Wedelolactone Enhances Osteoblastogenesis through ERK- and JNK-mediated BMP2 Expression and Smad/1/5/8 Phosphorylation
Abstract
:1. Introduction
2. Results and Discussion
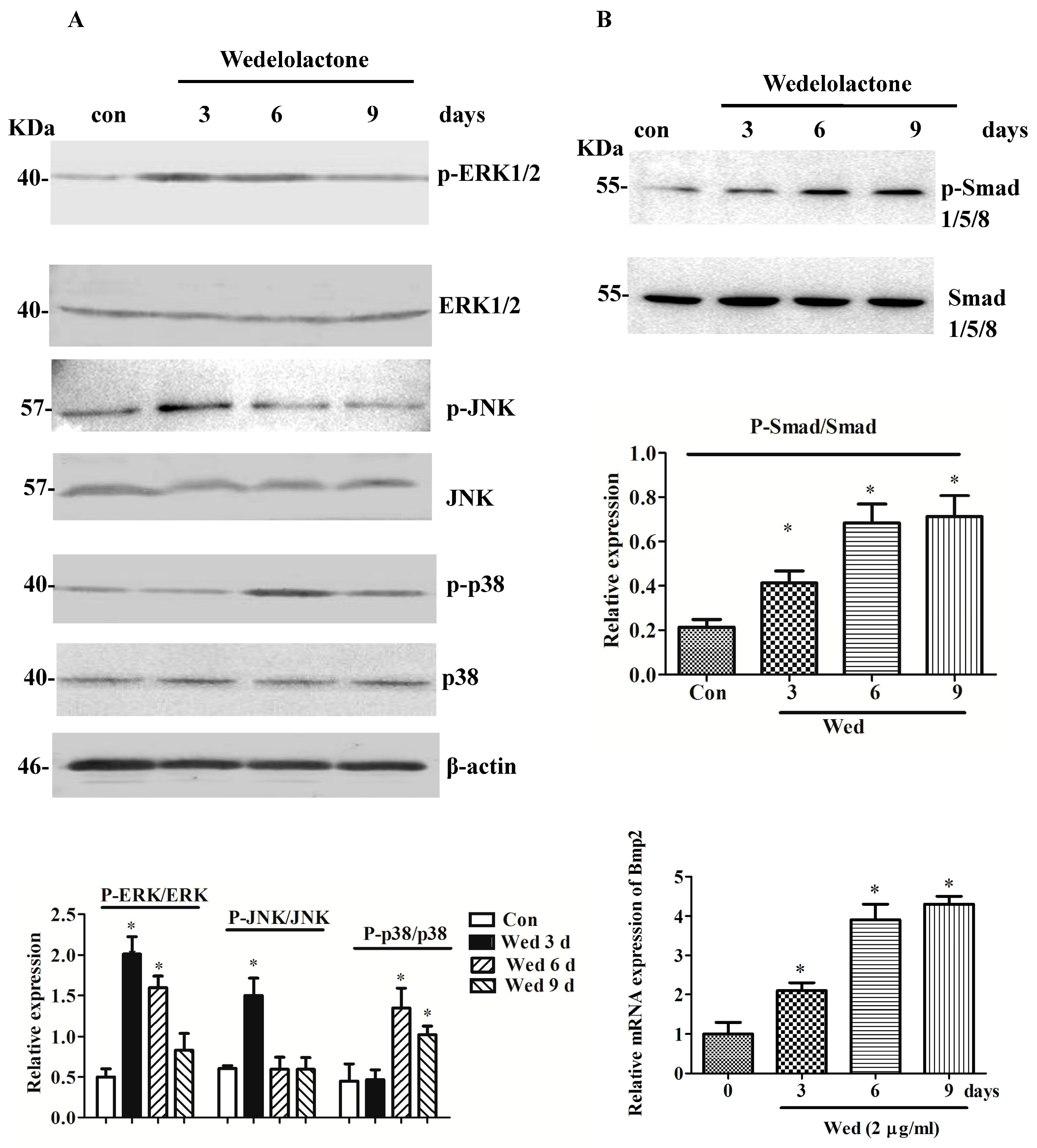
2.1. Effects of Wedelolactone on Phosphorylation of MAPK and Smad 1/5/8 and Expression of BMP2 mRNA
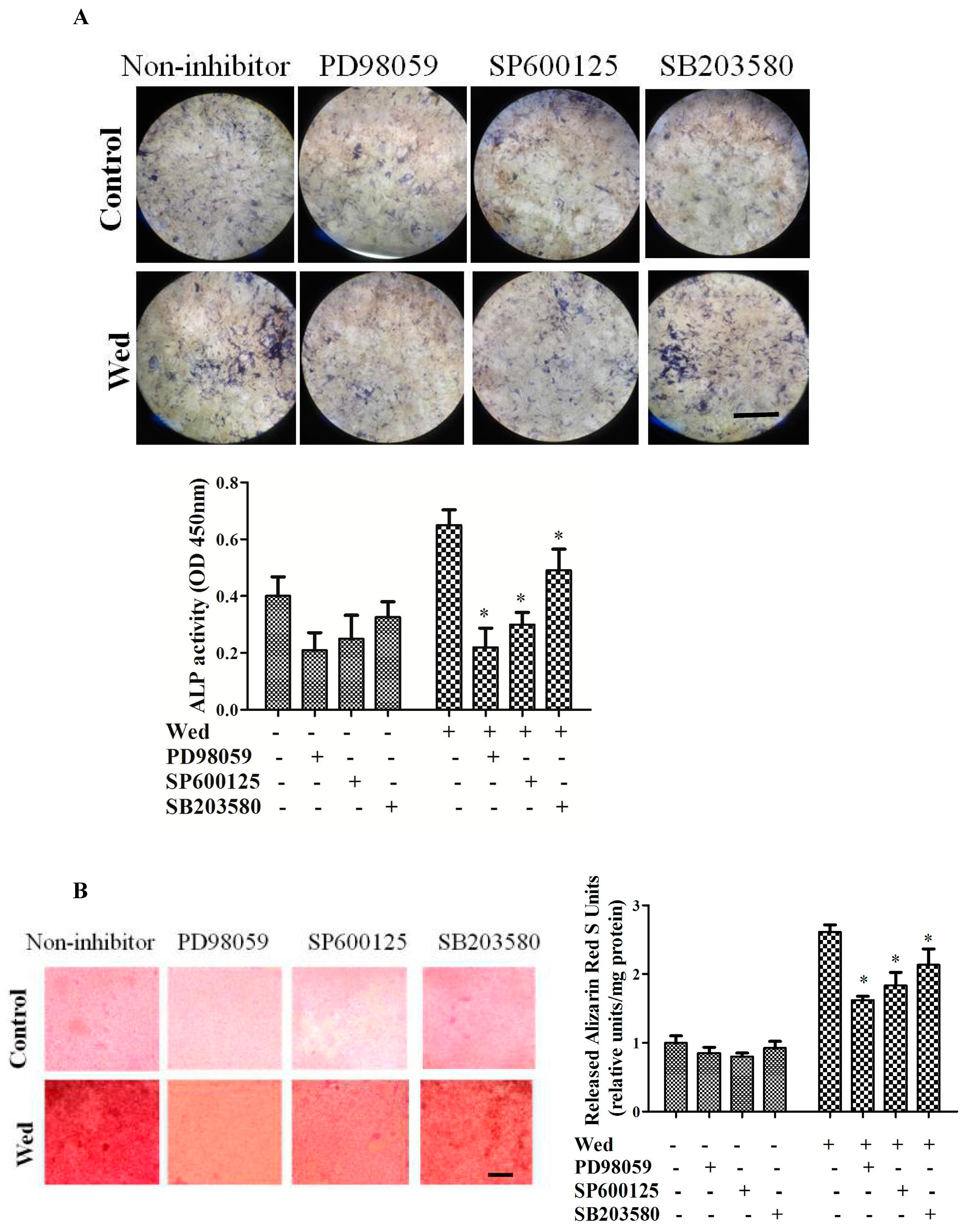
2.2. Effect of a MAPK Inhibitor on Wedelolactone-Enhanced ALP Activity and Bone Mineralization
2.3. MAPK Inhibitors Prevent mRNA Expression of Wedelolactone-Induced Osteoblast Marker Genes
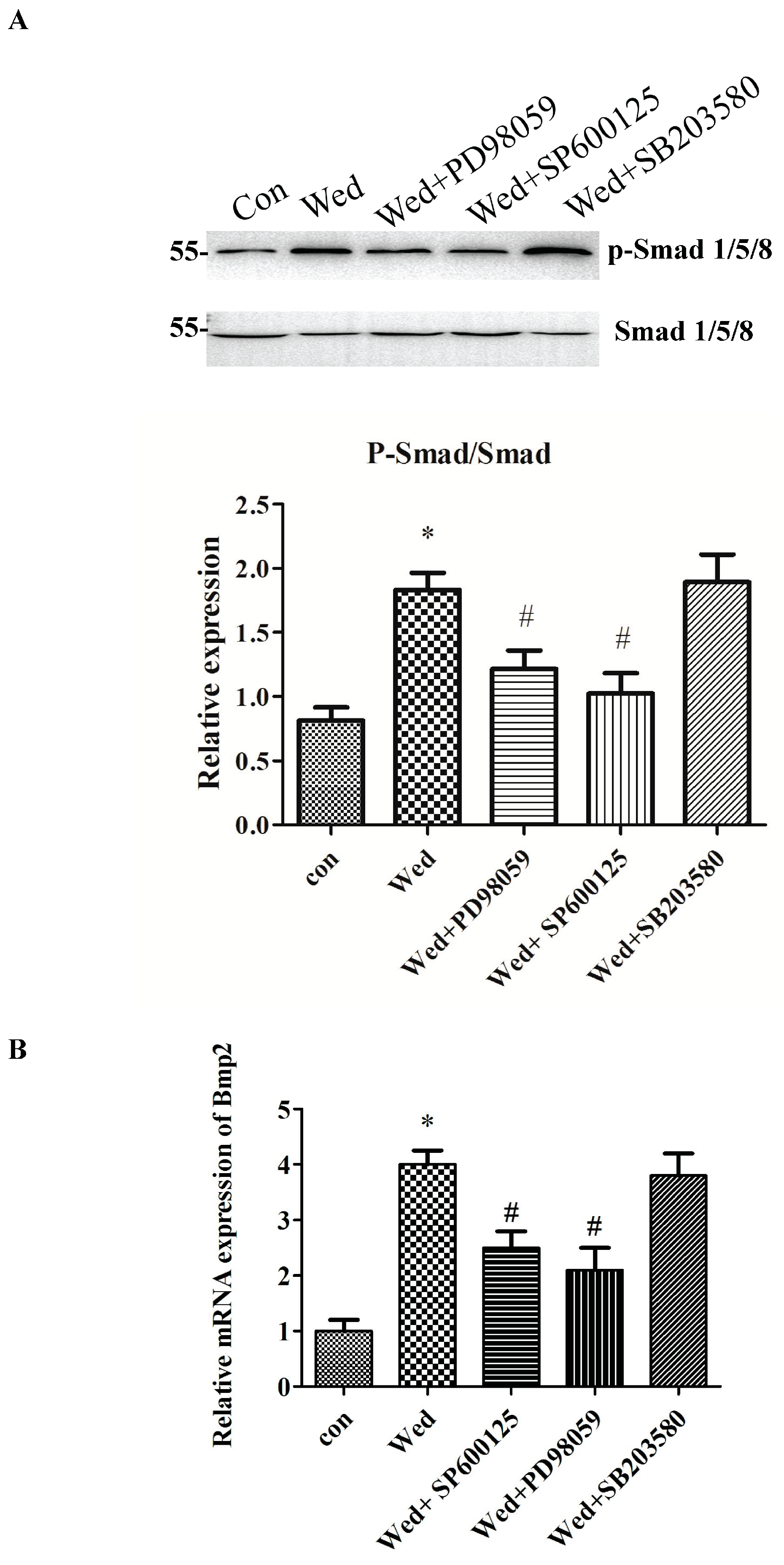
2.4. Activation of JNK and ERK Occurs Upstream of Smad 1/5/8 and BMP2 Signaling
3. Materials and Methods
3.1. Materials
3.2. Isolation and Culture of Mouse BMSCs
3.3. Assay to Measure ALP Activity
3.4. Alizarin Red Staining
3.5. Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
3.6. Western Blotting
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krebsbach, P.H.; Kuznetsov, S.A.; Bianco, P.; Robev, P.G. Bone marrow stromal cells: Characterization and clinical application. Crit. Rev. Oral Biol. Med. 1999, 10, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Xu, X.; Tong, Z.; Lin, J.; Yu, Q.; Lin, Y.; Kuang, W. Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: A possible mechanism of age related osteoporosis. Bone Res. 2015, 3, 15003. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, J.; Davies, J.E.; Stanford, W.L. Concise Review: Musculoskeletal Stem Cells to Treat Age-Related Osteoporosis. Stem Cells Transl. Med. 2017, 6, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Mostafavinia, A.; Dehdehi, L.; Ghoreishi, S.K.; Hajihossainlou, B.; Bayat, M. Effect of in vivo low-level laser therapy on bone marrow-derived mesenchymal stem cells in ovariectomy-induced osteoporosis of rats. J. Photochem. Photobiol. B Biol. 2017, 175, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Pelled, G.; Gazit, D. Stem cell therapy for osteoporosis. Curr. Osteoporos. Rep. 2014, 12, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.D.; Deepak, M.; Yogisha, S.; Chandrashekar, A.P.; Muddarachappa, K.A.; D’Souza1, P.; Agarwal1, A.; Venkataraman, B.V. Trypsin inhibitory effect of wedelolactone and demethylwedelolactone. Phytother. Res. 2003, 17, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Sarveswaran, S.; Gautam, S.C.; Ghosh, J. Wedelolactone, a medicinal plant-derived coumestan, induces caspase-dependent apoptosis in prostate cancer cells via downregulation of PKCɛ without inhibiting Akt. Int. J. Oncol. 2012, 41, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Fessler, B. In vitro 5-lipoxygenase inhibition by Eclipta alba extracts and the coumestan derivative wedelolactone. Planta Med. 1986, 52, 374–377. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, Q.; Fan, Y.; Liu, X.; Wang, L.; Xie, R.; Charlene, C.H.; Sun, W. A traditional Chinese herbal preparation Er-Zhi-Wan, prevent ovarietomy-induced osteoporosis in rats. J. Ethnopharmacol. 2011, 138, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Bai, D.; Liu, M.J.; Li, Y.; Pan, J.H.; Liu, H.; Wang, W.L.; Xiang, L.H.; Xiao, G.G.; Ju, D.H. Therapeutic effect of aqueous extract from Ecliptae herba on bone metabolism of ovariectomized rats. Menopause 2013, 20, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Hong, Z.L.; Zhan, L.B.; Chu, H.Y.; Zhang, X.Z.; Li, G.H. Wedelolactone enhances osteoblastogenesis by regulating Wnt/β-catenin signaling pathway but suppresses osteoclastogenesis by NF-κB/c-fos/NFATc1 pathway. Sci. Rep. 2016, 6, 32260. [Google Scholar] [CrossRef] [PubMed]
- Noël, D.; Gazit, D.; Bouquet, C.; Apparailly, F.; Bony, C.; Plence, P.; Millet, V.; Turgeman, G.; Perricaudet, M.; Sany, J.; et al. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells 2004, 22, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Kakita, A.; Suzuki, A.; Ono, Y.; Miura, Y.; Itoh, M.; Oiso, Y. Possible involvement of p38 MAP kinase in prostaglandin E1-induced ALP activity in osteoblastlike cells. Prostaglandin Leukot. Essent. Fatty Acid 2004, 70, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Daigang, L.; Jining, Q.; Jinlai, L.; Pengfei, W.; Chuan, S.; Liangku, H.; Ding, T.; Zhe, S.; Wei, W.; Zhong, L.; et al. LPS-stimulated inflammation inhibits BMP-9-induced osteoblastic differentiation through crosstalk between BMP/MAPK and Smad signaling. Exp. Cell Res. 2016, 341, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Palmer, G.; Bonjour, J.P.; Caverzasio, J. Regulation of alkaline phosphatase activity by p38 MAP kinase in response to activation of Gi protein-coupled receptors by epinephrine in osteoblast-like cells. Endocrinology 1999, 140, 3177–3182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, H.; Jing, H.; Li, Y.; Wang, X.; Zhang, H.; Jiang, L.; Ren, F. Lactoferrin stimulates osteoblast differentiation through PKA and p38 pathways independent of lactoferrin’s receptor LRP1. J. Bone Miner. Res. 2014, 29, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Xu, H.; Liang, Q.; Xu, L.; Zhao, Y.; Wang, Y. Over-expression of Sox2 in C3H10T1/2 cells inhibits osteoblast differentiation through Wnt and MAPK signalling pathways. Int. Orthop. 2012, 36, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Tang, T.; Miao, Y.; Zhang, S.; Qu, Z.; Dai, K. Stimulation of osteogenic differentiation and inhibition of adipogenic differentiation in bone marrow stromal cells by alendronate via ERK and JNK activation. Bone 2008, 43, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.B.; Kong, X.H.; Li, Y.H.; Fan, L.; Pan, Y.L.; Li, C.R.; Wu, X.L.; Lu, T.L.; Mei, Q.B. Radix Dipsaci total saponins stimulate MC3T3-E1 cell differentiation via the bone morphogenetic protein-2/MAPK/Smad-dependent Runx2 pathway. Mol. Med. Rep. 2015, 11, 4468–4472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xing, W.W.; Li, Y.S.; Zhu, Z.; Wu, J.Z.; Zhang, Q.Y.; Zhang, W.; Qin, L.P. Effect of a traditional Chinese herbal preparation on osteoblasts and osteoclasts. Maturitas 2009, 61, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Han, X.F.; Bo, J.X.; Ma, H.P. Wedelolactone Enhances Osteoblastogenesis but Inhibits Osteoclastogenesis through Sema3A/NRP1/PlexinA1 Pathway. Front. Pharmacol. 2016, 7, 375. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.L.; Yuan, Y.; Tu, J.; Zou, G.M.; Li, Q. Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 2014, 5, e1187. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jin, H.; Tang, D.; Huang, S.; Zuscik, M.J.; Chen, D. Smad1 plays an essential role in bone development and postnatal bone formation. Osteoarthr. Cartil. 2011, 19, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Phimphilai, M.; Zhao, Z.; Boules, H.; Roca, H.; Franceschi, R.T. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J. Bone Miner. Res. 2006, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Jiang, W.; MPhillips, F.; CHaydon, R.; Peng, Y.; Zhou, L.; Luu, H.H.; An, N.; Breyer, B.; Vanichakarn, P.; et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J. Bone Jt. Surg. Am. 2003, 85-A, 1544–1552. [Google Scholar] [CrossRef]
- Hen, B.; Wei, A.; Whittaker, S.; Williams, L.A.; Tao, H.; Ma, D.D.; Diwan, A.D. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J. Cell. Biochem. 2010, 109, 406–416. [Google Scholar]
- Ghosh-Choudhury, N.; Abboud, S.L.; Nishimura, R.; Celeste, A.; Mahimainathan, L.; Choudhury, G.G. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and mad-dependent BMP-2 gene transcription. J. Biol. Chem. 2002, 277, 33361–33368. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Ikebe, K.; Wada, M.; Nokubi, T. Osteoblast response to titanium regulates transcriptional activity of Runx2 through MAPK pathway. J. Biomed. Mater. Res. A 2007, 81, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kang, H.J.; Park, J.Y.; Lee, J. Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells. Exp. Mol. Med. 2015, 47, e128. [Google Scholar] [CrossRef] [PubMed]
- Kawaki, H.; Kubota, S.; Suzuki, A.; Suzuki, M.; Kohsaka, K.; Hoshi, K.; Fujii, T.; Lazar, N.; Ohgawara, T.; Maeda, T.; et al. Differential roles of CCN family proteins during osteoblast differentiation: Involvement of Smad and MAPK signaling pathways. Bone 2011, 49, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Selvamurugan, N.; Kwok, S.; Alliston, T.; Reiss, M.; Partridge, N.C. Transforming growth factor-beta 1 regulation of collagenase-3 expression in osteoblastic cells by cross-talk between the Smad and MAPK signaling pathways and their components, Smad2 and Runx2. J. Biol. Chem. 2004, 279, 19327–19334. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Lazarenko, O.P.; Wu, X.; Kang, J.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/β-catenin canonical Wnt signaling. J. Bone Miner. Res. 2010, 25, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Zhan, L.B.; Liu, T.; Cheng, M.C.; Liu, X.Y.; Xiao, H.B. Inhibitory effect of Ecliptae herba extract and its component wedelolactone on pre-osteoclastic proliferation and differentiation. J. Ethnopharmacol. 2014, 157, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Li, G.; Chan, C.Y.; Meng, C.L.; Lin, M.C.; Chen, Y.C.; He, M.L.; Leung, P.C.; Kung, H.F. Flavonoids of Herba Epimedii regulate osteogenesis of human mesenchymal stem cells through BMP and Wnt/β-catenin signaling pathway. Mol. Cell. Endocrinol. 2010, 314, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Egermann, M.; Goldhahn, J.; Schneider, E. Animal models for fracture treatment in osteoporosis. Osteoporos. Int. 2005, 16, S129–S138. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.S.; Hashimoto, S.; Lotz, M.; Pritzker, K.; Goding, J.; Terkeltaub, R. Up-regulated expression of the phosphodiesterase nucleotide pyrophosphatase family member PC-1 is a marker and pathogenic factor for knee meniscal cartilage matrix calcification. Arthritis Rheum. 2001, 44, 1071–1081. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds wedelolactone are available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Deng, X.; Han, X.-F.; Sun, X.-X.; Pan, T.-W.; Zheng, L.-P.; Liu, Y.-Q. Wedelolactone Enhances Osteoblastogenesis through ERK- and JNK-mediated BMP2 Expression and Smad/1/5/8 Phosphorylation. Molecules 2018, 23, 561. https://doi.org/10.3390/molecules23030561
Zhu D, Deng X, Han X-F, Sun X-X, Pan T-W, Zheng L-P, Liu Y-Q. Wedelolactone Enhances Osteoblastogenesis through ERK- and JNK-mediated BMP2 Expression and Smad/1/5/8 Phosphorylation. Molecules. 2018; 23(3):561. https://doi.org/10.3390/molecules23030561
Chicago/Turabian StyleZhu, Di, Xue Deng, Xiao-Fei Han, Xiao-Xin Sun, Tao-Wen Pan, Lu-Ping Zheng, and Yan-Qiu Liu. 2018. "Wedelolactone Enhances Osteoblastogenesis through ERK- and JNK-mediated BMP2 Expression and Smad/1/5/8 Phosphorylation" Molecules 23, no. 3: 561. https://doi.org/10.3390/molecules23030561
APA StyleZhu, D., Deng, X., Han, X.-F., Sun, X.-X., Pan, T.-W., Zheng, L.-P., & Liu, Y.-Q. (2018). Wedelolactone Enhances Osteoblastogenesis through ERK- and JNK-mediated BMP2 Expression and Smad/1/5/8 Phosphorylation. Molecules, 23(3), 561. https://doi.org/10.3390/molecules23030561





