Comparison of Structural and Functional Properties of Starches from the Rhizome and Bulbil of Chinese Yam (Dioscorea opposita Thunb.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Soluble Sugar and Starch Contents in Rhizome and Bulbil
2.2. Morphology and Granule Size Distribution of Starches from Rhizome and Bulbil
2.3. Amylose Content in Starches from Rhizome and Bulbil
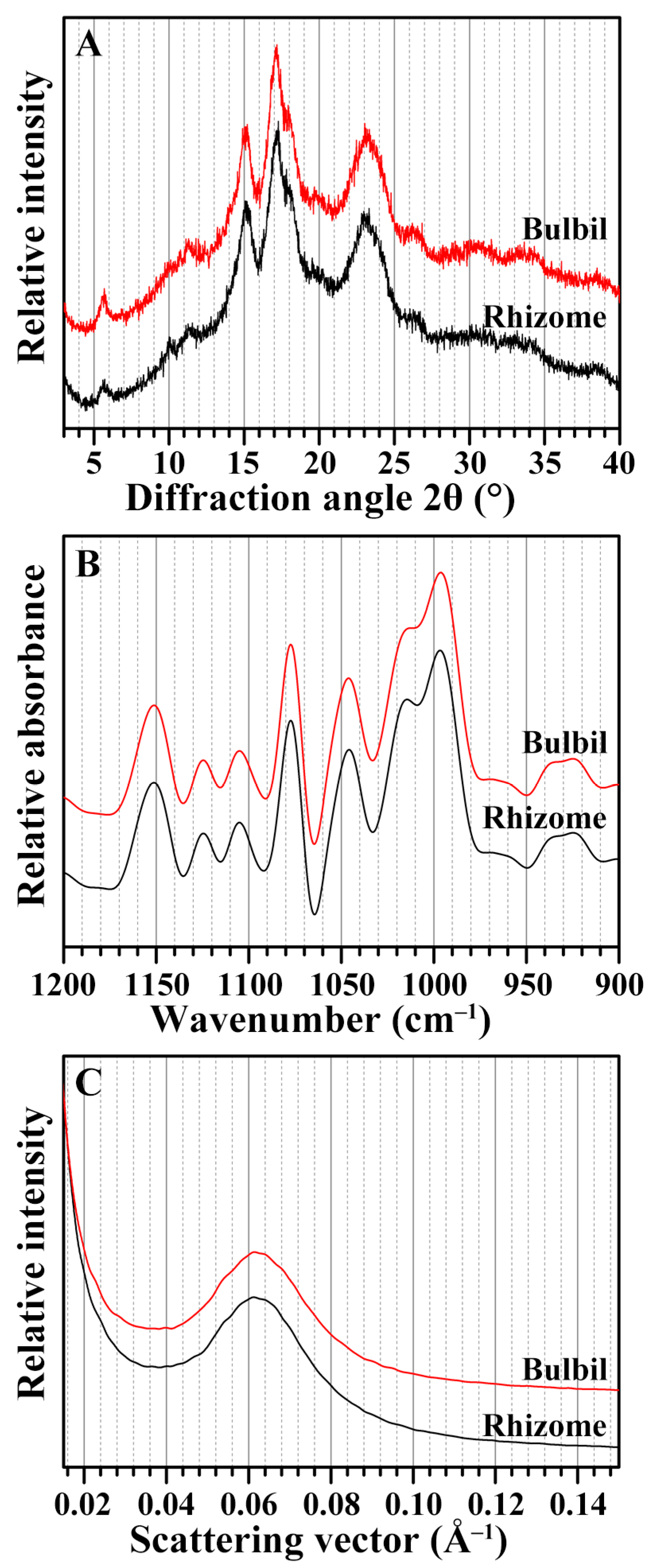
2.4. Crystalline Structure of Starches from Rhizome and Bulbil
2.5. Short-Range Ordered Structure of Starches from Rhizome and Bulbil
2.6. Lamellar Structure of Starches from Rhizome and Bulbil
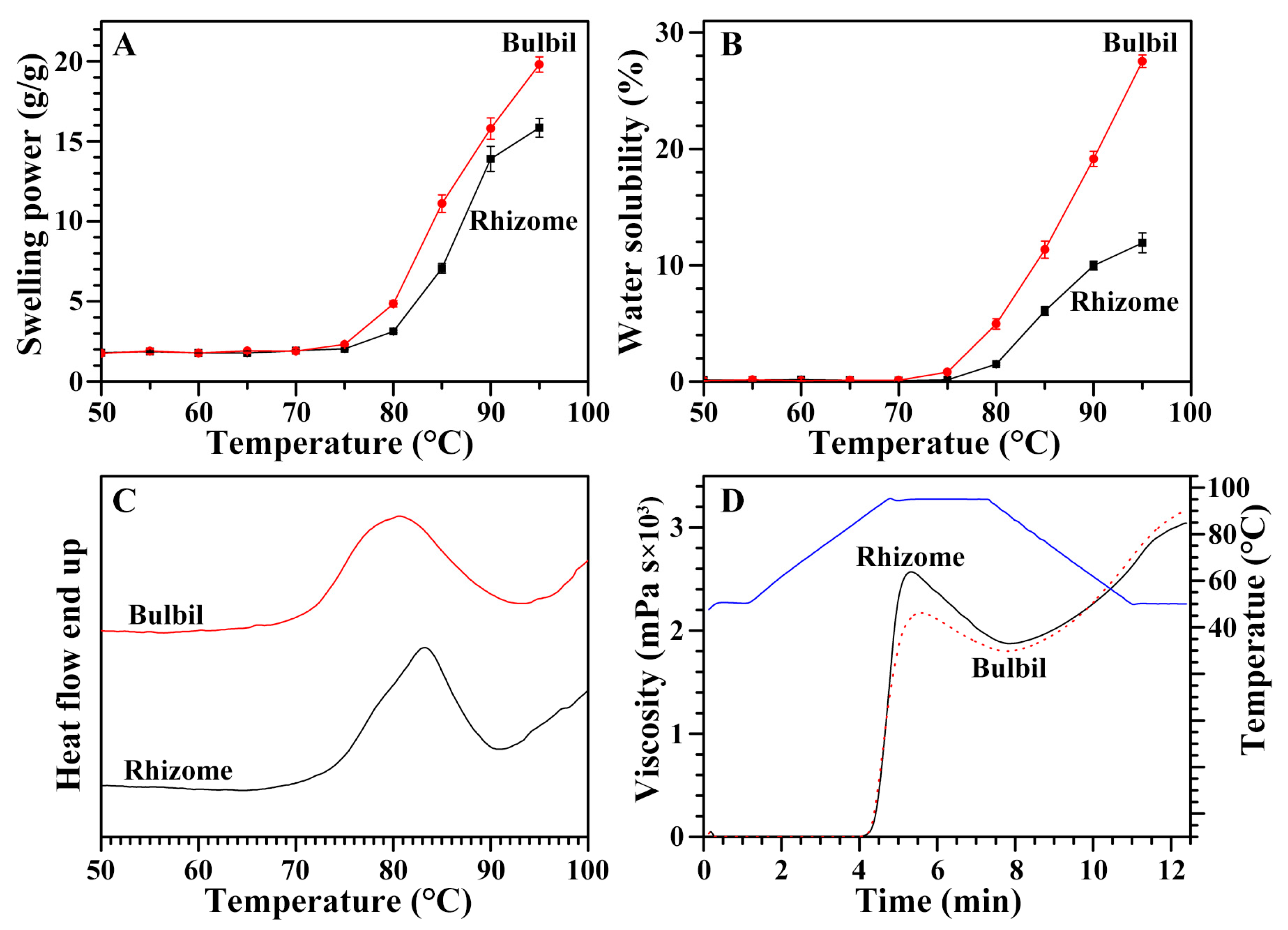
2.7. Swelling Power and Water Solubility of Starches from Rhizome and Bulbil
2.8. Thermal Properties of Starches from Rhizome and Bulbil
2.9. Pasting Properties of Starches from Rhizome and Bulbil
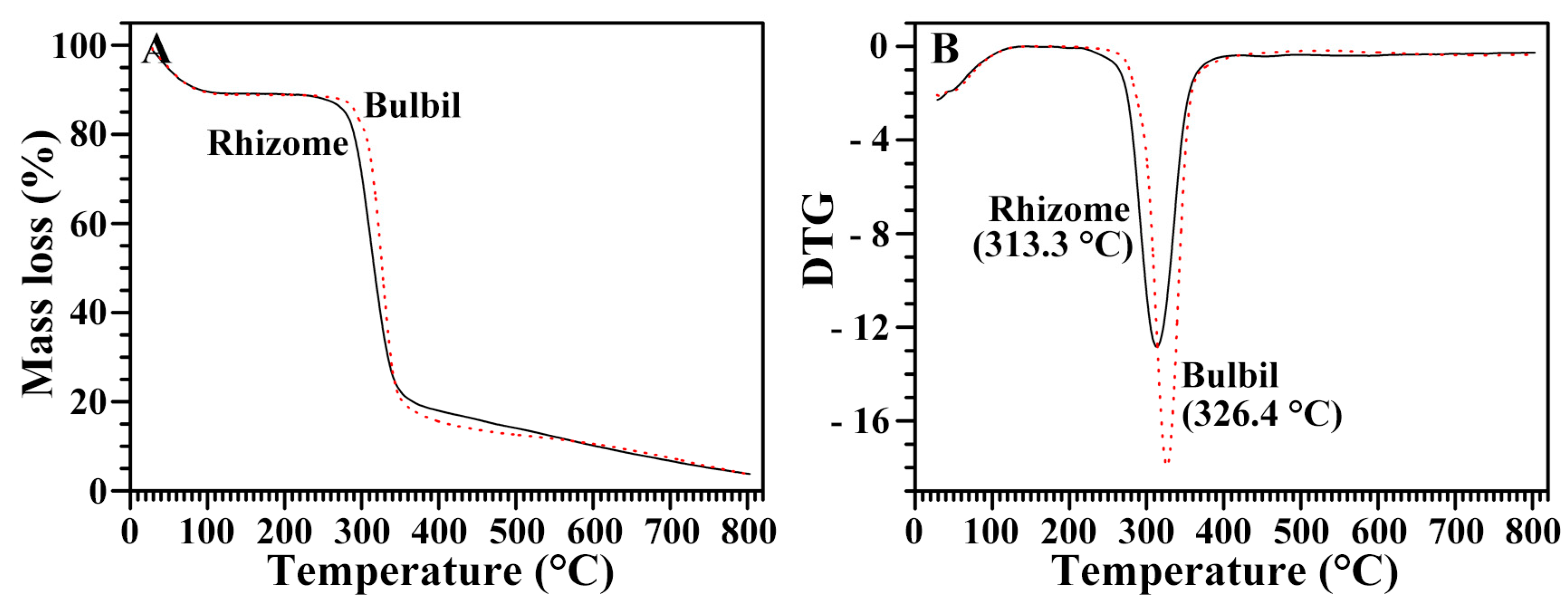
2.10. Thermal Stability of Starches from Rhizome and Bulbil
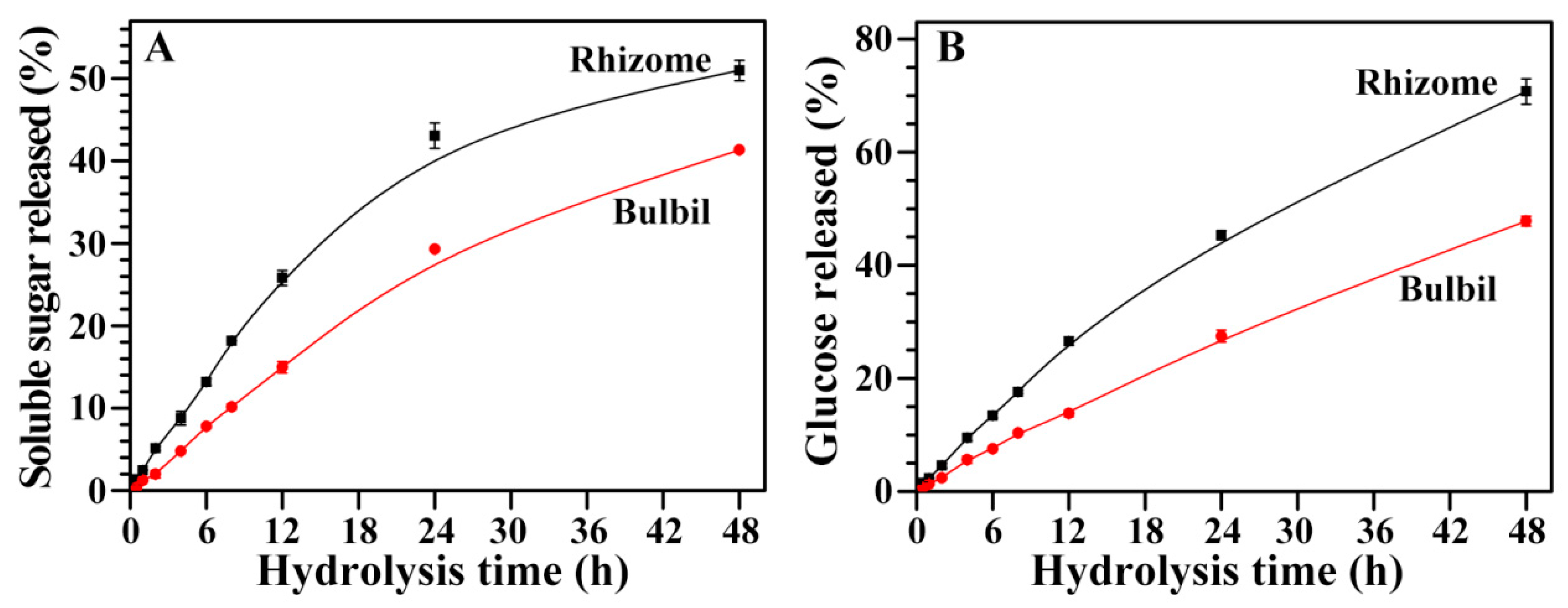
2.11. Hydrolysis of Starches from Rhizome and Bulbil
2.12. In Vitro Digestion Properties of Starches from Rhizome and Bulbil
3. Materials and Methods
3.1. Plant Materials
3.2. Measurements of Soluble Sugar and Starch Contents in Rhizome and Bulbil
3.3. Isolation of Starch
3.4. Morphology Observation and Size Analysis of Starch
3.5. Measurement of Amylose Content in Starch
3.6. Analysis of Crystalline Structure of Starch
3.7. Analysis of Short-Range Ordered Structure of Starch
3.8. Determination of Swelling Power and Water Solubility of Starch
3.9. Analysis of Thermal Properties of Starch
3.10. Analysis of Pasting Properties of Starch
3.11. Thermogravimetric Analysis of Starch
3.12. Measurement of PPA Hydrolysis of Starch
3.13. Analysis of In Vitro Digestion of Starch
3.14. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, J.; Fang, X.; Sun, Y.; Dou, X. Physicochemical properties of new starches isolated from Dioscorea opposite Thunb. bulbils. Starch 2012, 64, 290–296. [Google Scholar] [CrossRef]
- Jane, J.L.; Kasemsuwan, T.; Leas, S.; Zobel, H.; Robyt, J.F. Anthology of starch granule morphology by scanning electron microscopy. Starch 1994, 46, 121–129. [Google Scholar] [CrossRef]
- He, W.; Wei, C. Progress in C-type starches from different plant sources. Food Hydrocoll. 2017, 73, 162–175. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, thermal and rheological properties of starches from different botanical source-a review. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Emmambux, M.N.; Taylor, J.R.N. Morphology, physical, chemical, and functional properties of starches from cereals, legumes, and tubers cultivated in Africa: A review. Starch 2013, 65, 715–729. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Hamdani, A.M.; Gani, A.; Bhat, N.A.; Shah, A. Isolation, composition, and physicochemical properties of starch from legumes: A review. Starch 2016, 68, 1–12. [Google Scholar] [CrossRef]
- Wang, P.; Huang, J.; Zhao, L.; Chen, Y.; Wei, C. Structural and functional properties of endosperm starch and flour from dicotyledon Mirabilis jalapa. Starch 2015, 67, 328–337. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Huai, H.; Li, E.; Zhang, F.; Wei, C. Structural and functional properties of starches from wild Trapa quadrispinosa, japonica, mammillifera and incise. Food Hydrocoll. 2015, 48, 117–126. [Google Scholar] [CrossRef]
- Liu, C.; Wang, S.; Chang, X.; Wang, S. Structural and functional properties of starches from Chinese chestnuts. Food Hydrocoll. 2015, 43, 568–576. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, S.; Lin, L.; Zhao, L.; Liu, A.; Wei, C. Properties of new starches from tubers of Arisaema elephas, yunnanense and erubescens. Food Hydrocoll. 2016, 61, 183–190. [Google Scholar] [CrossRef]
- FAOSTAT (Statistics Division of Food and Agriculture Organization of the United Nations). Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 8 February 2018).
- Kim, S.K.; Lee, S.C.; Lee, B.H.; Choi, H.J.; Kim, K.U.; Lee, I.J. Bulbil formation and yield responses of Chinese yam to application of gibberellic acid, mepiquat chloride and trinexapac-ethyl. J. Agron. Crop Sci. 2003, 189, 255–260. [Google Scholar] [CrossRef]
- Zhu, F. Isolation, composition, structure, properties, modifications, and uses of yam starch. Compr. Rev. Food Sci. Food Saf. 2015, 14, 357–386. [Google Scholar] [CrossRef]
- Wang, S.; Yu, J.; Gao, W.; Liu, H.; Xiao, P. New starches from traditional Chinese medicine (TCM)—Chinese yam (Dioscorea opposite Thunb.) cultivars. Carbohydr. Res. 2006, 341, 289–293. [Google Scholar]
- Wang, S.; Liu, H.; Gao, W.; Chen, H.; Yu, J.; Xiao, P. Characterization of new starches separated from different Chinese yam (Dioscorea opposite Thunb.) cultivars. Food Chem. 2006, 99, 30–37. [Google Scholar]
- Wang, S.; Gao, W.; Liu, H.; Chen, H.; Yu, J.; Xiao, P. Studies on the physicochemical, morphological, thermal and crystalline properties of starches separated from different Dioscorea opposita cultivars. Food Chem. 2006, 96, 591–596. [Google Scholar]
- Zeng, H.; Huang, C.; Chen, P.; Zheng, B.; Liu, J.; Zhang, Y. Comparative study on the structural and physicochemical properties of flour and starch from Dioscorea opposite Thunb. Chin. J. Struct. Chem. 2017, 36, 1825–1836. [Google Scholar]
- Yang, P.; Xu, L.; Xu, H.; Tang, Y.; He, G.; Gao, Y.; Feng, Y.; Yuan, S.; Ming, J. Histological and transcriptomic analysis during bulbil formation in Lilium lancifolium. Front. Plant Sci. 2017, 8, 1508. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Shon, T.K.; Park, S.Y.; Lee, S.C.; Kim, H.Y.; Sohn, E.Y.; Jang, S.W.; Choo, Y.S.; Kim, K.U.; Lee, I.J. Endogenous gibberellins in bulbils of Chinese yam during growth and storage. Plant Prod. Sci. 2005, 8, 181–185. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Shao, S.; Yu, H.; Xiong, F.; Wang, Z. Morphological and physicochemical properties of bulb and bulbil starches from Lilium lancifolium. Starch 2015, 67, 448–458. [Google Scholar] [CrossRef]
- Cai, C.; Wei, C. In situ observation of crystallinity disruption patterns during starch gelatinization. Carbohydr. Polym. 2013, 92, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.S.; Singh, N.; Kaur, M. Characteristics of the different corn types and their grain fractions: Physicochemical, thermal, morphological and rheological properties of starches. J. Food Eng. 2004, 64, 119–127. [Google Scholar] [CrossRef]
- Pycia, K.; Juszczak, L.; Gałkowska, D.; Witczak, M. Physicochemical properties of starches obtained from Polish potato cultivars. Starch 2012, 64, 105–114. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef]
- Man, J.; Cai, J.; Cai, C.; Xu, B.; Huai, H.; Wei, C. Comparison of physicochemical properties of starches from seed and rhizome of lotus. Carbohydr. Polym. 2012, 88, 676–683. [Google Scholar] [CrossRef]
- Blazek, J.; Gilbert, E.P. Application of small-angle X-ray and neutron scattering techniques to the characterization of starch structure: A review. Carbohydr. Polym. 2011, 85, 281–293. [Google Scholar] [CrossRef]
- Carcea, M.; Acquistucci, R. Isolation and physicochemical characterization of Fonio (Digitaria exilis Stapf) starch. Starch 1997, 49, 131–135. [Google Scholar] [CrossRef]
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starch: A review. Starch 2004, 56, 89–99. [Google Scholar] [CrossRef]
- Mazurs, E.G.; Schoch, T.J.; Kite, F.E. Graphical analysis of the Brabender viscosity curves of various starches. Cereal Chem. 1957, 34, 141–152. [Google Scholar]
- Simi, C.K.; Abraham, T.E. Physicochemical rheological and thermal properties of Njavara rice (Oryza sativa) starch. J. Agric. Food Chem. 2008, 56, 12105–12113. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ren, C.; Shin, M. Physicochemical properties of starch isolated from eight different varieties of Korean sweet potatoes. Starch 2013, 65, 923–930. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, L.; Ezekiel, R.; Guraya, H.S. Microstructural, cooking and textural characteristics of potato (Solanum tuberosum L.) tubers in relation to physicochemical and functional properties of their flours. J. Sci. Food Agric. 2005, 85, 1275–1284. [Google Scholar] [CrossRef]
- Wang, L.; Xie, B.; Xiong, G.; Du, X.; Qiao, Y.; Liao, L. Study on the granular characteristics of starches separated from Chinese rice cultivars. Carbohydr. Polym. 2012, 87, 1038–1044. [Google Scholar] [CrossRef]
- Li, J.H.; Vasanthan, T.; Hoover, R.; Rossnagel, B.G. Starch from hull-less barley: V. In-vitro susceptibility of waxy, normal, and high-amylose starches towards hydrolysis by alpha-amylases and amyloglucosidase. Food Chem. 2004, 84, 621–632. [Google Scholar] [CrossRef]
- Blazek, J.; Gilbert, E.P. Effect of enzymatic hydrolysis on native starch granule structure. Biomacromolecules 2010, 11, 3275–3289. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.H.M.; Lee, B.H.; Chang, W.J. Small intestine mucosal α-glucosidase: A missing feature of in vitro starch digestibility. Food Hydrocoll. 2016, 53, 163–171. [Google Scholar] [CrossRef]
- Zhang, B.; Dhital, S.; Gidley, M.J. Densely packed matrices as rate determining features in starch hydrolysis. Trends Food Sci. Technol. 2015, 43, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Atichokudomchai, N.; Varavinit, S.; Chinachoti, P. A study of ordered structure in acid-modified tapioca starch by 13C CP/MAS solid-state NMR. Carbohydr. Polym. 2004, 58, 383–389. [Google Scholar] [CrossRef]
- Noda, T.; Takigawa, S.; Matsuura-Endo, C.; Suzuki, T.; Hashimoto, N.; Kottearachchi, N.S.; Yamauchi, H.; Zaidul, I.S.M. Factors affecting the digestibility of raw and gelatinized potato starches. Food Chem. 2008, 110, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cai, J.; Han, W.; Huai, H.; Chen, Y.; Wei, C. Comparison of starches isolated from three different Trapa species. Food Hydrocoll. 2014, 37, 174–181. [Google Scholar] [CrossRef]
- Lin, L.; Guo, D.; Zhao, L.; Zhang, X.; Wang, J.; Zhang, F.; Wei, C. Comparative structure of starches from high-amylose maize inbred lines and their hybrids. Food Hydrocoll. 2016, 52, 19–28. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Q.; Zhang, L.; Wei, C. Evaluation of the molecular structural parameters of normal rice starch and their relationships with its thermal and digestion properties. Molecules 2017, 22, 1526. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of rhizome and bulbil starches of Chinese yam are available from the authors. |

| Tissues | Soluble Sugar Content (%db) | Starch Content (%db) |
|---|---|---|
| Rhizome | 1.1 ± 0.1 | 84.6 ± 1.8 |
| Bulbil | 1.7 ± 0.1 ** | 66.9 ± 2.9 ** |
| Tissues | Size Distribution b | Amylose Content (%) | ||||
|---|---|---|---|---|---|---|
| D[4,3] (μm) | D[3,2] (μm) | d(0.1) (μm) | d(0.5) (μm) | d(0.9) (μm) | ||
| Rhizome | 17.74 ± 0.02 | 9.36 ± 0.01 | 10.51 ± 0.01 | 17.81 ± 0.02 | 26.03 ± 0.03 | 35.2 ± 0.5 |
| Bulbil | 16.42 ± 0.01 *** | 8.48 ± 0.01 *** | 10.17 ± 0.01 *** | 16.58 ± 0.01 *** | 23.64 ± 0.01 *** | 38.3 ± 0.1 ** |
| Tissues | Relative Crystallinity (%) | IR Ratio (cm−1) | Lamellar Structure Parameters b | |||
|---|---|---|---|---|---|---|
| 1045/1022 | 1022/995 | Smax (Å−1) | D (nm) | Imax (Counts) | ||
| Rhizome | 27.9 ± 1.8 | 0.71 ± 0.01 | 0.75 ± 0.04 | 0.062 ± 0.001 | 10.15 ± 0.08 | 180.7 ± 16.7 |
| Bulbil | 26.8 ± 1.8 | 0.70 ± 0.01 | 0.76 ± 0.01 | 0.062 ± 0.001 | 10.03 ± 0.20 | 161.4 ± 9.5 |
| Tissues | To (°C) b | Tp (°C) b | Tc (°C) b | ΔT (°C) b | ΔH (J/g) b |
|---|---|---|---|---|---|
| Rhizome | 73.6 ± 0.5 | 83.1 ± 0.1 | 88.6 ± 0.5 | 15.0 ± 0.9 | 12.3 ± 0.6 |
| Bulbil | 71.7 ± 0.3 ** | 81.1 ± 0.4 ** | 89.2 ± 0.8 | 17.5 ± 0.6 * | 13.0 ± 0.2 |
| Tissues | PV (mPa s) b | HV (mPa s) b | BV (mPa s) b | FV (mPa s) b | SV (mPa s) b | PT (min) b | PTemp (°C) b |
|---|---|---|---|---|---|---|---|
| Rhizome | 5145 ± 108 | 3752 ± 171 | 1393 ± 63 | 6065 ± 179 | 2313 ± 20 | 5.36 ± 0.04 | 88.7 ± 0.1 |
| Bulbil | 4288 ± 48 *** | 3542 ± 53 | 747 ± 14 *** | 6293 ± 37 | 2751 ± 27 *** | 5.58 ± 0.04 ** | 88.4 ± 0.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Guo, K.; Lin, L.; Wei, C. Comparison of Structural and Functional Properties of Starches from the Rhizome and Bulbil of Chinese Yam (Dioscorea opposita Thunb.). Molecules 2018, 23, 427. https://doi.org/10.3390/molecules23020427
Zhang B, Guo K, Lin L, Wei C. Comparison of Structural and Functional Properties of Starches from the Rhizome and Bulbil of Chinese Yam (Dioscorea opposita Thunb.). Molecules. 2018; 23(2):427. https://doi.org/10.3390/molecules23020427
Chicago/Turabian StyleZhang, Biao, Ke Guo, Lingshang Lin, and Cunxu Wei. 2018. "Comparison of Structural and Functional Properties of Starches from the Rhizome and Bulbil of Chinese Yam (Dioscorea opposita Thunb.)" Molecules 23, no. 2: 427. https://doi.org/10.3390/molecules23020427
APA StyleZhang, B., Guo, K., Lin, L., & Wei, C. (2018). Comparison of Structural and Functional Properties of Starches from the Rhizome and Bulbil of Chinese Yam (Dioscorea opposita Thunb.). Molecules, 23(2), 427. https://doi.org/10.3390/molecules23020427





