Effect of Atomic Charges on Octanol–Water Partition Coefficient Using Alchemical Free Energy Calculation
Abstract
:1. Introduction
2. Results and Discussion
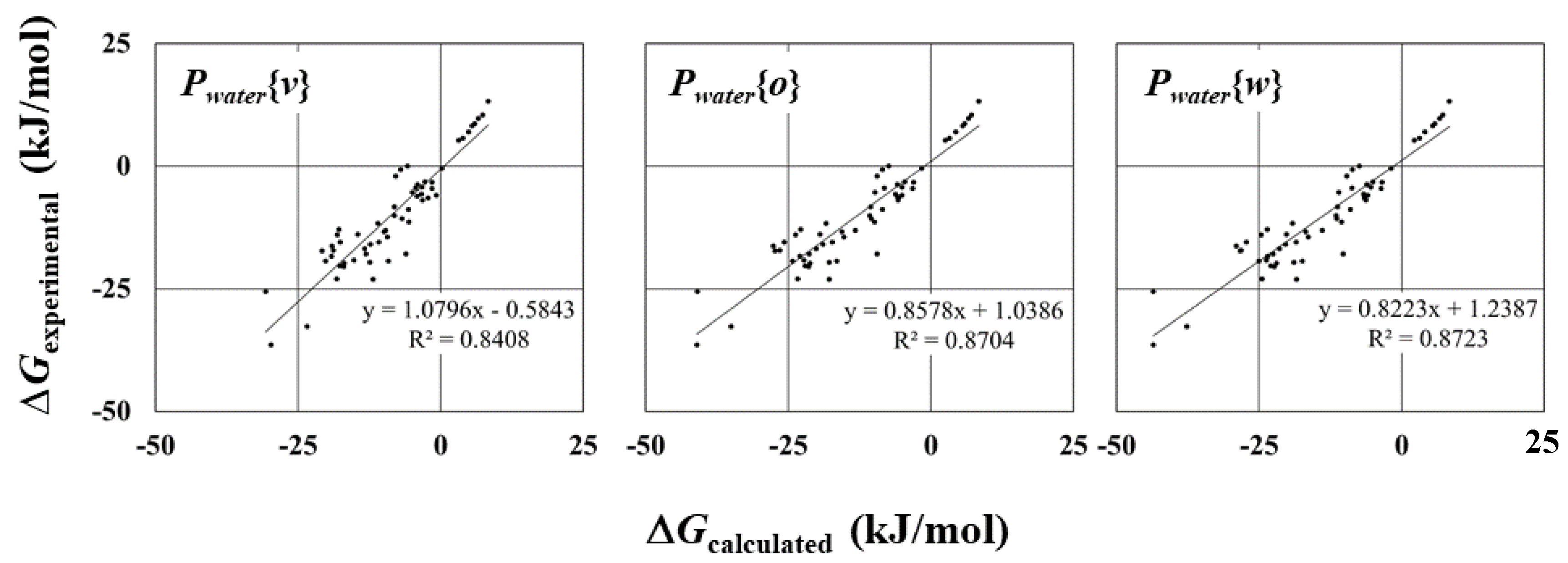
2.1. Solvation Free Energy in Water
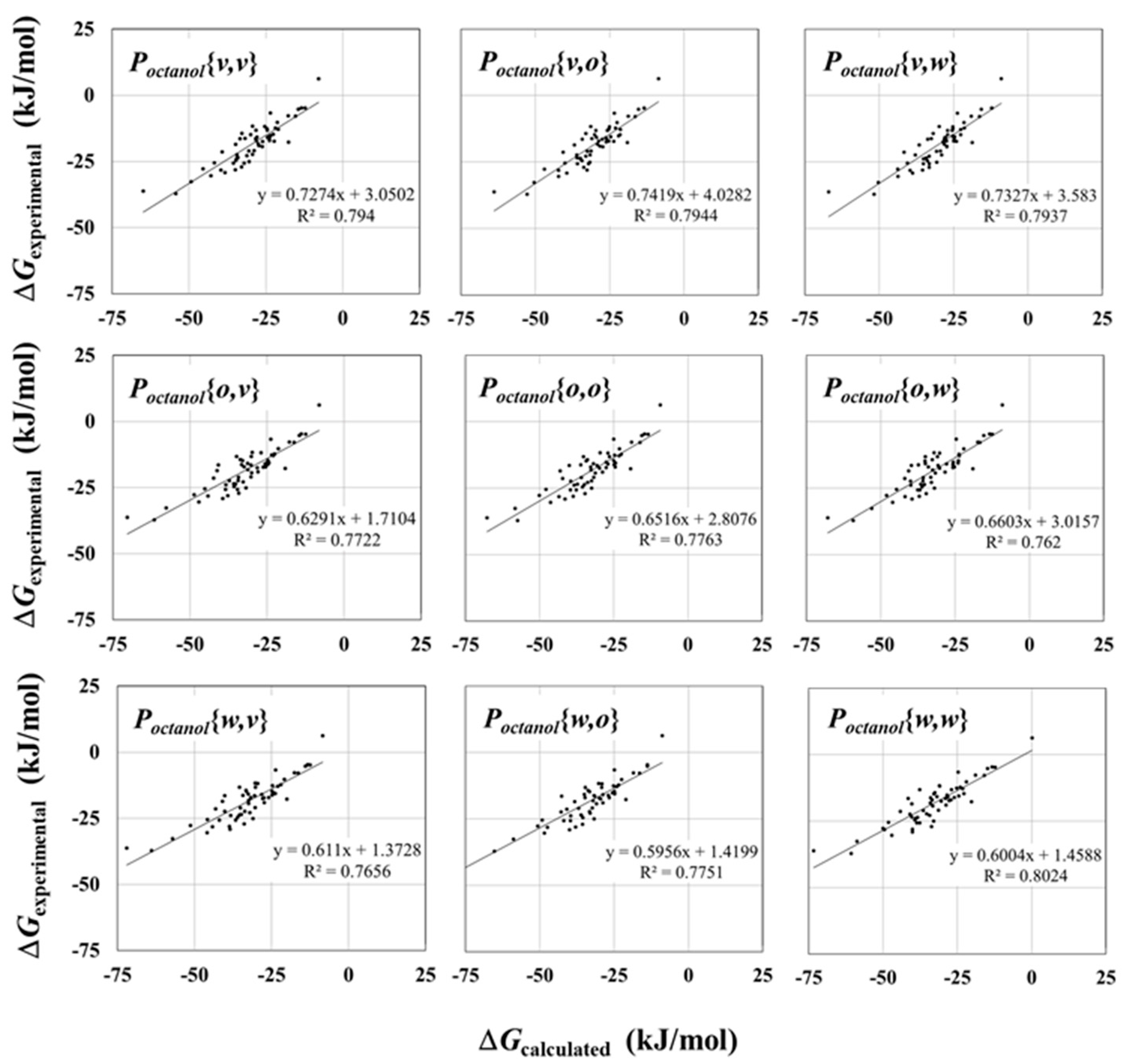
2.2. Solvation Free Energy in Octanol
2.3. Effect of Water and Octanol Solvents on Free Energy
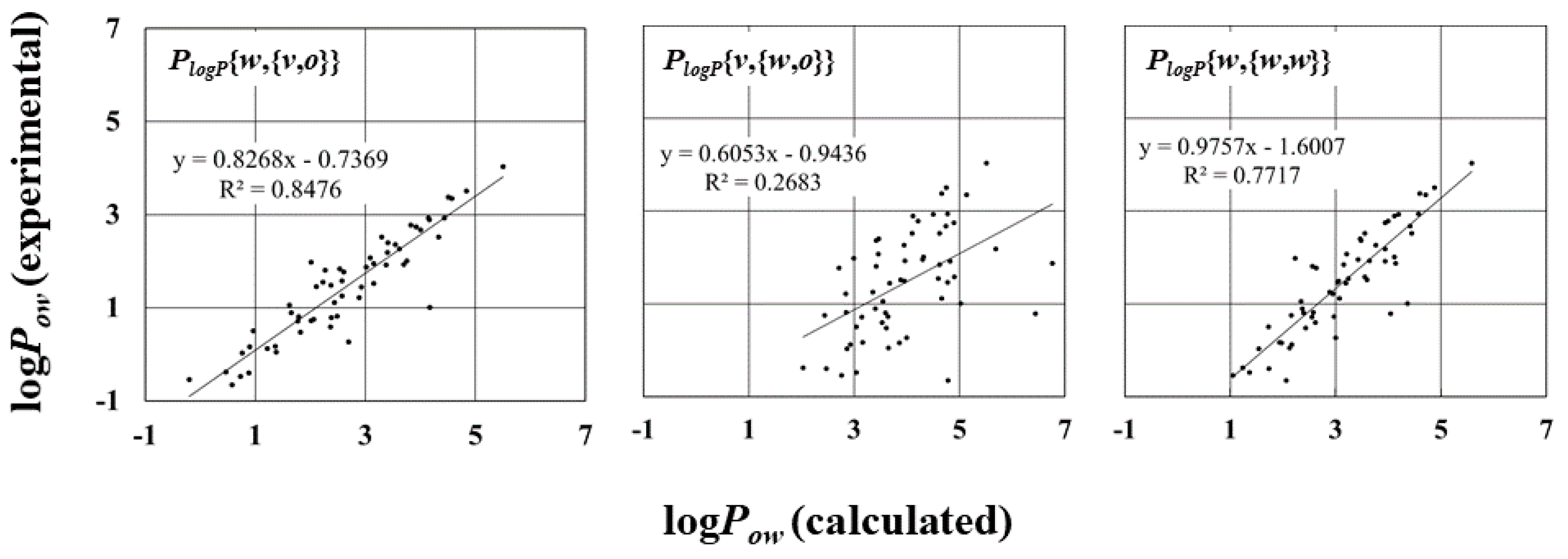
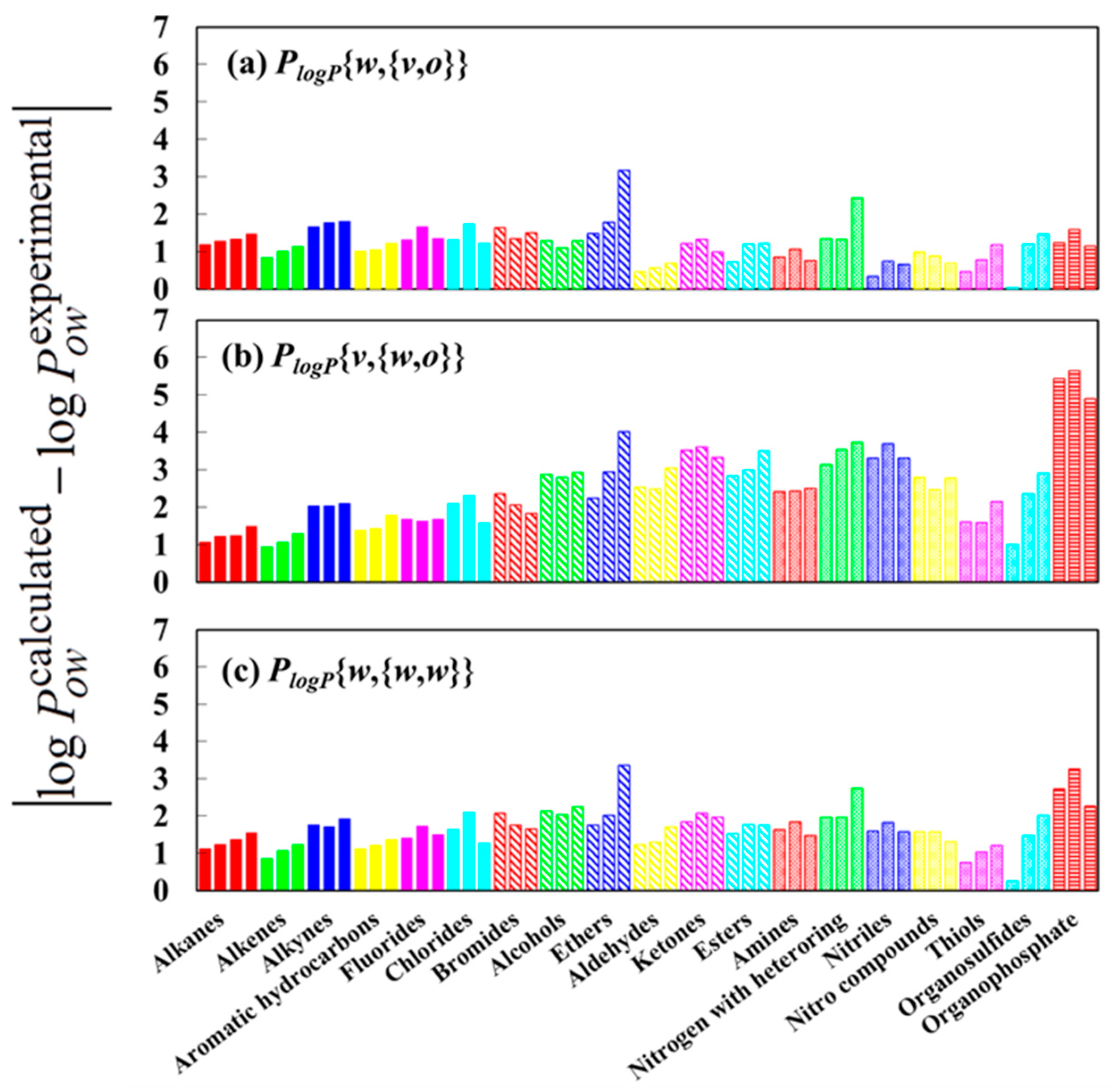
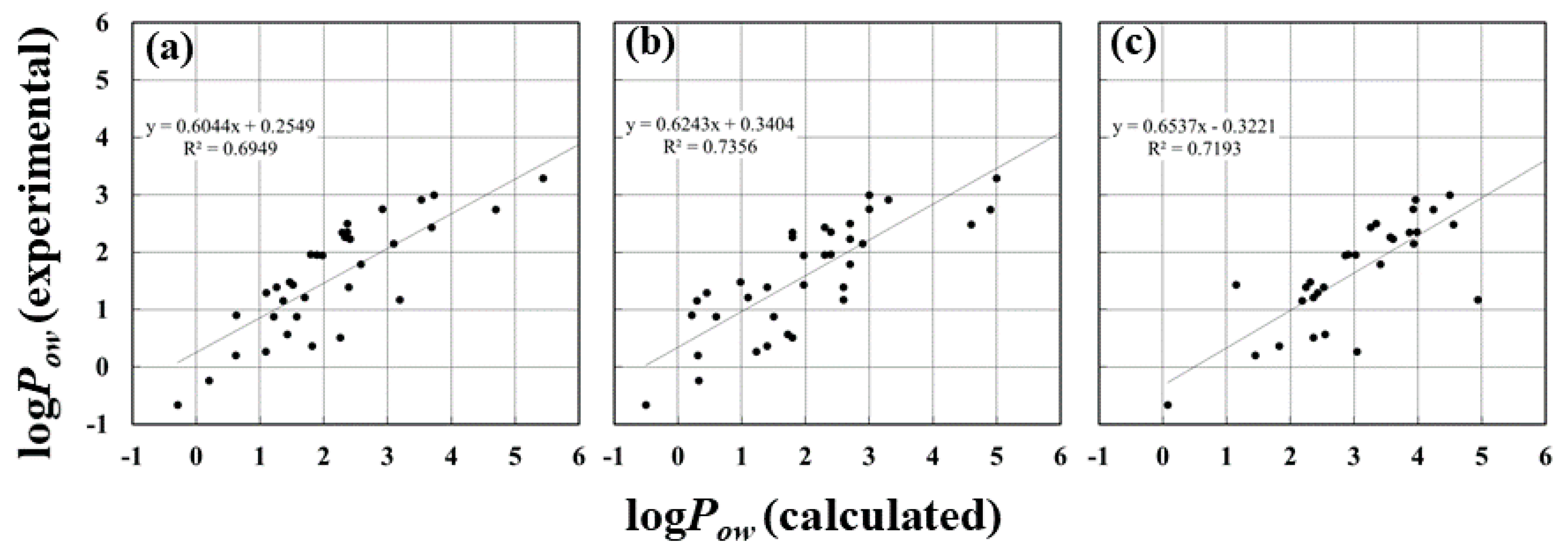
2.4. LogPow for Tested Compounds
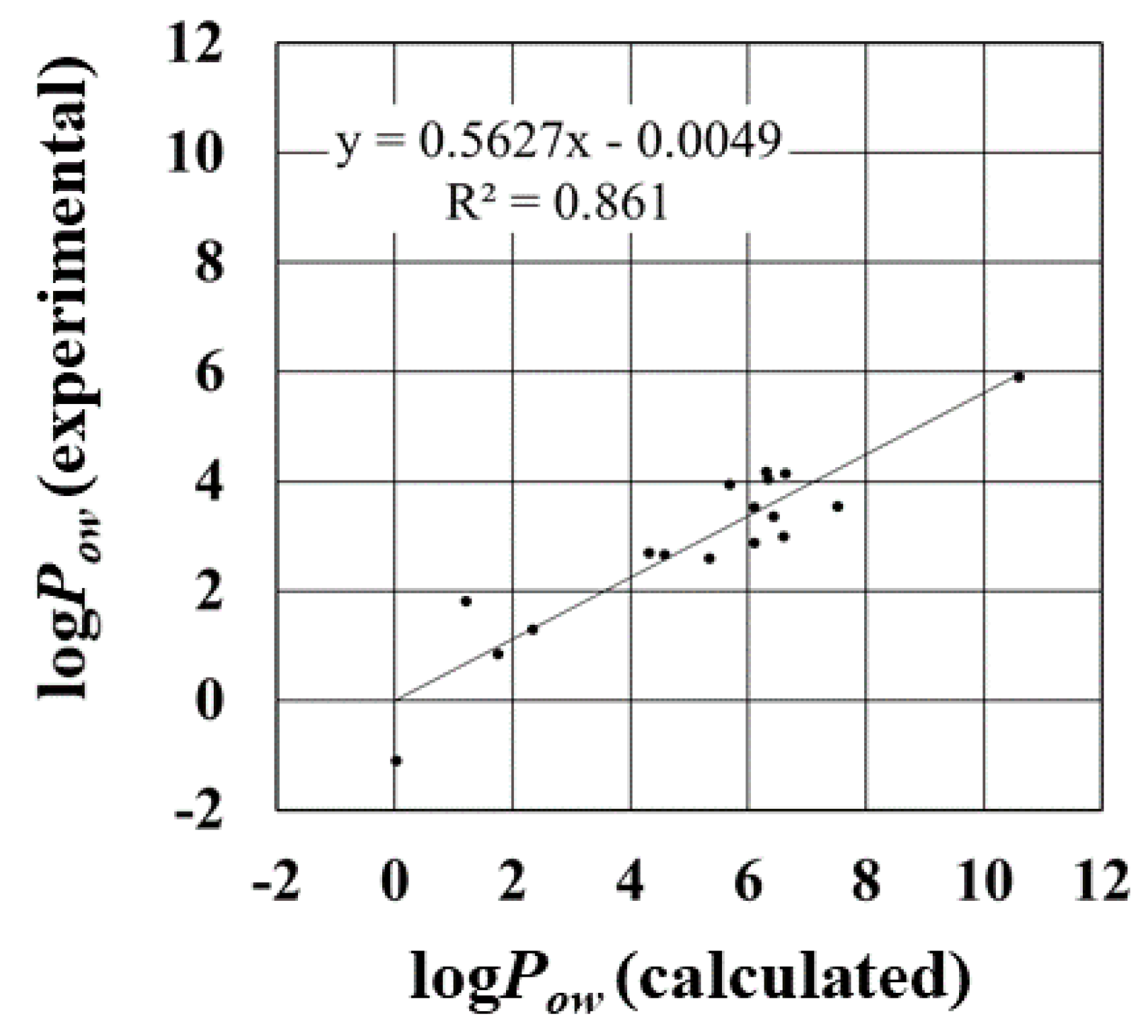
2.5. LogPow for 17 Compounds
3. Materials and Methods
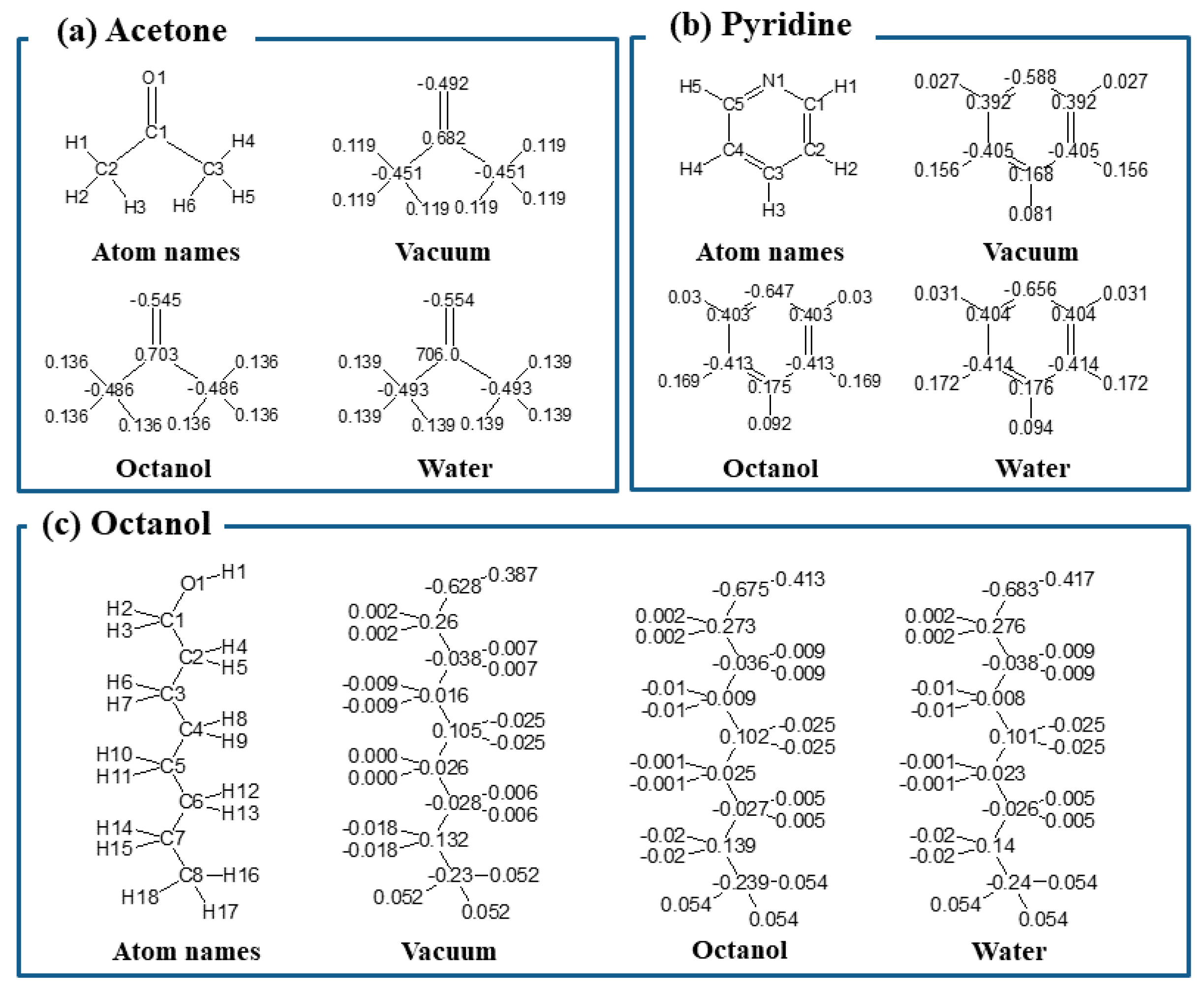
3.1. Setting Parameters of Compounds
3.2. Initial Models of Compounds in Solvent Box
3.3. Free Energy Calculation with BAR Method
3.4. Calculation of LogPow
3.5. Test Compounds
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hansch, C.; Fujita, T. p-σ-π analysis. A method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Leo, A.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- Sangster, J. Octanol–water partition coefficients of simple organic compounds. J. Phys. Chem. Ref. Data 1989, 18, 1111–1229. [Google Scholar] [CrossRef]
- Fujita, T.; Iwasa, J.; Hansch, C. A new substituent constant, π, derived from partition coefficients. J. Am. Chem. Soc. 1964, 86, 5175–5180. [Google Scholar] [CrossRef]
- Devoe, H.; Miller, M.M.; Wasik, S.P. Generator columns and high pressure liquid chromatography for determining aqueous solubilities and octanol–water partition coefficients of hydrophobic substances. J. Res. Nat. Bur. Stand. 1981, 86, 361–366. [Google Scholar] [CrossRef]
- Opperhuizen, A.; Serne, P.; Van der Steen, J.M.D. Thermodynamics of fish/water and octan-1-ol/water partitioning of some chlorinated benzenes. Environ. Sci. Technol. 1988, 22, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, X.; Li, Y.; Wang, R.; Lai, L. Computation of octanol–water partition coefficients by guiding an additive model with knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Meylan, W.M.; Howard, P.H. Estimating log P with atom/fragments and water solubility with log P. Perspect. Drug Discov. Des. 2000, 19, 67–84. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Bolton, E.E.; Wang, Y.; Thiessen, P.A.; Bryant, S.H. Chapter 12—Pubchem: Integrated platform of small molecules and biological activities. In Annual Reports in Computational Chemistry; Ralph, A.W., David, C.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 4, pp. 217–241. [Google Scholar]
- Irwin, J.J.; Sterling, T.; Mysinger, M.M.; Bolstad, E.S.; Coleman, R.G. Zinc: A free tool to discover chemistry for biology. J. Chem. Inf. Model. 2012, 52, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, J.G. Statistical mechanics of fluid mixtures. J. Chem. Phys. 1935, 3, 300–313. [Google Scholar] [CrossRef]
- Zwanzig, R.W. High-temperature equation of state by a perturbation method. I. Nonpolar gases. J. Chem. Phys. 1954, 22, 1420–1426. [Google Scholar] [CrossRef]
- Shirts, M.R.; Pitera, J.W.; Swope, W.C.; Pande, V.S. Extremely precise free energy calculations of amino acid side chain analogs: Comparison of common molecular mechanics force fields for proteins. J. Chem. Phys. 2003, 119, 5740–5761. [Google Scholar] [CrossRef]
- Wolf, M.G.; Groenhof, G. Evaluating nonpolarizable nucleic acid force fields: A systematic comparison of the nucleobases hydration free energies and chloroform-to-water partition coefficients. J. Comput. Chem. 2012, 33, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Chodera, J.D.; Mobley, D.L.; Shirts, M.R.; Dixon, R.W.; Branson, K.; Pande, V.S. Alchemical free energy methods for drug discovery: Progress and challenges. Curr. Opin. Struct. Biol. 2011, 21, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, T.; Labahn, A. Towards accurate free energy calculations in ligand protein-binding studies. Curr. Med. Chem. 2010, 17, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Kuczera, K.; Tidor, B.; Karplus, M. Hidden thermodynamics of mutant proteins: A molecular dynamics analysis. Science 1989, 244, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Gao, J.; Tidor, B.; Brady, J.W.; Karplus, M. Solvent effect on the anomeric equilibrium in D-glucose: A free energy simulation analysis. J. Am. Chem. Soc. 1991, 113, 1553–1557. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Ravimohan, C. Monte carlo simulation of differences in free energies of hydration. J. Chem. Phys. 1985, 83, 3050–3054. [Google Scholar] [CrossRef]
- Kollman, P. Free energy calculations: Applications to chemical and biochemical phenomena. Chem. Rev. 1993, 93, 2395–2417. [Google Scholar] [CrossRef]
- Bash, P.; Singh, U.; Langridge, R.; Kollman, P. Free energy calculations by computer simulation. Science 1987, 236, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.F.; McCammon, J.A. Dynamics and design of enzymes and inhibitors. J. Am. Chem. Soc. 1986, 108, 3830–3832. [Google Scholar] [CrossRef]
- Merz, K.M.; Kollman, P.A. Free energy perturbation simulations of the inhibition of thermolysin: Prediction of the free energy of binding of a new inhibitor. J. Am. Chem. Soc. 1989, 111, 5649–5658. [Google Scholar] [CrossRef]
- Kitamura, K.; Tamura, Y.; Ueki, T.; Ogata, K.; Noda, S.; Himeno, R.; Chuman, H. Binding free-energy calculation is a powerful tool for drug optimization: Calculation and measurement of binding free energy for 7-azaindole derivatives to glycogen synthase kinase-3β. J. Chem. Inf. Model. 2014, 54, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Okada, O.; Yamashita, H.; Takedomi, K.; Ono, S.; Sunada, S.; Kubodera, H. Prediction of the binding affinity of compounds with diverse scaffolds by MP-CAFEE. Biophys. Chem. 2013, 180–181, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, H.; Tanida, Y.; Ito, M.; Jayachandran, G.; Snow, C.D.; Shirts, M.R.; Sorin, E.J.; Pande, V.S. Direct calculation of the binding free energies of fkbp ligands. J. Chem. Phys. 2005, 123, 084108. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, H.; Tanida, Y.; Matsuura, A. Massively parallel computation of absolute binding free energy with well-equilibrated states. Phys. Rev. E 2009, 79, 021914. [Google Scholar] [CrossRef] [PubMed]
- DeBolt, S.E.; Kollman, P.A. Investigation of structure, dynamics, and solvation in 1-octanol and its water-saturated solution: Molecular dynamics and free-energy perturbation studies. J. Am. Chem. Soc. 1995, 117, 5316–5340. [Google Scholar] [CrossRef]
- Huang, W.; Blinov, N.; Kovalenko, A. Octanol–water partition coefficient from 3D-RISM-KH molecular theory of solvation with partial molar volume correction. J. Phys. Chem. B 2015, 119, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, N.; Kamath, G.; Chelst, I.; Potoff, J.J. Direct calculation of 1-octanol–water partition coefficients from adaptive biasing force molecular dynamics simulations. J. Chem. Phys. 2012, 137, 014502. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.; Hünenberger, P.H.; van Gunsteren, W.F. Efficient combination of environment change and alchemical perturbation within the enveloping distribution sampling (eds) scheme: Twin-system eds and application to the determination of octanol–water partition coefficients. J. Chem. Theory Comput. 2013, 9, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Siepmann, J.I. Partitioning of alkane and alcohol solutes between water and (dry or wet) 1-octanol. J. Am. Chem. Soc. 2000, 122, 6464–6467. [Google Scholar] [CrossRef]
- Pranata, J.; Jorgensen, L.W. Monte carlo simulations yield absolute free energies of binding for guanine—Cytosine and adenine—Uracil base pairs in chloroform. Tetrahedron 1991, 47, 2491–2501. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Buckner, J.K.; Boudon, S.; Tirado-Rives, J. Efficient computation of absolute free energies of binding by computer simulations. Application to the methane dimer in water. J. Chem. Phys. 1988, 89, 3742–3746. [Google Scholar] [CrossRef]
- Bennett, C.H. Efficient estimation of free energy differences from monte carlo data. J. Comput. Phys. 1976, 22, 245–268. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- De Leeuw, S.W.; Perram, J.W.; Smith, E.R. Simulation of electrostatic systems in periodic boundary conditions. I. Lattice sums and dielectric constants. Proc. R. Soc. A. Math. Phys. Sci. 1980, 373, 27–56. [Google Scholar] [CrossRef]
- Zhang, C.; Hutter, J.; Sprik, M. Computing the kirkwood G-factor by combining constant maxwell electric field and electric displacement simulations: Application to the dielectric constant of liquid water. J. Phys. Chem. Lett. 2016, 7, 2696–2701. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Abascal, J.L.F. Simulating water with rigid non-polarizable models: A general perspective. Phys. Chem. Chem. Phys. 2011, 13, 19663–19688. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, M.; Frank, E.U. Static dielectric constant of water and steam. J. Phys. Chem. Ref. Data 1980, 9, 1291–1306. [Google Scholar] [CrossRef]
- Smyth, C.P.; Stoops, W.N. The dielectric polarization of liquids. Vi. Ethyl iodide, ethanol, normal-butanol and normal-octanol. J. Am. Chem. Soc. 1929, 51, 3312–3329. [Google Scholar] [CrossRef]
- Smyth, C.P.; Stoops, W.N. The dielectric polarization of liquids. Vii. Isomeric octyl alcohols and molecular orientation. J. Am. Chem. Soc. 1929, 51, 3330–3341. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef] [PubMed]
- Bannan, C.C.; Calabró, G.; Kyu, D.Y.; Mobley, D.L. Calculating partition coefficients of small molecules in octanol/water and cyclohexane/water. J. Chem. Theory Comput. 2016, 12, 4015–4024. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Kollmann, P.A. Application of RESP charges to calculate conformational energies, hydrogen bond energies, and free energies of solvation. J. Am. Chem. Soc. 1993, 115, 9620–9631. [Google Scholar] [CrossRef]
- Cieplak, P.; Cornell, W.D.; Bayly, C.; Kollman, P.A. Application of the multimolecule and multiconformational RESP methodology to biopolymers: Charge derivation for DNA, RNA, and proteins. J. Comput. Chem. 1995, 16, 1357–1377. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Shirts, M.R.; Bair, E.; Hooker, G.; Pande, V.S. Equilibrium free energies from nonequilibrium measurements using maximum-likelihood methods. Phys. Rev. Lett. 2003, 91, 140601–140604. [Google Scholar] [CrossRef] [PubMed]
- Beutler, T.C.; Mark, A.E.; van Schaik, R.C.; Gerber, P.R.; van Gunsteren, W.F. Avoiding singularities and numerical instabilities in free energy calculations based on molecular simulations. Chem. Phys. Lett. 1994, 222, 529–539. [Google Scholar] [CrossRef]
- Zacharias, M.; Straatsma, T.P.; McCammon, J.A. Separation-shifted scaling, a new scaling method for lennard-jones interactions in thermodynamic integration. J. Chem. Phys. 1994, 100, 9025–9031. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Huo, S.; Lee, M.; Kollman, P.A. Solvation model based on weighted solvent accessible surface area. J. Phys. Chem. B 2001, 105, 5055–5067. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds not available. |
| Parameter | R | R2 | RMSE a (kJ/mol) | MAE b (kJ/mol) |
|---|---|---|---|---|
| Pwater{v} | 0.92 | 0.84 | 4.47 | 3.63 |
| Pwater{o} | 0.93 | 0.87 | 5.05 | 3.74 |
| Pwater{w} | 0.93 | 0.87 | 5.69 | 4.28 |
| Parameter | R | R2 | RMSE a (kJ/mol) | MAE b (kJ/mol) |
|---|---|---|---|---|
| Poctanol{v, v} | 0.89 | 0.80 | 11.18 | 10.36 |
| Poctanol{v, o} | 0.90 | 0.81 | 11.23 | 10.49 |
| Poctanol{v, w} | 0.90 | 0.81 | 11.26 | 10.56 |
| Poctanol{o, v} | 0.88 | 0.78 | 13.59 | 12.53 |
| Poctanol{o, o} | 0.89 | 0.78 | 13.70 | 12.77 |
| Poctanol{o, w} | 0.89 | 0.79 | 13.65 | 12.72 |
| Poctanol{w, v} | 0.88 | 0.77 | 14.18 | 13.05 |
| Poctanol{w, o} | 0.89 | 0.79 | 14.15 | 13.18 |
| Poctanol{w, w} | 0.89 | 0.79 | 14.23 | 13.26 |
| Solvents | Charge | ε | <μ> (Debye) | Flu c (Debye2) | <V> (Å3) | vdW (kJ/mol) | Elec. (kJ/mol) | |
|---|---|---|---|---|---|---|---|---|
| Exp. | Sim. | |||||||
| Water | 78.49 (25 °C) a | 92.70 | 2.35 | 20,914.2 | 22,290.7 | 14,371.9 | −33,641.5 | |
| Octanol | Vacuum | 9.34 (32.1 °C) b | 4.21 | 2.08 | 836.1 | 26,547.7 | −3860.9 | −2778.5 |
| Octanol | 3.63 | 2.24 | 678.0 | 26,320.5 | −3768.3 | −3687.8 | ||
| Water | 3.42 | 2.27 | 625.2 | 26,347.2 | −3714.9 | −3879.3 | ||
| Parameters | R | R2 | RMSE 1 (kJ/mol) | MAE 2 (kJ/mol) |
|---|---|---|---|---|
| PlogP{v, {v, v}} | 0.77 | 0.59 | 2.16 | 2.04 |
| PlogP{v, {v, o}} | 0.80 | 0.64 | 2.17 | 2.07 |
| PlogP{v, {v, w}} | 0.82 | 0.68 | 2.17 | 2.07 |
| PlogP{v, {o, v}} | 0.55 | 0.31 | 2.61 | 2.42 |
| PlogP{v, {o, o}} | 0.60 | 0.36 | 2.63 | 2.46 |
| PlogP{v, {o, w}} | 0.62 | 0.38 | 2.62 | 2.45 |
| PlogP{v, {w, v}} | 0.49 | 0.24 | 2.72 | 2.51 |
| PlogP{v, {w, o}} | 0.54 | 0.29 | 2.72 | 2.53 |
| PlogP{v, {w, w}} | 0.54 | 0.29 | 2.74 | 2.55 |
| PlogP{o, {v, v}} | 0.92 | 0.84 | 1.40 | 1.31 |
| PlogP{o, {v, o}} | 0.92 | 0.85 | 1.42 | 1.34 |
| PlogP{o, {v, w}} | 0.92 | 0.85 | 1.43 | 1.35 |
| PlogP{o, {o, v}} | 0.85 | 0.73 | 1.79 | 1.69 |
| PlogP{o, {o, o}} | 0.87 | 0.76 | 1.82 | 1.73 |
| PlogP{o, {o, w}} | 0.88 | 0.77 | 1.81 | 1.72 |
| PlogP{o, {w, v}} | 0.82 | 0.67 | 1.90 | 1.78 |
| PlogP{o, {w, o}} | 0.84 | 0.71 | 1.90 | 1.81 |
| PlogP{o, {w, w}} | 0.84 | 0.71 | 1.92 | 1.82 |
| PlogP{w, {v, v}} | 0.92 | 0.85 | 1.26 | 1.17 |
| PlogP{w, {v, o}} | 0.92 | 0.86 | 1.29 | 1.20 |
| PlogP{w, {v, w}} | 0.92 | 0.85 | 1.31 | 1.21 |
| PlogP{w, {o, v}} | 0.88 | 0.78 | 1.63 | 1.55 |
| PlogP{w, {o, o}} | 0.90 | 0.80 | 1.66 | 1.59 |
| PlogP{w, {o, w}} | 0.90 | 0.81 | 1.66 | 1.58 |
| PlogP{w, {w, v}} | 0.86 | 0.75 | 1.73 | 1.64 |
| PlogP{w, {w, o}} | 0.88 | 0.77 | 1.75 | 1.66 |
| PlogP{w, {w, w}} | 0.88 | 0.77 | 1.76 | 1.68 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogata, K.; Hatakeyama, M.; Nakamura, S. Effect of Atomic Charges on Octanol–Water Partition Coefficient Using Alchemical Free Energy Calculation. Molecules 2018, 23, 425. https://doi.org/10.3390/molecules23020425
Ogata K, Hatakeyama M, Nakamura S. Effect of Atomic Charges on Octanol–Water Partition Coefficient Using Alchemical Free Energy Calculation. Molecules. 2018; 23(2):425. https://doi.org/10.3390/molecules23020425
Chicago/Turabian StyleOgata, Koji, Makoto Hatakeyama, and Shinichiro Nakamura. 2018. "Effect of Atomic Charges on Octanol–Water Partition Coefficient Using Alchemical Free Energy Calculation" Molecules 23, no. 2: 425. https://doi.org/10.3390/molecules23020425
APA StyleOgata, K., Hatakeyama, M., & Nakamura, S. (2018). Effect of Atomic Charges on Octanol–Water Partition Coefficient Using Alchemical Free Energy Calculation. Molecules, 23(2), 425. https://doi.org/10.3390/molecules23020425






