanol-induced liver injury through SIRT1-mediated deacetylation of p53 in rats. Toxicol. Lett. 2014, 228, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.D.; Wang, L.; Shen, J.C.; Hao, S.J.; Ming, A.M.; Wang, X.D.; Su, F.; Zhang, Z. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res. Bull. 2015, 115, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, G.B.; Liu, P.; Song, J.H.; Liang, Y.; Yan, X.J.; Xu, F.; Wang, B.-S.; Mao, J.-H.; Shen, Z.-X.; et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effecti ve treatment for promyelocy tic leukemia. Proc. Natl. Acad. Sci. USA 2008, 105, 4826–4831. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.B.; Chen, Y.; Li, X.; Tan, X.B.; Fan, C.Y.; Li, L.D. New thoughts and methods of studying material base of traditional Chinese herbal formula. Chin. J. Tradit. Chin. Med. Pharm. Chin. 2008, 23, 420–425. [Google Scholar]
- Jin, A.; Li, X.; Zhu, Y.Y.; Yu, H.Y.; Pi, F.H.; Zhang, P.; Ruan, H.L. Four new compounds from the bulbs of Lycoris aurea with neuroprotective effects against CoCl2 and H2O2-induced SH-SY5Y cell injuries. Arch. Pharm. Res. 2014, 37, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Maltepe, E.; Goldwasser, E.; Mathieu, C.E.; Simon, M.C.; Schumacker, P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 1998, 29, 11715–11720. [Google Scholar] [CrossRef]
- Jung, J.Y.; Kim, W.J. Involvement of mitochondrial and Fas-mediated dual mechanism in CoCl2 induced apoptosis of rat PC12 cells. Neurosci. Lett. 2004, 371, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Sandner, P.; Wolf, K.; Bergmaier, U.; Gess, B.; Kurtz, A. Hypoxia and cobalt stimulate vascular endothelial growth factor receptor gene expression in rats. Pflugers Arch. 1997, 433, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Yan, M.; Xu, W.; Huo, H.; Sun, L.; Zheng, Z.; Liu, X. Cobalt chloride induces PC12 cells apoptosis through reactive oxygen species and accompanied by AP-1 activation. J. Neurosci. Res. 2001, 64, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.F.; Liu, Z.Q.; Cui, W.; Zhang, W.T.; Gong, J.P.; Wang, X.M.; Zhang, Y.; Yang, M.J. Antioxidant effect of salvianolic acid B on hippocampal CA1 neurons in mice with cerebral ischemia and reperfusion injury. Chin. J. Integr. Med. 2015, 21, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Obrenoviteh, T.P.; Seheller, D.; Matsumoto, T.; Tegtmeier, E.; Holler, M.; Symon, L. A rapid Redistribution of hydrogen ions is associated with depolarization and repolarization subsequent to cerebral ischemia reperfusion. J. Neurophysiol. 1990, 64, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Di Raimondo, D.; Tuttolomondo, A.; Buttà, C.; Miceli, S.; Licata, G.; Pinto, A. Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. Curr. Pharm. Des. 2012, 18, 4385–4413. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Pecoraro, R.; Casuccio, A.; Di Raimondo, D.; Buttà, C.; Clemente, G.; Della Corte, V.; Guggino, G.; Arnao, V.; Maida, C.; et al. Peripheral frequency of CD4+ CD28- cells in acute ischemic stroke: Relationship with stroke subtype and severity markers. Medicine 2015, 94, 813. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Di Sciacca, R.; Di Raimondo, D.; Pedone, C.; La Placa, S.; Pinto, A.; Licata, G. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: A retrospective chart review from the GIFA study. Int. J. Cardiol. 2011, 151, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke:an integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Olivot, J.; Mlynash, M.; Thijs, V.N.; Purushotham, A.; Kemp, S.; Lansberg, L.M.G.; Wechsler, L.; Gold, G.E.; Bammer, R.; Marks, M.P.; et al. Structure and evolution of diffusion and perfusion lesions in diffusion and perfusion imaging evaluation for understanding stroke evolution. Stroke 2009, 40, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; EI-Deiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A.; et al. Classification of cell death: Recommendations of the nomenclature committee on cell death. Cell Death Differ. 2005, 2, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar] [PubMed]
- Tsakiri, N.; Kimber, I.; Rothwell, N.J.; Pinteaux, E. Interleukin 1-induced interleu kin-6 synthe sis is mediated by the neutral sphingomyelinase/Src kinase pathway in neurones. Br. J. Pharmacol. 2008, 153, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, S.; Dong, Y.; Fan, C.; Zhao, L.; Yang, X.; Li, J.; Di, S.; Yue, L.; Liang, G.; et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015, 58, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.; Chan, S.H.; Chu, P.M.; Tsai, K.L. Homocysteine facilitates LOX-1 activation and endothelial death through the PKCbeta and SIRT1/HSF1 mechanism: Relevance to human hyperhomocysteinaemia. Clin. Sci. 2015, 129, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Kou, D.Q.; Jiang, Y.L.; Qin, J.H.; Huang, Y.H. Magnolol attenuates the inflammation and apoptosis through the activation of SIRT1 in experimental stroke rats. Pharmacol. Rep. 2017, 69, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.J.; Zhang, C.; Bai, X.; Zhang, J.; Zhao, X.M.; Chen, L.Y.; Wang, L.; Zhu, C.H.; Cui, L.L.; et al. Nobiletin promotes antioxidant and anti-inflammatory responses and elicits protection against ischemic stroke in vivo. Brain Res. 2016, 1636, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.; Khoutorova, L.; Zhao, W.; Vigdorehik, A.; Belayev, A.; Busto, R.; Magal, E.; Ginsberg, M.D. Neuroprotective effect of darbepoetin alfa,a novel recombinant erythropoietic protein, in focal cerebral ischemia in rats. Stroke 2005, 36, 1065–1070. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, C.; Liang, J.; Zhang, C.; Li, R.; Mou, Q.; Qin, J.; Li, X.; Wang, J. Synergistic Effects of Salvianolic Acid B and Puerarin on Cerebral Ischemia Reperfusion Injury. Molecules 2018, 23, 564. https://doi.org/10.3390/molecules23030564
Ling C, Liang J, Zhang C, Li R, Mou Q, Qin J, Li X, Wang J. Synergistic Effects of Salvianolic Acid B and Puerarin on Cerebral Ischemia Reperfusion Injury. Molecules. 2018; 23(3):564. https://doi.org/10.3390/molecules23030564
Chicago/Turabian StyleLing, Chengli, Jianming Liang, Chun Zhang, Ruixiang Li, Qianqian Mou, Jin Qin, Xiaofang Li, and Jianxin Wang. 2018. "Synergistic Effects of Salvianolic Acid B and Puerarin on Cerebral Ischemia Reperfusion Injury" Molecules 23, no. 3: 564. https://doi.org/10.3390/molecules23030564
APA StyleLing, C., Liang, J., Zhang, C., Li, R., Mou, Q., Qin, J., Li, X., & Wang, J. (2018). Synergistic Effects of Salvianolic Acid B and Puerarin on Cerebral Ischemia Reperfusion Injury. Molecules, 23(3), 564. https://doi.org/10.3390/molecules23030564



